MaxEnt Modeling for Predicting the Potential Geographical Distribution of Camellia oleifera Abel Under Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution Data of Species
2.2. Environmental Variables
2.3. Ecological Niche Modeling
2.4. Niche Overlap and Range Overlap Analysis
3. Results
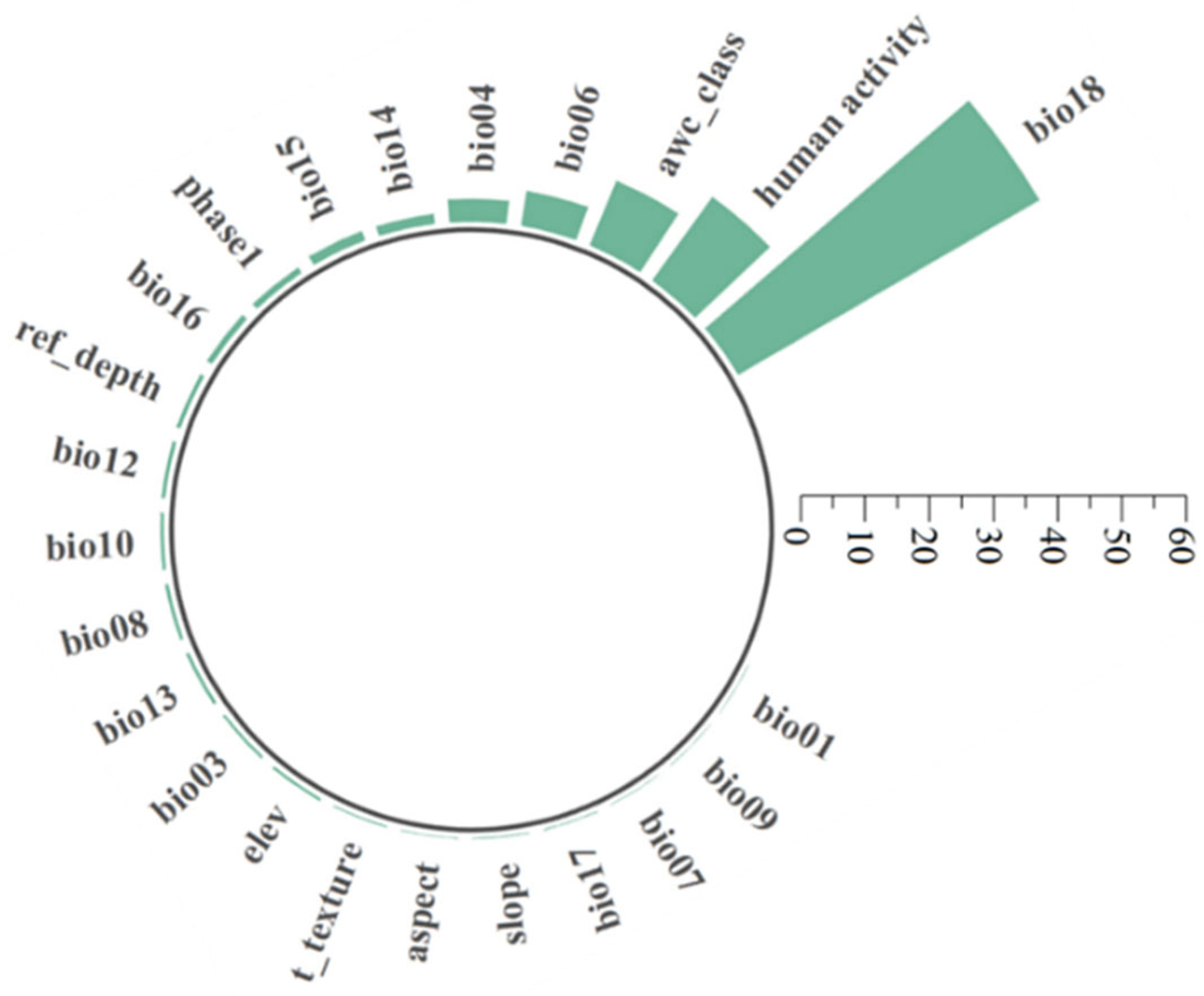
3.1. Main Environment Variables
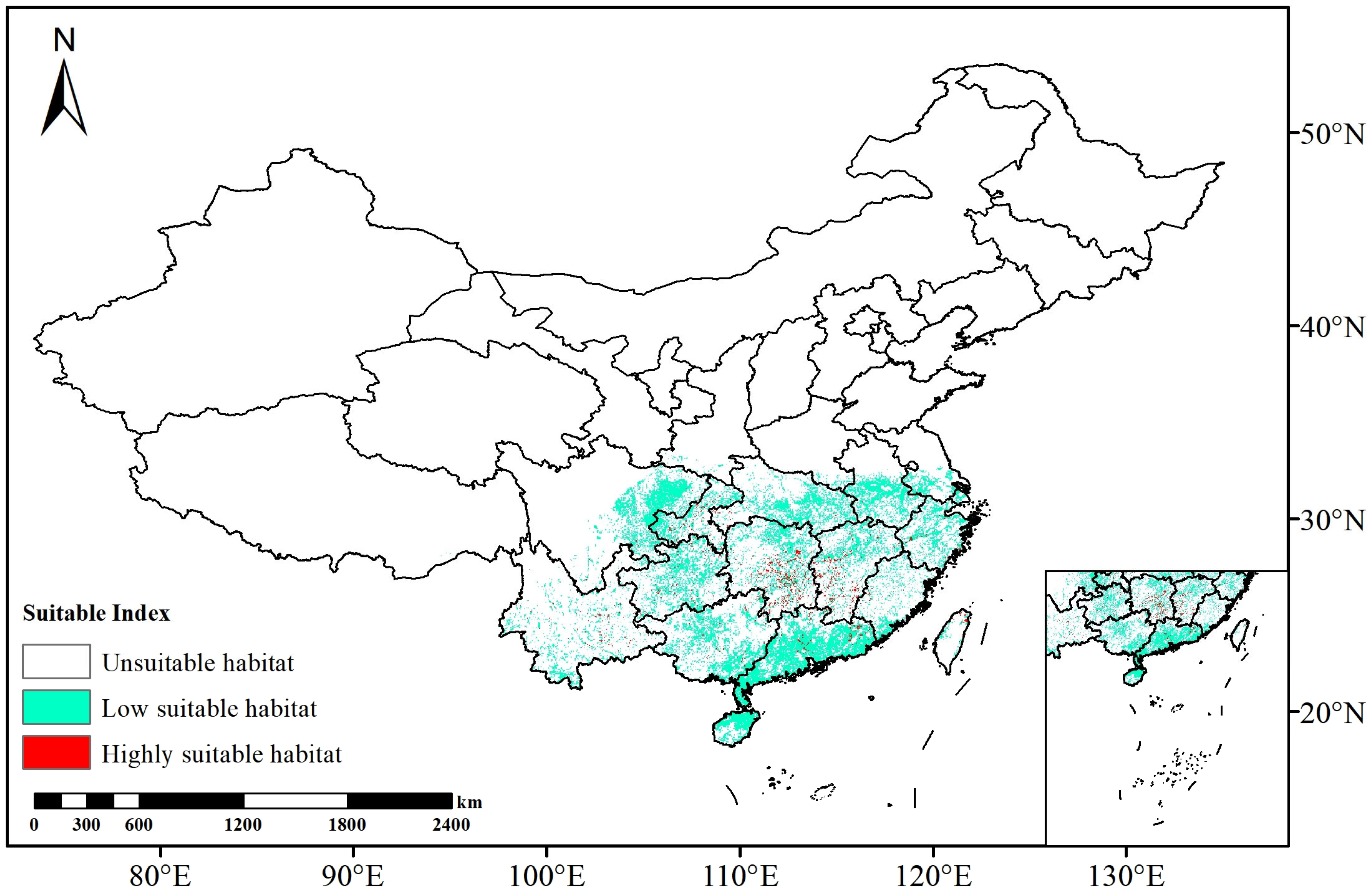
3.2. Distribution of Suitable Habitat and Model Accuracy Under Current Climate Conditions
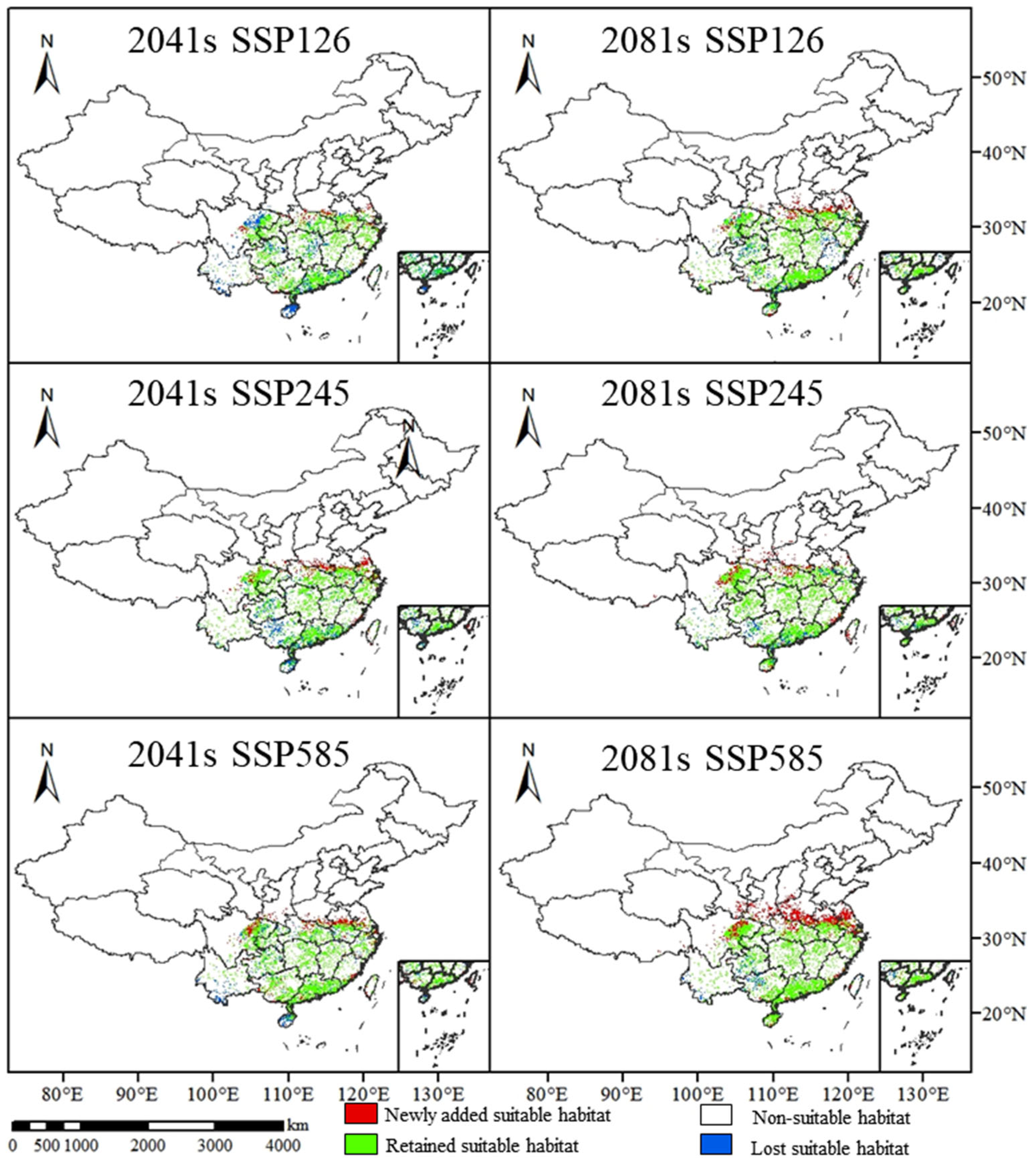
3.3. Range Changes in C. oleifera Under Future Climate Change
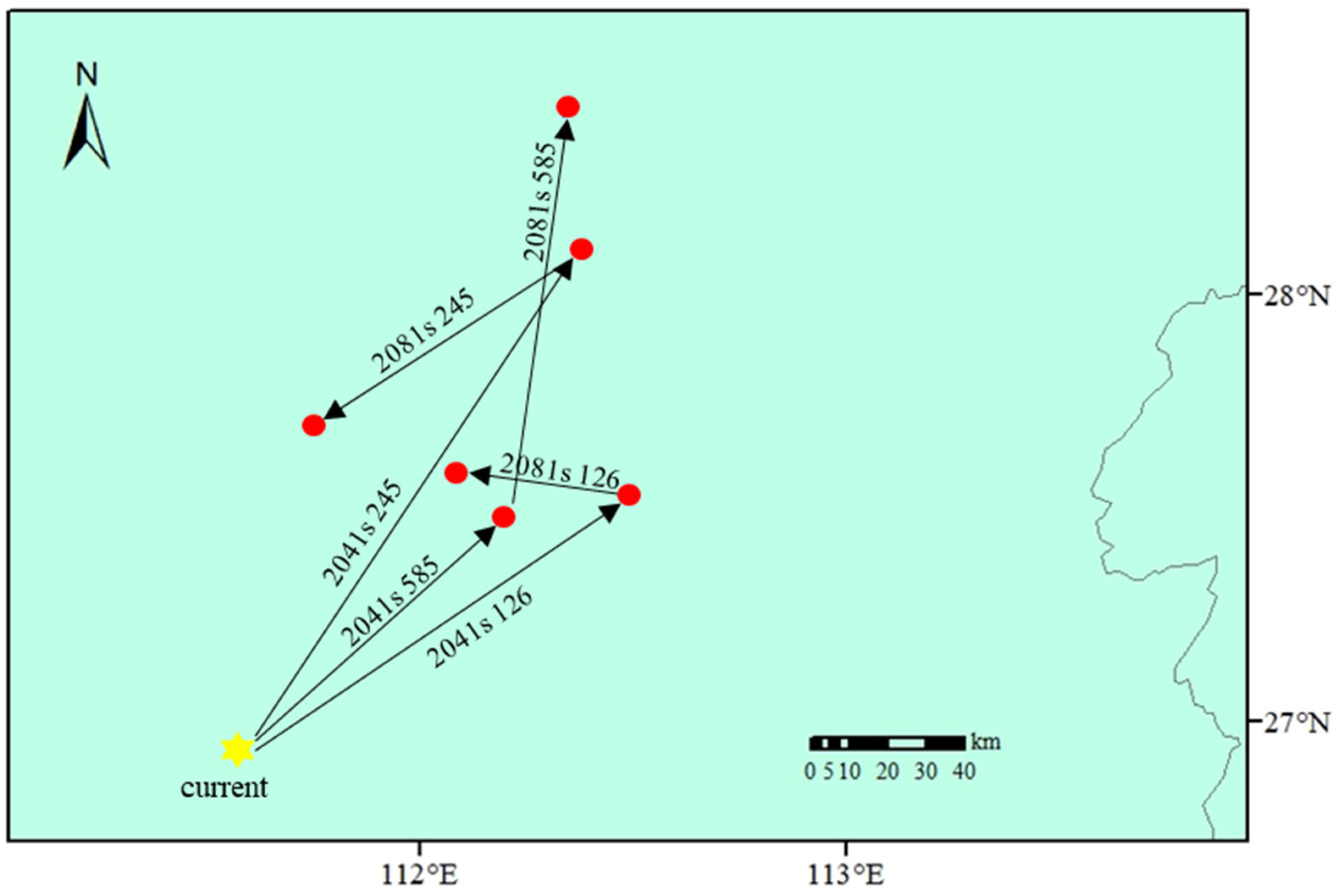
3.4. Future Changes in the Suitable Habitat and Centroid Migration of C. oleifera
3.5. Niche Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Rovzar, C.; Gillespie, T.W.; Kawelo, K. Landscape to site variations in species distribution models for endangered plants. For. Ecol. Manag. 2016, 369, 20–28. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Sax, D.F.; Early, R.; Bellemare, J. Niche syndromes, species extinction risks, and management under climate change. Trends Ecol. Evol. 2013, 28, 517–523. [Google Scholar] [CrossRef]
- Graham, E.M.; Reside, A.E.; Atkinson, I.; Baird, D.; Hodgson, L.; James, C.S.; Vanderwal, J.J. Climate change and biodiversity in Australia: A systematic modelling approach to nationwide species distributions. Australas. J. Environ. Manag. 2019, 26, 112–123. [Google Scholar] [CrossRef]
