Abstract
Sterculia foetida L., commonly known as the Java olive, is a tropical tree species native to regions of East Africa, tropical Asia, and northern Australia. This study employs species distribution modeling (SDM) to predict the potential geographic distribution of S. foetida under current and future climate scenarios. Using 1425 occurrence data and 19 environmental variables, we applied an ensemble modelling approach of three algorithms: Boosting Regression Trees (BRT), Generalized Linear Model (GLM), and Random Forests (RF), to generate distribution maps. Our models showed high accuracy (mean AUC = 0.98) to indicate that S. foetida has a broad ecological niche, with high suitability in tropical and subtropical regions of north Australia (New Guinea and Papua), Southeast Asia (India, Thailand, Myanmar, Taiwan, Philippines, Malaysia, Sri Lanka), Oman and Yemen in the southwest of Asia, Central Africa (Guinea, Ghana, Nigeria, Congo, Kenya and Tanzania), the Greater and Lesser Antilles, Mesoamerica, and the north of South America (Colombia, Panama, Venezuela, Ecuador and Brazil). Indeed, the probability of occurrence of S. foetida positively correlates with the Maximum temperature of warmest month (bio5), Mean temperature of wettest quarter (bio8) and Precipitation of wettest month (bio13). The model results showed a suitability area of 4,744,653 km2, representing 37.86% of the total study area, classified into Low (14.12%), Moderate (8.71%), and High suitability (15.02%). Furthermore, the study found that habitat suitability for S. foetida showed similar trends under both near future climate scenarios (SSP1-2.6 and SSP5-8.5 for 2041–2060), with a slight loss in potential distribution (0.24% and 0.25%, respectively) and moderate gains (1.98% and 2.12%). In the far future (2061–2080), the low scenario (SSP1-2.6) indicated a 0.29% loss and a 2.52% gain, while the high scenario (SSP5-8.5) showed a more dramatic increase in both loss (0.6%) and gain areas (3.79%). These findings are crucial for conservation planning and management, particularly in regions where S. foetida is considered invasive and could become problematic. The study underscores the importance of incorporating climate change projections in SDM to better understand species invasiveness dynamics and inform biodiversity conservation strategies.
1. Introduction
Climate change alters the distribution, structure, and functioning of plant species in ecosystems [1,2]. The current global rate of plant extinction is rising, and it may increase tenfold within the next century [3]. The major threats to biodiversity and ecosystem functioning are population growth, agricultural intensification, and infrastructure development [4], which all contribute to the rise in greenhouse gas emissions and resulting climate change [5]. Plant traits are strongly influenced by environmental and climatic factors [6]. Specially, precipitation and temperature govern plant communities’ growth, development, geographical distribution, diversity, and richness [7,8]. Habitats where species thrive natively or are regularly planted may become unsuitable for them in the future due to trees’ incapacity to match the rapid rate of climate change [9,10]. Therefore, assessing the potential adaptability of tree species is crucial for effective forest management planning [11]. In recent years, various tools and indicators have been developed and used to predict species distribution ranges affected by climate change, offering significant scientific insights into habitat loss and fragmentation across diverse ecosystems [12,13,14].
Ecological niche modeling (ENM), also known as species distribution modeling (SDM) or habitat suitability modeling, is gaining popularity over the other tools of analysis available for ecologists to predict the distribution and niches of species using occurrence records and environmental variables [15,16]. SDMs have been extensively utilized in various research domains, including species distribution prediction under current and future scenarios [17,18,19,20], conservation, invasive species prediction, plant disease prediction and forest management planning [21,22,23], identifying potential introduction and cultivation areas [24,25,26]. A variety of SDM tools are accessible, including Boosted Regression Trees (BRT) [27], Support Vector Machine (SVM) [28], Random Forests (RF) [29], Maximum Entropy (MaxEnt) [30], Generalized Additive Models (GAMs), Generalized Linear Models (GLMs) [31], and Genetic Algorithm for Rule-set Prediction (GARP) [32]. Using several models at once to estimate species distribution modeling can lead to substantial changes in predictions and probable biases linked to the selected model [33]. Therefore, we combined SDM models into an ensemble model to improve robustness [17].
Sterculia foetida L. (Family: Malvaceae), also known as wild almond, java olive, and bastard poon tree, inhabits tropical and subtropical areas [34]. It is a deciduous tree which grows to a height of up to 40 m, mainly found along roadsides and parks in shady places [34]. It contains various important bioactive compounds such as fatty acids, terpenoids, tannins, protein, lipid, flavonoids, alkaloids, phenols, saponins, sterols, glycoside, coumarin, and carbohydrate [35,36]. The seeds of S. foetida are the richest natural source of sterculic acid, containing up to 78% [37]. Sterculic acid is a cyclopropane fatty acid known for its wide range of biological effects and capacity to treat several alignments such as Alzheimer’s disease, cancer, non-alcoholic steatohepatitis, and retinal disorders. Various chemical activities have been identified in this species such as anti-inflammatory, anti-nociceptive, antioxidant, anti-cancer, anti-inflammatory, anti-microbial, anti-allergic, anti-fertility, anti-obesity, anti-osteosarcoma, and anti-diabetic properties [36,38,39]. The seed oil, resin, leaves, and bark of S. foetida are known for their notable medicinal benefits; the leaves are used as herbal medicine for their laxative, diuretic, and insect-repelling effects. It is traditionally used to treat several ailments including rheumatism, skin diseases, dysentery, cirrhosis, arthritis, wounds, gonorrhea, obesity, and edema [40]. The resin of this species includes vital minerals needed for metabolic processes in the body, such as potassium, calcium, zinc, magnesium, ferrite, manganese, aluminum, and more [40]. The oil extracted from the seeds is rich in fatty acids, and is utilized for cooking and baking [41]. The seed oil possesses a high acid value, making it a significant potential candidate for biofuel production [42,43]. This important tree species is facing threats due to over-exploitation and unscientific methods of extracting resin [44]. Thus, estimation of the potential habitat suitability under current and future climatic scenarios of S. foetida can be valuable in developing strategies for its conservation and sustainable utilization. The present study aimed to do the following: (1) Predict suitable habitats for Sterculia foetida using an ensemble analysis that combines three algorithms: Boosted Regression Trees (BRT), Random Forest (RF), and Generalized Linear Model (GLM). (2) Determine the environmental factors influencing the distribution of S. foetida habitats. (3) Identify potential habitat suitability of S. foetida under present climate conditions (1970–2000). (4) Project changes in suitable habitats for S. foetida under climate change scenarios for the 2060s and 2080s, considering the risks of naturalization and invasiveness of the species outside of its native range.