- Ma, T.S.; Deng, X.W.; Chen, L.; Xiang, W.H. The soil properties and their effects on plant diversity in different degrees of rocky desertification. Sci. Total Environ. 2020, 736, 139667. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xiong, D.H.; Wu, H.; Zhang, S.; Zhang, B.J.; Dahal, N.M.; Liu, L.; Li, W.X.; Zhang, W.D.; Shi, L.T. Spatial variation of soil physical properties and its relationship with plant biomass in degraded slopes in dry-hot valley region of Southwest China. J. Soils Sediments 2020, 20, 2354–2366. [Google Scholar] [CrossRef]
- Kouhi, S.M.M.; Erfanian, M. Predicting the Present and Future Distribution of Medusahead and Barbed Goatgrass in Iran. Ecopersia 2020, 8, 41–46. [Google Scholar]
- Su, Q.T.; Du, Z.X.; Luo, Y.; Zhou, B.; Xiao, Y.A.; Zou, Z.R. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios. Biology 2024, 13, 745. [Google Scholar] [CrossRef]
- Su, Q.T.; Du, Z.X.; Xue, Y.X.; Li, H.; Zhang, Y.X.; Zhang, S.J.; Huang, X.Y.; Zhou, B.; Qian, H.; Xiao, Y.A.; et al. Habitat Suitability Modeling of Endemic Genus Chimonanthus in China under Climate Change. Forests 2024, 15, 1625. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.L.; Su, E.R.; Chen, Y.Z.; Cao, F.L. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Yang, L.; Gao, C.; Xie, J.J.; Qiu, J.; Deng, Q.N.; Zhou, Y.C.; Liao, D.S.; Deng, C.Y. Fruit economic characteristics and yields of 40 superior Camellia oleifera Abel plants in the low-hot valley area of Guizhou Province, China. Sci. Rep. 2022, 12, 7068. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, L.; Ma, J.; Xia, S.T.; Gong, S.M.; Yin, Y.L.; Chen, Y.Z. Camellia (Camellia oleifera Abel.) Seed Oil Regulating of Metabolic Phenotype and Alleviates Dyslipidemia in High Fat-Fed Mice through Serum Branch-Chain Amino Acids. Nutrients 2022, 14, 2424. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.F.; Liu, F.D.; Zhao, J.R.; Yuan, J.; Xiao, S.X.; Masabni, J.; Zou, F.; Yuan, D.Y. Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China. Horticulturae 2022, 8, 818. [Google Scholar] [CrossRef]