2. Materials and Methods
2.1. Studied Species and Occurrence Data
Sterculia foetida L. is a large, deciduous tree that can grow up to 40 m tall and 3 m wide, with a trunk diameter of 100–120 cm [38]. Its branches spread horizontally, and the bark changes from smooth and grey in young trees to dark brown and rough in mature trees. The leaves are clustered at the branch tips, arranged in a digitate formation with seven to nine leaflets per leaf, emitting an unpleasant odor [40]. The tree produces racemose panicles of flowers that are green to dull purple and dioecious, with male and female flowers on separate trees. The fruit bears large, scarlet follicles containing 10–15 seeds, which are exalbuminous and encased in a hard shell [38]. This species grows in tropical lowlands and moderate highlands up to 1500 m above sea level, predominantly found in East Asia, including India, Sri Lanka, Myanmar, and Thailand [45]. It thrives in tropical lowlands with hot climates, preferring daytime temperatures of 18–32 °C and annual rainfall of 1100–1800 mm. It grows best in deep, fertile, well-drained soils under sunny conditions and can tolerate a pH range of 5–8 [46]. It is typically found in lowland dry woodlands, primary and secondary forests, and coastal areas. The tree prefers riverbanks, sandstone rocks along the coast, and thickets. Seeds should be sown immediately after collection due to rapid sprouting. Scarification is required to break physical dormancy, and ideal germination occurs at 20–30 °C. Approximately 95% of seeds germinate within two weeks with proper pretreatment [46]. For most of human history, S. foetida has been a valuable supplier of natural timber and non-timber materials. Its leaves and bark have traditionally been used as a diaphoretic, aperitif, and diuretic. It grows throughout the dry seasons and is now found in the tropical zones of Africa, Australia, and Asia. It is native to Vietnam, Thailand, Sumatra, Sri Lanka, the Philippines, Myanmar, Malaya, the Lesser Sunda Is., Laos, India, Cambodia, Bangladesh, Assam, and has been introduced into the Windward Is., Trinidad and Tobago, Puerto Rico, Pakistan, the Leeward Is., Jamaica, Hainan, the Gulf of Guinea Is., Cuba, Comoros, and southeast China [47]. The research history of this species, particularly regarding its phytochemical and pharmacological properties, has been previously documented [40].
However, few details are available about the introduction of S. foetida outside its native range. Although the flowers have an unpleasant smell, it is an attractive tree used as an ornamental. There is a report of the species being cultivated in Saint Vincent in the 1800s [46]. Evidence from herbarium specimens shows the species present in Puerto Rico and Cuba since 1923 and 1927, respectively (New York Botanical Garden herbarium specimens), and in Guadeloupe since 1932 (Smithsonian National Museum of Natural History herbarium specimens) [46]. Despite being known for its low to medium risk of introduction in tropical and subtropical areas, S. foetida has been reported as invasive in Cuba. Although first identified as invasive in Brazil, it has recently been confirmed that the species is not considered invasive in the country. In Cuba, it is recognized as a transformer species that has a tendency to expand throughout the country [46]. It is categorized as a weed in Egypt and Puerto Rico; however, there is no evidence of human effect or management of the species [48]. Hence, its current status is Possibly Invasive [46].
Interestingly, S. foetida is found in over 200 locations across various countries, including India, Sri Lanka, Myanmar, Thailand, and the Philippines, covering an area of 18,725,503 km2. Despite habitat decline, it is classified as Least Concern due to its widespread distribution and presence in at least four protected sites [45]. Population density varies significantly, with eight to 20 mature trees per hectare in intact forests and fewer than five trees per hectare in fragmented areas. Major threats include deforestation and habitat destruction due to agriculture and urbanization. Conservation efforts include protection in at least four areas and ex situ collections in 45 locations. It was assessed as Least Concern in Sri Lanka in 2020 [45].
Information on occurrence sites of Sterculia foetida used in the current paper was compiled from the following sources: 1—National Herbaria: the United States National Herbarium (US), the Herbarium of the University of Puerto Rico Río Piedras Campus (UPRRP), Indian Virtual Herbarium, Botanical Survey of India (https://ivh.bsi.gov.in, accessed on 1 December 2024), Chinese virtual Herbarium, China (https://www.cvh.ac.cn/, accessed on 1 December 2024), Singapore Herbarium Online (https://herbaria.plants.ox.ac.uk/bol/sing, accessed on 1 December 2024); 2—Botanical Databases: Plants of South Asia Database (https://data.nhm.ac.uk/search, accessed on 1 December 2024), Global plants on JSTOR (https://plants.jstor.org/collection/TYPSPE, accessed on 1 December 2024), Global Biodiversity Information Facility [49], iNaturalist (https://www.inaturalist.org/taxa/346407-Sterculia-foetida, accessed on 1 December 2024); and 3—field surveys. A total of 1425 distribution sites were documented in the initial study. This work was implemented in ArcGIS 10.8 (ESRI) (Figure 1).
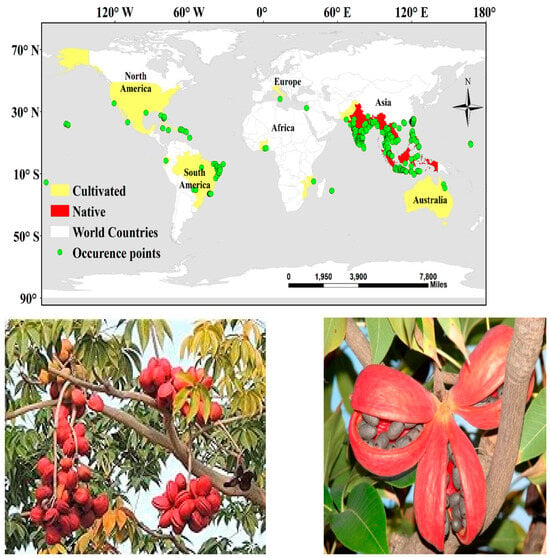
Figure 1.
Study area (above) showing the occurrence of Sterculia foetida, indicating locations of the collected occurrence records; (below) Sterculia foetida growth in its natural habitat (plant photos obtained from [46]).
2.2. Environmental Data
The WorldClim Global Climate Database was used to acquire 19 environmental variables at a geographical resolution of 2.5 arc minutes (~5 km) ([50], http://www.worldclim.org, accessed on 1 January 2025), in an initial study. These climatic variables have been generated from monthly temperature and precipitation data, as well as seasonal variations and meteorological extreme indices from 1970 to 2000. Environmental variables employed in SDMs may either indirectly or directly affect the effectiveness of the model [51] (Table 1).

Table 1.
Environmental variables used in the study (sources: http://worldclim.org/version2.1, accessed on 22 April 2025).
To determine the relevance of the environmental factors, preliminary exploratory data analyses and a multicollinearity test were performed. Variance inflation factors (VIFs) were utilized to discover multicollinearity and identify the influential variables that would be used to predict the analyzed species distribution. Variables with a VIF value greater than 5 were ignored since their contributions were insignificant. VIF was implemented using the “usdm” package [52]. The vifcor and vifstep functions of the usdm package [53] were used within the framework of R 4.2.1 [54].
2.3. Modeling Construction
IPSL-CM6A-LR general circulation models (GCMs) were used to forecast the global spread of S. foetida in the near future. This model was chosen because it accurately represents the observed contemporary climate and the geographical patterns of global and zonal precipitation and temperature distribution. GCMs accurately simulate global warming and multi-decadal variations in temperature and precipitation, projecting a larger increase in the mean annual temperature than other models over the same time frame [55,56]. Temperature and precipitation estimates were analyzed for 23 GCMs and four Shared Socio-economic Pathways (SSPs) in the IPCC’s sixth climate assessment report (AR6), which discusses various different emission scenarios (126, 245, 370, and 585 SSPs).
In this study, the future distribution of the studied species was predicted using IPSL-CM6A-LR GCMs for the future periods of the 2060s (averaged over 20 years from 2041 to 2060) and 2080s (averaged over 20 years from 2061 to 2080), and two Shared Socioeconomic Pathways (SSP1-2.6 and SSP5-8.5). This was undertaken to portray the variances in the future projections by the GCMs and have insights into the future potential of the researched species under different emission scenarios both in the short-term and long-term.
Furthermore, it was assumed that the global mean temperature will increase by 0.3–4.8 °C by 2100 throughout all SSPs [57]. All future climate projection data were downloaded from the WorldClim Database (http://www.worldclim.org, accessed on 1 January 2025). All current and future data had a spatial resolution of 2.5 arc min (approx. ∼5 km resolution at the equator).
2.4. Ensemble Modeling
The ensemble model technique (EM) is considered to be superior to other standard modeling techniques in terms of model performance transferability and optimization [58,59,60]; therefore, it was utilized in this study to minimize uncertainties in model predictions. Three algorithms were used to build the ensemble model for species distribution. The three algorithms included Boosting Regression Trees (BRT: [27,61]), the Generalized Linear Model (GLM: [62]) as a parametric technique, and Random Forests (RF: [29,63]) as a non-parametric machine-learning technique. In comparison to other models, the chosen methods exhibit good stability and transferability [60,64,65]. Furthermore, GLM and RF behave best on both cross-validation and external validation [66]. The ensemble of the species distribution models was conducted using the ‘sdm’ R package version 1.1-8 [52]. The distribution of the studied species was predicted using each of the three modelling techniques under current climatic conditions using 70% of the data set for training while keeping the remaining 30% for model testing and evaluation [60]. The EM weighted the modeling methods by the True Skill Statistic (TSS) and the Maximum training sensitivity plus specificity (MTSS) criterion using the sdm package in R 4.2.1 [52]. The MTSS criterion is recommended as threshold rule because it reduces both the commission (over-prediction) and omission (under-prediction) errors [58,67]. To address concerns regarding the absence of true absence data in species distribution modeling, the ‘sdm’ package in R provides a robust solution by generating background points, also known as pseudo-absences. These points are randomly sampled from the study area where no presence data has been recorded, allowing the model to contrast environmental conditions at presence locations with those at background locations. This approach is particularly useful when only presence data are available, which is common in ecological studies due to limitations in survey efforts or detection probabilities. By using presence-only data alongside background points, the ‘sdm’ package enables the construction of reliable models that estimate species–environment relationships and predict potential distributions, while accounting for sampling bias and spatial autocorrelation [52].
2.5. Model Evaluation
Evaluation of model performance is a critical stage in niche modeling. The area under the curve (AUC) for the receiver operating characteristic (ROC) [68] and the true skill statistic (TSS; sensitivity + specificity − 1) [69] were used to estimate the performance of the model according to [70]. AUC value varies from 0 to 1, where a value below 0.5 can be taken as a random prediction; 0.5–0.7 indicates poor model performance; 0.7–0.9 shows moderate performance; and a value over 0.9 is deemed to have “excellent’’ discriminating capabilities [71]. The TSS takes into consideration both omission and commission errors, with values ranging from −1 to 1, with 1 indicating perfect agreement and 0 representing random fit. Excellent model performance is reflected by TSS values near to 1 [69].
To analyze the impact of environmental variables on Sterculia foetida distribution, the relative variable importance was employed to calculate the importance and percentage contributions of each variable. To maximize predictive information and simplify future analysis, suitable habitat areas for Sterculia foetida were reclassified into four levels: unsuitable habitat (no risk), low habitat suitability (low risk), moderate habitat suitability (medium risk), and high habitat suitability. To account for heterogeneity between GCMs, a final projected environmental suitability map for S. foetida was generated by averaging estimates from all future climate scenarios. The reclassification function in the Spatial Analyst Tools in the context of ArcGIS 10.8 was used to perform the categorization. To measure the change in the distribution of the suitable habitat in terms of loss/gain or persistence under future scenarios, the “Map Algebra” functions within the framework of ArcGIS 10.8 were applied to binary maps resulting from the transformed current and future habitat suitability probability maps. The estimation of the changes in the distribution of suitable habitat between the current and the future scenarios in each period was calculated by SDM toolbox v2.5 within the framework of ArcGIS 10.8, and the potential change maps for each period under different scenarios were obtained. Through the estimation of the changes in the potential distribution of habitat using the produced binary maps of the species studied under different climate scenarios, the tendency for geographical shift in the species’ distribution was analyzed (Figure 2).
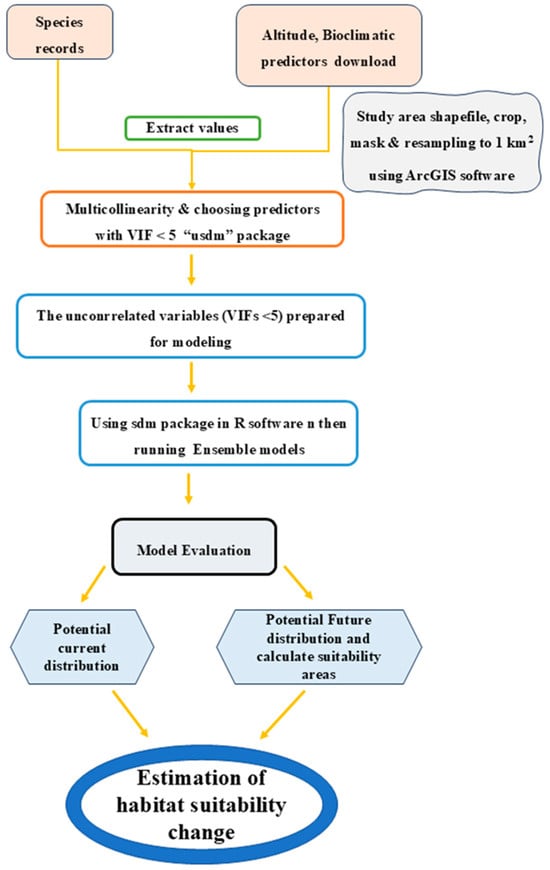
Figure 2.
Flow chart of the ensemble ecological niche modeling algorithms utilized for habitat suitability modeling of Sterculia foetida.
3. Results
3.1. Model Performance for Potential Distribution
The study used seven climatic variables (Table 2): bio2 (Mean diurnal range (Mean of monthly (max temp-min temp)), bio4 (Temperature Seasonality (standard deviation × 100)), bio5 (Max temperature of warmest month), bio8 (Mean temperature of wettest quarter), bio13 (Precipitation of wettest month), bio14 (Precipitation of driest month), and bio19 (Precipitation of coldest quarter) based on multicollinearity analysis of the total 19 bioclimatic variables for habitat suitability modeling of S. foetida.

Table 2.
Employed environmental variables to model the distribution probability of Sterculia foetida in this study. Problems related to collinearity were avoided by removing variables with VIF values > 5. The variables highlighted were selected depending on a multi-collinearity test and were used in the modeling process.
Ensemble models of GLM, BRT, and RF demonstrated great accuracy and performance, with an AUC value of 0.98 ± 0.021 and a TSS value of 0.90 ± 0.054 (Figure 3). Temperature seasonality (bio4), maximum temperature of the warmest month (bio5), and mean diurnal range (bio2) were the most important variables explaining the probable distribution of Sterculia foetida with relative importance higher than 10% to 40% (Figure 4).
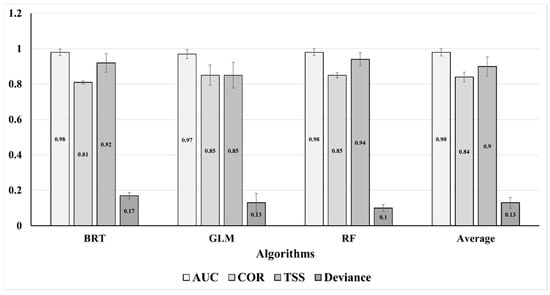
Figure 3.
Accuracy measures used for evaluation of the models predicting the potential distribution of Sterculia foetida.