- Quan, W.X.; Wang, A.P.; Gao, C.; Li, C.C. Applications of Chinese Camellia oleifera and its By-Products: A Review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef]
- Chen, P.J.; Hu, C.S.; Gu, J.; Lin, X.Y.; Yang, C.L.; Leu, S.Y.; Guan, L.T. Pyrolysis characteristics of tea oil camellia (Camellia oleifera Abel.) shells and their chemically pre-treated residues: Kinetics, mechanisms, product evaluation and joint optimization. J. Anal. Appl. Pyrolysis 2022, 164, 105526. [Google Scholar] [CrossRef]
- Chaudhary, N.; Bhardwaj, J.; Seo, H.J.; Kim, M.Y.; Shin, T.S.; Kim, J.D. Camellia sinensis fruit peel extract inhibits angiogenesis and ameliorates obesity induced by high-fat diet in rats. J. Funct. Foods 2014, 7, 479–486. [Google Scholar] [CrossRef]
- Gao, H.; Ouyang, Z.Y.; Chen, S.B.; van Koppen, C.S.A. Role of culturally protected forests in biodiversity conservation in Southeast China. Biodivers. Conserv. 2013, 22, 531–544. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, F.L.; Ouyang, Z.Y.; Tu, N.M.; Xu, W.H.; Wang, X.K.; Miao, H.; Li, X.Q.; Tian, Y.X. Impacts of reforestation approaches on runoff control in the hilly red soil region of Southern China. J. Hydrol. 2008, 356, 174–184. [Google Scholar] [CrossRef]
- Yang, S.X.; Wang, X.N.; Zhang, Z.; Chen, L.S.; Wang, R.; Chen, Y.Z. Camellia Resources in Hunan Province. GX For. Sci. 2022, 52, 583–590. [Google Scholar]
- Ai, J.X.; Zhen, D.J.; Wu, X. Progress in Germplasm Resources, Chemical Composition and Pharmacological Activities of Camellia oleifera. Food Drug 2025, 27, 209–218. [Google Scholar]