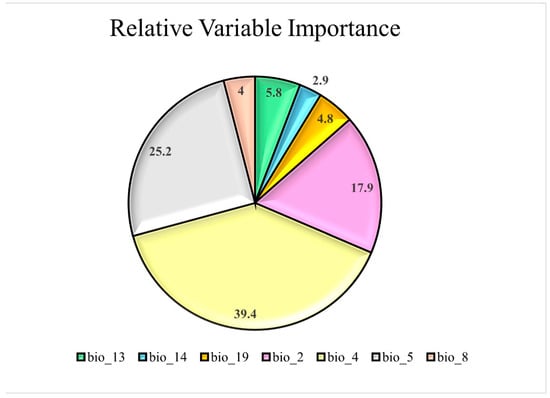
Figure 4.
The relative importance (%) of ten environmental predictors used in the ecological niche modelling of Sterculia foetida. For abbreviations, see Table 1.
The response curves revealed that there is a positive relationship between the Max bio5, bio8 and bio13 and the probability of presence of Sterculia foetida that means the increase of these variables lead to an increase in the probability of occurrence (Figure 4). In contrast, bio2, bio4, bio14, and bio19 have a negative relationship with the probability of occurrence of Sterculia foetida (Figure 5).
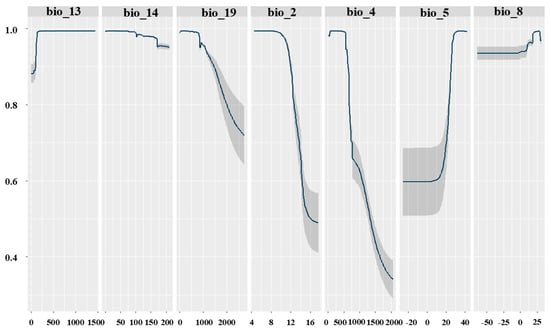
Figure 5.
The response curves of seven environmental predictors used in the ecological niche modelling based on ensemble models for Sterculia foetida. For abbreviations, see Table 1.
3.2. Current and Future Potential Distribution of Sterculia foetida
Area Under the Curve (AUC) and true skill statistic (TSS) results demonstrate that the models developed for the training data set are reliable (mean AUC value of 0.98 ± 0.021 and mean TSS value of 0.90 ± 0.05). Ensemble SDM presents high habitat suitability of Sterculia foetida in parts of north Australia (New Guinea and Papua), Southeast Asia (India, Thailand, Myanmar, Taiwan, Philippines, Malaysia, Sri Lanka), Oman and Yemen in the southwest of Asia, Central Africa (Guinea, Ghana, Nigeria, Congo, Kenya and Tanzania), Greater and Lesser Antilles, Mesoamerica, and the north of South America (Colombia, Panama, Venezuela, Ecuador and Brazil) (Figure 6). The model results show that the suitability area is equal to 4,744,653 km2, which represents 37.86% of the total study area. The suitability area was classified into Low suitable (1,769,711 km2 (14.12%)), Moderate suitable (1,092,161 km2 (8.71%)) and High suitable (1,882,781 km2 (15.02%)).
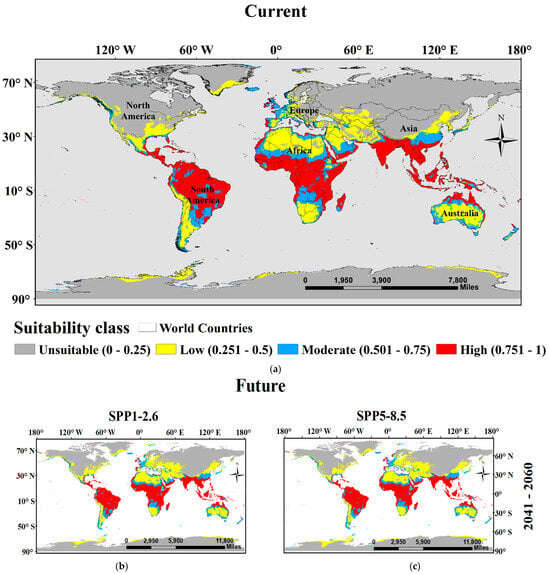
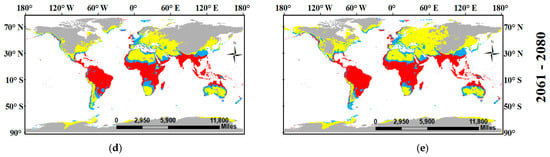
Figure 6.
Location maps showing the potential current (a) and future habitat (under two global warming scenarios SSPs1-2.6 ((b) for years 2041–2060 and (d) for years 2061–2080) and SSPs5-8.5 ((c) for years 2041–2060—(e) for years 2061–2080) of Sterculia foetida. Based on ensemble models, the habitat suitability classes include unsuitable (0–25), Low potential (0.251–0.50), Moderate potential (0.501–0.75), and High potential (0.751–1).
The potential future changes in habitat suitability of Sterculia foetida were similar under both climate change scenarios (Figure 6 and Figure 7 and Table 3). Predictions under the SSP1-2.6 scenario of the IPSL-CM6A-LR GCM model for the period 2041–2060 revealed a projected expansion of the current area of distribution, with the projected suitable area covering 5,173,383 km2 (41.28% of the total study area). This suitable area was classified into a High suitable area which covered 2,072,686 km2 (that is, 16.54% of the study area), Moderate suitable area covering 1,023,116 km2 (8.16%) and Low suitable area covering 2,077,581 km2 (16.58% of the study area). The suitable area for Sterculia foetida distribution will increase in the future by 2080 under the same scenario to reach 5,432,642 Km2 (43.35% of the study area); of this total area the High, Moderate, and Low suitable areas will cover 2,278,046, 1,030,293, and 2,124,303 Km2, respectively (18.18, 8.22 and 16.95% of the study area).
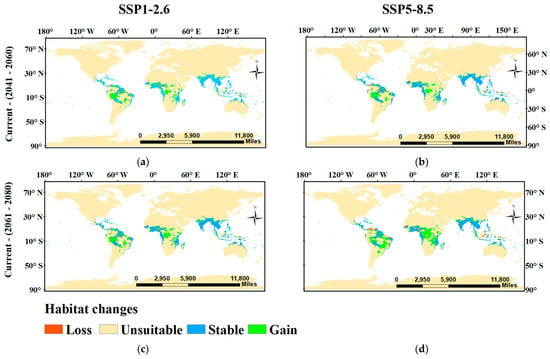
Figure 7.
Percentage of habitat suitability classes of Sterculia foetida predicted by the ensemble model based on the IPSL-CM6A-LR general climate model under the scenarios SSP1-2.6 and SSP5-8.5 during the period 2041–2060 and 2060–2080. (a) under SSP1-2.6 for 2041–2060, (b) under SSP1-2.6 for 2061–2080, (c) under SSP5-8.5 for 2041–2060, (d) under SSP5-8.5 for 2061–2080.

Table 3.
The area (km2) and percentage of the suitable habitat and the potential changes in habitat for Sterculia foetida under current and future climate change based on Ensemble models predictions.
Under the higher emission scenario SSP5-8.5 of the IPSL-CM6A-LR GCM model for the period 2041–2060, the suitable area will cover 5,272,943 km2. All classes were expanded; High and Low suitability areas decreased as compared to the current potential, to cover 2,088,843 and 2,157,453 km2 (respectively 16.67 and 17.21% of the study area). However, the Moderate suitability area will decrease as compared to the current potential to cover 1,026,647 km2, which is 8.19% of the study area. Under the same scenario, by 2080 the suitable area will expand to 6,550,460 km2 (that is, 52.27% of the study area), with the High, Moderate, and Low suitability areas covering 2,341,121, 1,069,011 and 3,140,328 km2, respectively (18.68, 8.53, and 25.06% of the study area).
3.3. Habitat Changes of Sterculia foetida Under Different Future Scenarios
The potential changes in habitat suitability of Sterculia foetida were similar under both near future climate change scenarios (SSP1-2.6 (2041–2060) and SSP5-8.5 (2041–2060)). Both showed a loss in the potential distribution of Sterculia foetida with a percentage of 29,494 km2 (0.24%) and 31,196 km2 (0.25%), respectively. The gain areas in the future were 248,639 km2 (1.98%) and 265,260 km2 (2.12%) of the study area (Figure 8, Table 4).
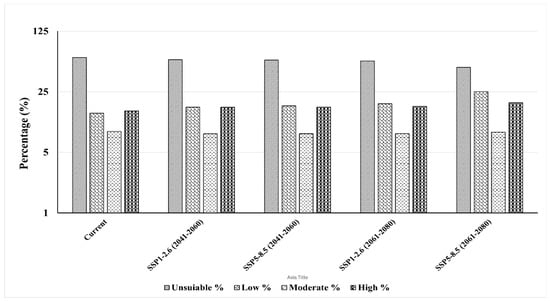
Figure 8.
The predicted distribution of Sterculia foetida based on the ensemble model under two global warming scenarios, SSPs1-2.6.

Table 4.
The area (km2) and percentage of the potential changes in habitat for Sterculia foetida under future climate change based on ensemble models predictions.
However, under the far future low scenario of climate change SSP1-2.6 (2061–2080), the loss area was 0.29% (35,730 km2). Indeed, there was a notable increase in gain areas under the SSP1-2.6 scenario to 2.52% (316,416 km2). Meanwhile, there was a substantial increase in loss areas of 0.6% (75,364 km2) and gain areas of 3.79% (475,424 km2) under the distant future scenario of SSP5-8.5 (2061–2080) (Figure 8, Table 4).
4. Discussion
Species distribution modeling has become a commonly used tool in identifying suitable habitats for endangered and medicinal species [72,73,74,75], invasive species management [76], and mapping of crop-associated pathogens [77]. This study aimed to assess the effects of climate change on the suitable habitats of S. foetida at the global level by using an ensemble modeling approach that integrates three models (BRT, GLM, and RF). Using an ensemble of Boosting Regression Trees (BRT), Generalized Linear Models (GLM), and Random Forests (RF) offers several advantages over Maxent. Ensembles improve predictive performance by combining multiple models, which enhances accuracy and robustness while reducing overfitting. They can handle various data types and manage missing data more effectively. Additionally, GLM provides straightforward interpretation, and BRT and RF offer insights into variable importance and interactions. Ensembles also balance bias and variance, leading to more stable predictions, and modern implementations are optimized for computational efficiency, making them suitable for large datasets. While Maxent is valuable for presence-only data, the ensemble approach offers superior performance, flexibility, and interpretability for complex datasets [65,66,67].
The AUC and TSS values showed outstanding predictive accuracy observed by all three models (BRT, GLM, and RF) in this study. The excellent performance of BRT, GLM, and RF (AUC > 0.97) highlights the effectiveness of these methods in species distribution modeling, and was also reported in the previous studies on Prosopis juliflora [78], Fontainea australis and F. oraria [79], and Uvaria chamae [80].
The habitat suitability of S. foetida was mainly influenced by three environmental variables: temperature seasonality (bio4), max temperature of warmest month (bio5), and mean diurnal range (bio2). The authors of [81] also reported that the temperature seasonality (bio4), max temperature of warmest month (bio5), and mean diurnal range (bio2) were the key variables for the distribution of Sterculia setigera, while the authors of [82] also found that the diurnal temperature range (bio2) was the key variable for Sterculia apetala. S. foetida is well-suited to hot tropical environments with medium to heavy rainfall and a dry season of 3 to 4 months. It is a photophilous species that cannot endure shade or prolonged cold conditions [46]. The optimum temperature for seed germination in this species occurs between 20 °C to 30 °C, which means the bio4, bio5, and bio2 variables could play a significant role in the seed germination of S. foetida.
In this study, the suitable habitat of S. foetida under current climatic conditions is distributed across parts of north Australia (New Guinea and Papua), Southeast Asia (India, Thailand, Myanmar, Taiwan, Philippines, Malaysia, Sri Lanka), Oman and Yemen in the southwest of Asia, Central Africa (Guinea, Ghana, Nigeria, Congo, Kenya and Tanzania), the Caribbean (Cuba), Mesoamerica (Nicaragua), and the north of South America (Colombia, Panama, Venezuela, Ecuador and Brazil). These regions are mainly located in the tropical zone, where the annual average temperature exceeds 18 °C [83]; moreover, this species prefers to grow at temperatures ranging between 18 °C and 32 °C [46]. The temperature seasonality and annual mean temperature were also reported as key drivers in other plant species’ distribution at the global level [84].
The worldwide reduction of the medicinal species population is caused by multiple factors, such as the loss of their natural habitats due to overharvesting, urbanization expansion, and the adverse effects of climate change [85,86]. Climate change influences alterations to species’ habitat ranges by leading to niche shifts, expansions, or contractions [87]. Global warming can lead to habitat loss for Sterculia foetida through several mechanisms. Increased temperatures may alter growth rates and distribution, making some areas unsuitable for its survival. Changes in precipitation patterns can result in droughts, reducing water availability crucial for the tree’s growth. More frequent and severe storms can damage habitats, increasing tree mortality. Coastal habitats may be affected by rising sea levels, leading to habitat loss. Additionally, higher temperatures and drought conditions can increase the frequency and intensity of wildfires, destroying habitats. These factors combined can significantly impact the distribution and health of Sterculia foetida populations [2]. Interestingly, assessments of future habitat suitability show more expansion than reduction under climate change scenarios. The lower contraction and greater expansion of S. foetida’s habitat range under future climate scenarios (2060 and 2080) indicates that the possibilities of more suitable environmental conditions will occur in the future. However, the effects of global climate change on habitat suitability vary depending on geographical location. The possibilities of increased environmental suitability in future scenarios may be due to dominant climatic variables such as temperature seasonality (Bio4), maximum temperature of the warmest month (Bio5), and mean diurnal range (Bio2), which will dominate in future climate scenarios. Similar patterns of habitat expansion in future climate scenarios have been reported for various other species in tropical regions, such as Elaeis guineensis Jacq. (Arecaceae), Mangifera indica L. (Anacardiaceae), Borassus aethiopum Mart. (Arecaceae) [88], Afzelia rhomboidea (Blanco) Fern.-Vill. (Fabaceae), Koordersiodendron pinnatum (Blanco) Merr. (Anacardiaceae), Mangifera altissima Blanco (Anacardiaceae), Pentacme contorta (S.Vidal) Merr. & Rolfe (Dipterocarpaceae), Rubroshorea palosapis (Blanco) P.S.Ashton & J.Heck. (Dipterocarpaceae), Rubroshorea polysperma (Blanco) P.S.Ashton & J.Heck. (Dipterocarpaceae) and Vitex parviflora A.Juss. (Lamiaceae) [75,89].