- Luan, F.; Zeng, J.S.; Yang, Y.; He, X.R.; Wang, B.J.; Gao, Y.B.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Foods 2021, 75, 104242. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Zeng, J.R.; Li, C.M.; Liu, J.Z.; Li, Y.Y.; Hu, Z.Z.; He, M.L.; Zhang, H.Y.; Yan, H.J. Ecological assessment of current and future Pogostemon cablin Benth. potential planting regions in China based on MaxEnt and ArcGIS models. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100308. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. ORIGINAL ARTICLE: Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2015, 17, 43–57. [Google Scholar] [CrossRef]
- Zhang, X.F.; Nizamani, M.M.; Jiang, C.; Fang, F.Z.; Zhao, K.K. Potential planting regions of Pterocarpus santalinus (Fabaceae) under current and future climate in China based on MaxEnt modeling. Ecol. Evol. 2024, 14, e11409. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, X.H.; Li, Y.; Wu, C.H.; Zhao, B.; Peng, M.C.; Chen, W.; Wang, C.Y. Response of Extremely Small Populations to Climate Change—A Case of Trachycarpus nanus in Yunnan, China. Biology 2024, 13, 240. [Google Scholar] [CrossRef]
- Leimbach, M.; Giannousakis, A. Burden sharing of climate change mitigation: Global and regional challenges under shared socio-economic pathways. Clim. Change 2019, 155, 273–291. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, L.Y.; Zhou, X.Y.; Chen, R.F.; Zhao, G.H.; Zhang, F.G. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Lun, X.C.; Li, C.; Zhou, R.B.; Zhao, Z.; Wang, J.; Zhang, Q.F.; Liu, Q.Y. Predicting the Potential Global Distribution of Amblyomma americanum (Acari: Ixodidae) under Near Current and Future Climatic Conditions, Using the Maximum Entropy Model. Biology 2021, 10, 1057. [Google Scholar] [CrossRef]