The trend for more expansion than reduction observed in suitable habitats in this study under future climate change scenarios might reflect the suitable climatic conditions that would occur for Sterculia foetida in future scenarios. However, 19 bioclimatic variables were used in this study to predict the current and future habitat suitability. Nonetheless, further research is necessary to assess how additional climatic and non-climatic factors such as forest composition, soil types, land use and land cover, and human activities influence habitat predictions for S. foetida.
This study represents the first investigation using an ensemble modeling approach to assess the effects of global climate change on the habitat distribution range and future habitat suitability of Sterculia foetida at a global scale. The findings of this study will assist in identifying the most favorable sites for the conservation and cultivation of S. foetida under current and future climate scenarios at a global scale. The predicted suitable habitat of S. foetida will expand more than reduce from the present to the years 2060 and 2080. Therefore, regions with ideal habitat conditions for S. foetida under climate change scenarios will benefit from strategic planning for its future cultivation, conservation, and sustainable utilization.
5. Conclusions
This study highlights the broad ecological niche of Sterculia foetida and its high suitability in tropical and subtropical regions. The species distribution models (SDMs) demonstrated high accuracy, with mean AUC values of 0.98 ± 0.021, indicating that S. foetida is likely to thrive in various regions across the globe. The ensemble SDM revealed high habitat suitability of S. foetida in parts of northern Australia (New Guinea and Papua), Southeast Asia (India, Thailand, Myanmar, Taiwan, Philippines, Malaysia, Sri Lanka), Oman and Yemen in southwest Asia, Central Africa (Guinea, Ghana, Nigeria, Congo, Kenya, and Tanzania), southern North America (Cuba and Nicaragua), and northern South America (Colombia, Panama, Venezuela, Ecuador, and Brazil). Future climate change scenarios predict similar trends in habitat suitability for S. foetida. Under the SSP1-2.6 scenario for 2041–2060, the suitable area is projected to expand to 5,173,383 km2 (41.28% of the study area), while the suitable area will increase to 5,432,642 km2 (43.35%) under the same scenario by 2080. Under the higher emission scenario SSP5-8.5 for 2041–2060, the suitable area will cover 5,272,943 km2, while the suitable area will expand dramatically to 6,550,460 km2 (52.27%) by 2080. Overall, the gain areas are projected to be significantly larger than the loss areas over time. This suggests that the species will find new suitable habitats, potentially increasing its presence in regions where it is currently found and spreading to new areas. These results are crucial for conservation planning and management, particularly in regions where S. foetida is invasive.
Effective management strategies for S. foetida, especially given its potential for range expansion, should focus on prevention and early detection through strict quarantine protocols, public awareness campaigns, conservation of tropical forests, and regular monitoring to detect early signs of spread. Rapid response plans are essential to quickly address new infestations, involving coordinated efforts to remove or control the species before it becomes established. Integrated Pest Management (IPM) should be employed, using a combination of biological, chemical, and mechanical control methods tailored to specific situations. Habitat restoration is crucial to support native species and ecosystems, reducing the competitive advantage of S. foetida and promoting biodiversity. Ongoing research and monitoring are necessary to understand the species’ ecology, distribution, and impacts, allowing for the adaptation of management strategies as needed. Collaboration and partnerships with local communities, governments, NGOs, and other stakeholders can ensure a comprehensive and effective approach to managing the spread of S. foetida and mitigating its potential impacts on ecosystems and biodiversity.
Author Contributions
Conceptualization, H.B. and H.C.S.; methodology, H.B.; software, H.B. and A.R.M.; validation, H.B. and A.R.M.; formal analysis, H.B.; investigation, H.B.; resources, H.B. and H.C.S.; data curation, H.B. and H.C.S.; writing—original draft preparation, M.M.E.-K., A.R.M. and H.C.S.; writing—review and editing, H.B., H.C.S., A.R.M. and M.M.E.-K.; visualization, A.R.M.; supervision, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, L.; Li, H. Projecting the potential distribution and analyzing the bioclimatic factors of four Rhododendron subsect. Tsutsusi species under climate warming. J. For. Res. 2023, 34, 1707–1721. [Google Scholar]
- Bedair, H.; Alghariani, M.S.; Omar, E.; Anibaba, Q.A.; Remon, M.; Bornman, C.; Kiboi, S.K.; Rady, H.A.; Salifu, A.-M.A.; Ghosh, S.; et al. Global Warming Status in the African Continent: Sources, Challenges, Policies, and Future Direction. Int. J. Environ. Res. 2023, 17, 45. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Dinerstein, E.; Boucher, T.; Brooks, T.M.; Butchart, S.H.M.; Hoffmann, M.; Lamoreux, J.F.; Morrison, J.; Parr, M.; Pilgrim, J.D.; et al. Pinpointing and preventing imminent extinctions. Proc. Natl. Acad. Sci. USA 2005, 102, 18497–18501. [Google Scholar] [CrossRef] [PubMed]
- Bargali, S.S.; Shahi, C.; Bargali, K.; Negi, B.; Khatri, K. Energy and monetary efficiencies at the different altitudinal agroecosystems in central Himalaya, India. Heliyon 2022, 8, e11500. [Google Scholar] [CrossRef]
- Arora, V.K.; Scinocca, J.F.; Boer, G.J.; Christian, J.R.; Denman, K.L.; Flato, G.M.; Kharin, V.V.; Lee, W.G.; Merryfield, W.J. Carbon emission limits required to satisfy future representative concentration pathways of greenhouse gases: Allowable future carbon emissions. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Bozkurt, A.E.; Şahan, E.A.; Köse, N. Growth responses of Pinus sylvestris L. to climate from the southeastern limit of its natural distribution area, Turkey. Dendrochronologia 2021, 70, 125897. [Google Scholar] [CrossRef]
- Mo, L.; Liu, J.; Zhang, H.; Xie, Y. The predicted effects of climate change on local species distributions around Beijing, China. J. For. Res. 2020, 31, 1539–1550. [Google Scholar] [CrossRef]
- Dakhil, M.A.; Zhang, L.; El-Barougy, R.F.; Bedair, H.; Hao, Z.; Yuan, Z.; Feng, Y.; Halmy, M.W.A. Diversity pattern of Symplocos tree species in China under climate change scenarios: Toward conservation planning. Glob. Ecol. Conserv. 2024, 54, e03198. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Bedair, H.; Badawy, N.K.; Morsy, A.; Rashad, H.; Dakhil, M.A. Impact of climate change on the spatial distribution of the endemic shrub Rubus asirensis in the Arabian Peninsula. Plant Ecol. 2024, 225, 441–450. [Google Scholar] [CrossRef]