- Beale, C.M.; Lennon, J.J. Incorporating uncertainty in predictive species distribution modelling. Philos. Trans. R. Soc. B 2012, 367, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Ren, Z.X.; Wang, W.J.; Xu, X.; Liu, J.; Zhao, Y.H.; Wang, H. Predicting the spatial distribution of three Astragalusspecies and their pollinating bumblebees in the Sino-Himalayas. Biodivers. Sci. 2021, 29, 759–769. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Lu, Z.Q.; Zhao, J.L.; Li, Q.J. Genomic data reveal two distinct species from the widespread alpine ginger Roscoea tibetica Batalin (Zingiberaceae). J. Syst. Evol. 2020, 59, 1232–1243. [Google Scholar] [CrossRef]
- Zhao, J.L.; Gugger, P.F.; Xia, Y.M.; Li, Q.J. Ecological divergence of two closely related Roscoea species associated with late Quaternary climate change. J. Biogeogr. 2016, 43, 1990–2001. [Google Scholar] [CrossRef]
- Marcer, A.; Lloren, S.; Molowny-Horas, R.; Pons, X.; Pino, J. Using species distribution modelling to disentangle realised versus potential distributions for rare species conservation. Biol. Conserv. 2013, 166, 221–230. [Google Scholar] [CrossRef]
- Ngarega, B.K.; Masocha, V.F.; Schneider, H. Forecasting the effects of bioclimatic characteristics and climate change on the potential distribution of Colophospermum mopane in southern Africa using Maximum Entropy (Maxent). Ecol. Inform. 2021, 65, 101419. [Google Scholar] [CrossRef]
- Wang, H.R.; Xia, J.M.; Sun, Y.J. Effect of short-term low temperature stress on non-structural carbohydrates in Camellia oleifera leaves. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2025, 53, 392–400. [Google Scholar]
- Lan, X.H.; Wang, J.L.; Fu, C.; Li, L.M.; Yuan, M.Q.; Tan, T.T.; Du, F.G. Prediction of Suitable Area of Magnolia siboldii in China Based on the Optimized Maxent Model. J. Northwest For. Univ. 2022, 37, 100–106. [Google Scholar]
- Leng, X.H.; Xue, L.; Wang, J.; Li, S.; Yang, Z.L.; Ren, H.D.; Yao, X.H.; Wu, Z.Y.; Li, J.Y. Physiological Responses of Handeliodendron bodinieri (Levl.) Rehd. to Exogenous Calcium Supply under Drought Stress. Forests 2020, 11, 69. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, T.L.; Hu, C.G.; Zhang, J.Z. The Role of Drought and Temperature Stress in the Regulation of Flowering Time in Annuals and Perennials. Agronomy 2023, 13, 3034. [Google Scholar] [CrossRef]
- Liang, J.H.; Wu, Z.; Xu, T.F.; Li, X.F.; Jiang, F.; Wang, H.Q. Overexpression of HANABA TARANU in cultivated strawberry delays flowering and leads to defective flower and fruit development. Plant Sci. 2022, 321, 111307. [Google Scholar] [CrossRef] [PubMed]
- Negoro, S.; Hirabayashi, T.; Iwasaki, R.; Torii, K.U.; Uchida, N. EPFL peptide signalling ensures robust self-pollination success under cool temperature stress by aligning the length of the stamen and pistil. Plant Cell Environ. 2022, 46, 451–463. [Google Scholar] [CrossRef]
- Wang, M.; Guan, Q.; Xie, Y. Prediction of potential suitable areas for Broussonetia papyrifera in China using the MaxEnt model and CIMP6 data. J. Plant Ecol. 2023, 16, rtad006. [Google Scholar] [CrossRef]
- Piao, S.L.; Yin, G.D.; Tan, J.G.; Cheng, L.; Huang, M.T.; Li, Y.; Liu, R.G.; Mao, J.F.; Myneni, R.B.; Peng, S.S.; et al. Detection and attribution of vegetation greening trend in China over the last 30 years. Glob. Change Biol. 2015, 21, 1601–1609. [Google Scholar] [CrossRef]