- Liu, D.; Lei, X.; Gao, W.; Guo, H.; Xie, Y.; Fu, L.; Lei, Y.; Li, Y.; Zhang, Z.; Tang, S. Mapping the potential distribution suitability of 16 tree species under climate change in northeastern China using Maxent modelling. J. For. Res. 2022, 33, 1739–1750. [Google Scholar] [CrossRef]
- Jung, J.B.; Park, G.E.; Kim, H.J.; Huh, J.H.; Um, Y. Predicting the Habitat Suitability for Angelica gigas Medicinal Herb Using an Ensemble Species Distribution Model. Forests 2023, 14, 592. [Google Scholar] [CrossRef]
- Wudu, K.; Abegaz, A.; Ayele, L.; Ybabe, M. The impacts of climate change on biodiversity loss and its remedial measures using nature based conservation approach: A global perspective. Biodivers. Conserv. 2023, 32, 3681–3701. [Google Scholar] [CrossRef]
- Zeraatkar, A.; Khajoei Nasab, F. Mapping the habitat suitability of endemic and sub-endemic almond species in Iran under current and future climate conditions. Environ. Dev. Sustain. 2023, 26, 14859–14876. [Google Scholar] [CrossRef]
- Tikhonov, G.; Opedal, Ø.H.; Abrego, N.; Lehikoinen, A.; de Jonge, M.M.; Oksanen, J.; Ovaskainen, O. Joint species distribution modelling with the R-package Hmsc. Methods Ecol. Evol. 2020, 11, 442–447. [Google Scholar] [CrossRef]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Model. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Amindin, A.; Pourghasemi, H.R.; Safaeian, R.; Rahmanian, S.; Tiefenbacher, J.P.; Naimi, B. Predicting Current and Future Habitat Suitability of an Endemic Species Using Data-Fusion Approach: Responses to Climate Change. Rangel. Ecol. Manag. 2024, 94, 149–162. [Google Scholar] [CrossRef]
- Bedair, H.; Shaltout, K.; Halmy, M.W.A. Stacked machine learning models for predicting species richness and endemism for Mediterranean endemic plants in the Mareotis subsector in Egypt. Plant Ecol. 2023, 224, 1113–1126. [Google Scholar] [CrossRef]
- El-Khalafy, M.M.; El-Kenany, E.T.; Al-Mokadem, A.Z.; Shaltout, S.K.; Mahmoud, A.R. Habitat suitability modeling to improve conservation strategy of two highly-grazed endemic plant species in saint Catherine Protectorate, Egypt. BMC Plant Biol. 2025, 25, 485. [Google Scholar] [CrossRef]
- Mahmoud, A.R.; Farahat, E.A.; Hassan, L.M.; Halmy, M.W.A. Remotely sensed data contribution in predicting the distribution of native Mediterranean species. Sci. Rep. 2025, 15, 12475. [Google Scholar] [CrossRef]
- Bazzichetto, M.; Malavasi, M.; Bartak, V.; Acosta, A.T.R.; Rocchini, D.; Carranza, M.L. Plant invasion risk: A quest for invasive species distribution modelling in managing protected areas. Ecol. Indic. 2018, 95, 311–319. [Google Scholar] [CrossRef]
- Bosso, L.; Luchi, N.; Maresi, G.; Cristinzio, G.; Smeraldo, S.; Russo, D. Predicting current and future disease outbreaks of Diplodia sapinea shoot blight in Italy: Species distribution models as a tool for forest management planning. For. Ecol. Manag. 2017, 400, 655–664. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A.R. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model—Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Peng, L.-P.; Cheng, F.-Y.; Hu, X.-G.; Mao, J.-F.; Xu, X.-X.; Zhong, Y.; Li, S.-Y.; Xian, H.-L. Modelling environmentally suitable areas for the potential introduction and cultivation of the emerging oil crop Paeonia ostii in China. Sci. Rep. 2019, 9, 3213. [Google Scholar] [CrossRef] [PubMed]
- Sheidai, M.; Noormohammadi, Z.; Alishah, O. Future cultivation of cotton for industrial use: Landscape cytogenetics and species distribution modeling. Genet. Resour. Crop Evol. 2024, 71, 4429–4440. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Dakhil, M.A.; Halmy, M.W.A.; Bedair, H.; Fouad, M.S. Towards Forest Conservation Planning: How Temperature Fluctuations Determine the Potential Distribution and Extinction Risk of Cupressus funebris Conifer Trees in China. Forests 2023, 14, 2234. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Palermino, A.; De Felice, A.; Canduci, G.; Biagiotti, I.; Costantini, I.; Centurelli, M.; Menicucci, S.; Gašparević, D.; Tičina, V.; Leonori, I. Modeling of the habitat suitability of European sprat (Sprattus sprattus, L.) in the Adriatic Sea under several climate change scenarios. Front. Mar. Sci. 2024, 11, 1383063. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Ahmad, M.; Shaheen, A.; Zafar, M.; Ullah, R.; Asma, M.; Sultana, S.; Munir, M.; Rashid, N.; Malik, K.; et al. Comparative Study of Liquid Biodiesel from Sterculia foetida (Bottle Tree) Using CuO-CeO2 and Fe2O3 Nano Catalysts. Front. Energy Res. 2019, 17, 4. [Google Scholar] [CrossRef]
- Jafri, A.; Bano, S.; Rais, J.; Khan, F.; Shivnath, N.; Sharma, A.K.; Arshad, M. Phytochemical screening of Sterculia foetida seed extract for anti-oxidant, anti-microbial activity, and detection of apoptosis through reactive oxygen species (ROS) generation, mitochondrial membrane potential (MMP) decrease, and nuclear fragmentation in human osteosarcoma cells. J. Histotechnol. 2019, 42, 68–79. [Google Scholar]
- Alam, N.; Banu, N.; Aziz, M.A.I.; Barua, N.; Ruman, U.; Jahan, I.; Chy, F.J.; Denath, S.; Paul, A.; Chy, M.N.U.; et al. Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida. Plants 2021, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Buist, P.H. 1.02 Unsaturated Fatty Acids. Chem. Biol. 2010, 1, 5–33. [Google Scholar]
- Swarnalatha, K.; Kishore Babu, C.V.; Hari Babu, B. Phytochemical screening, anti-diabetic and antioxidant activities of Kigelia africana (LAM.) and Sterculia foetida L. Rasayan J. Chem. 2019, 12, 907–914. [Google Scholar] [CrossRef]
- Amuthavalli, A.; Ramesh, T. Phytochemical, Fluorescence and GC-MS Analysis of Methanolic Extract of Sterculia foetida L. Seeds. Int. J. Environ. Agric. Biotechnol. 2021, 6, 046–053. [Google Scholar] [CrossRef]
- Kavitha, M.; Vadivu, R.; Radha, R. A Review on Sterculia foetida Linn. Res. J. Pharmacogn. Phytochem. 2015, 7, 239. [Google Scholar]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, C.H.; Reddy, J.R.C.; Rao, B.V.S.K.; Ravinder, T.; Chakrabarti, P.P.; Karuna, M.S.L.; Prasad, R.B.N. Preparation and Evaluation of Biodiesel from Sterculia foetida Seed Oil. J. Am. Oil Chem. Soc. 2012, 89, 891–896. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Yusaf, T.F. Production of biodiesel from Sterculia foetida and its process optimization. Fuel 2013, 111, 478–484. [Google Scholar] [CrossRef]
- Bhakshu, L.M.; Ratnam, K.V. Gum Karaya—Threats and Conservation. In Gum Karaya, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; pp. 156–166. Available online: https://www.taylorfrancis.com/books/9781003438960/chapters/10.1201/9781003438960-11 (accessed on 19 April 2025).
- de Kok, R.; IUCN. Sterculia foetida: The IUCN Red List of Threatened Species in 2023. 2024. e.T198991191A203234841. Available online: https://www.iucnredlist.org/species/198991191/203234841 (accessed on 19 April 2025).
- Vélez-Gavilán, J. Sterculia foetida (Java olive). CABI Compend. 2023, 51446. Available online: http://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.51446 (accessed on 19 April 2025).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2025. Available online: https://powo.science.kew.org/ (accessed on 20 January 2025).
- Acevedo-Rodríguez, P.; Strong, M.T. Catalogue of Seed Plants of the West Indies. Smithson. Contrib. Bot. 2012, 98, 1–1192. [Google Scholar] [CrossRef]
- GBIF. Annonaceae in GBIF Secretariat. 2021. GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://doi.org/10.15468/39omei (accessed on 15 September 2021).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog. Phys. Geogr. Earth Environ. 2006, 30, 751–777. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. SDM: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Naimi, B. Usdm: Uncertainty Analysis for Species Distribution Models. R package version 1.1–15. R Documentation. 2025. Available online: https://cran.r-project.org/web/packages/usdm/usdm.pdf (accessed on 25 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 February 2025).
- Andrews, M.B.; Ridley, J.K.; Wood, R.A.; Andrews, T.; Blockley, E.W.; Booth, B.; Burke, E.; Dittus, A.J.; Florek, P.; Gray, L.J.; et al. Historical Simulations with HadGEM3-GC3.1 for CMIP6. J. Adv. Model Earth Syst. 2020, 12, e2019MS001995. [Google Scholar] [CrossRef]
- Nooni, I.K.; Ogou, F.K.; Chaibou, A.A.S.; Nakoty, F.M.; Gnitou, G.T.; Lu, J. Evaluating CMIP6 Historical Mean Precipitation over Africa and the Arabian Peninsula against Satellite-Based Observation. Atmosphere 2023, 14, 607. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Change 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R, 1st ed.; Cambridge University Press: Cambridge, UK, 2017; Available online: https://www.cambridge.org/core/product/identifier/9781139028271/type/book (accessed on 6 December 2024).
- Turner, J.A.; Babcock, R.C.; Kendrick, G.A.; Hovey, R.K. How does spatial resolution affect model performance? A case for ensemble approaches for marine benthic mesophotic communities. J. Biogeogr. 2019, 46, 1249–1259. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karger, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Annual Statistics. 1 October 2001. Available online: https://projecteuclid.org/journals/annals-of-statistics/volume-29/issue-5/Greedy-function-approximation-A-gradient-boosting-machine/10.1214/aos/1013203451.full (accessed on 19 April 2025).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Routledge: London, UK, 2019; Available online: https://www.taylorfrancis.com/books/9781351445856 (accessed on 19 April 2025).
- Cutler, D.R.; Edwards, T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests FOR Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.G.; Gastón, A.; Aroca-Fernández, M.J.; Broennimann, O.; Guisan, A.; Saura, S.; García-Viñas, J.I. Hierarchical species distribution models in support of vegetation conservation at the landscape scale. J. Veg. Sci. 2019, 30, 386–396. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2015, 6, 337–348. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B.; Peterson, A.T.; Soberón, J.; Pearson, R.G. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; Available online: https://www.degruyter.com/document/doi/10.1515/9781400840670/html (accessed on 19 April 2025).
- Thampan, J.; Srivastava, J.; Saraf, P.N.; Samal, P. Habitat distribution modelling to identify areas of high conservation value under climate change for an endangered arid land tree Tecomella undulata. J. Arid. Environ. 2025, 227, 105317. [Google Scholar] [CrossRef]
- Wairokpam, B.; Wagh, V.V.; Singh, H.C.; Maurya, A.; Singh, L.A.; Rana, T.S. Integrating ecological niche modeling and natural regeneration assessment to identify conservation priorities for Garcinia pedunculata in India. Environ. Monit. Assess. 2025, 197, 476. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.C.; Maurya, A.; Wairokpam, B.; Tiwari, V.; Tiwari, A.; Rana, T.S. Predicting current and future suitable habitats for Bergenia ciliata in Indian Himalayan region. Landsc. Ecol. Eng. 2025, 21, 1–14. [Google Scholar] [CrossRef]
- Bedair, H.; Hazzazi, Y.; Hatab, A.A.; Halmy, M.W.A.; Dakhil, M.A.; Alghariani, M.S.; Sumayli, M.; El-Shabasy, A.; El-Khalafy, M.M. Predicting climate-driven shift of the East Mediterranean endemic Cynara cornigera Lindl. Front. Plant Sci. 2025, 16, 1461639. [Google Scholar] [CrossRef]
- Qin, F.; Han, B.; Bussmann, R.W.; Xue, T.; Liang, Y.; Zhang, W.; Liu, Q.; Chen, T.; Yu, S. Present status, future trends, and control strategies of invasive alien plants in China affected by human activities and climate change. Ecography 2023, 2024, 06919. [Google Scholar] [CrossRef]
- Nguru, W.; Mwongera, C. Predicting the future climate-related prevalence and distribution of crop pests and diseases affecting major food crops in Zambia. PLoS Clim. 2023, 2, e0000064. [Google Scholar] [CrossRef]
- Ahmed, N.; Atzberger, C.; Zewdie, W. Species Distribution Modelling performance and its implication for Sentinel-2-based prediction of invasive Prosopis juliflora in lower Awash River basin, Ethiopia. Ecol. Process. 2021, 10, 18. [Google Scholar] [CrossRef]
- Brunton, A.J.; Conroy, G.C.; Schoeman, D.S.; Rossetto, M.; Ogbourne, S.M. Seeing the forest through the trees: Applications of species distribution models across an Australian biodiversity hotspot for threatened rainforest species of Fontainea. Glob. Ecol. Conserv. 2023, 42, e02376. [Google Scholar] [CrossRef]
- Daï, E.H.; Houndonougbo, J.S.H.; Idohou, R.; Ouédraogo, A.; Kakaï, R.G.; Hotes, S.; Assogbadjo, A.E. Modeling current and future distribution patterns of Uvaria chamae in Benin (West Africa): Challenges and opportunities for its sustainable management. Heliyon 2023, 9, e13658. [Google Scholar] [CrossRef]
- Djaboutou, R.F.; Biaou, S.; Gouwakinnou, G.N.; Ouinsavi, C.A.I.N. Climate change impact on the distribution and priority areas for conservation of Sterculia setigera Delile in Benin, West Africa. Trees People 2025, 20, 100840. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Tomas, W.M.; Guedes, N.M.R.; Peterson, A.; Szabo, J.K.; Júnior, A.S.; Camilo, A.R.; Padovani, C.R.; Garcia, L.C. The relationship between scale and predictor variables in species distribution models applied to conservation. Biodivers. Conserv. 2021, 30, 1971–1990. [Google Scholar] [CrossRef]
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; van Dijk, A.I.J.M.; Miralles, D.G. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Chen, Y.; Fang, M.; Zheng, Y.; Yu, S. Environmental drivers of plant distributions at global and regional scales. Glob. Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar] [CrossRef]
- Groner, V.P.; Nicholas, O.; Mabhaudhi, T.; Slotow, R.; Akçakaya, H.R.; Mace, G.M.; Pearson, R.G. Climate change, land cover change, and overharvesting threaten a widely used medicinal plant in South Africa. Ecol. Appl. 2022, 32, e2545. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Saini, M.K. Impending threats to the plants with medicinal value in the Eastern Himalayas Region: An analysis on the alternatives to its non-availability. Phytomedicine Plus 2022, 2, 100151. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process. 2020, 9, 6. [Google Scholar] [CrossRef]
- Dogbo, S.F.; Salako, K.V.; Agoundé, G.; Dimobe, K.; Adiko, A.E.G.; Gebauer, J.; Yao, C.Y.A.; Kakaï, R.G. Potential impacts of future climate on twelve key multipurpose tree species in Benin: Insights from species distribution modeling for biodiversity conservation. Trees People 2024, 19, 100744. [Google Scholar] [CrossRef]
- Garcia, K.; Lasco, R.; Ines, A.; Lyon, B.; Pulhin, F. Predicting geographic distribution and habitat suitability due to climate change of selected threatened forest tree species in the Philippines. Appl. Geogr. 2013, 44, 12–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).