- He, L.M.; Zhang, Y.; Wang, R.; Chen, Y.Z.; Yang, D.Y.; He, Z.L.; Shen, J.C.; Ma, X.F.; Lai, H.G. Phenotypic Genetic Diversity Analysis of 378 Oil-tea Camellia Germplasm Resources. Mol. Plant Breed. 2024, 22, 2732–2749. [Google Scholar]
- Jiang, R.P.; Zou, M.; Qin, Y.; Tan, G.D.; Huang, S.P.; Quan, H.G.; Zhou, J.Y.; Liao, H. Modeling of the Potential Geographical Distribution of Three Fritillaria Species Under Climate Change. Front. Plant Sci. 2021, 12, 749838. [Google Scholar] [CrossRef] [PubMed]
- Faticov, M.; Abdelfattah, A.; Roslin, T.; Vacher, C.; Hambäck, P.; Blanchet, F.G.; Lindahl, B.D.; Tack, A.J.M. Climate warming dominates over plant genotype in shaping the seasonal trajectory of foliar fungal communities on oak. New Phytol. 2021, 231, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Y.; Wang, S.C.; Duan, Y.; Han, J.; Huang, D.H.; Zhou, J. Current and future distribution of the deciduous shrub Hydrangea macrophylla in China estimated by MaxEnt. Ecol Evol. 2021, 11, 16099–16112. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, D.C.; McGill, B.J. Trees have overlapping potential niches that extend beyond their realized niches. Science 2024, 385, 75–80. [Google Scholar] [CrossRef]
- Jacquemyn, H.; De Coensel, B.; Evans, A.; Wang, D.Y.; Merckx, V.S.F.T. The relationship between phylogeny, range size, niche breadth and niche overlap in European orchids (Orchidaceae). J. Biogeogr. 2024, 51, 409–421. [Google Scholar] [CrossRef]

| Training AUC | Test AUC | TSS | |
|---|---|---|---|
| current | 0.990 | 0.989 | 0.967 |
| 2041s 126 | 0.991 | 0.988 | 0.960 |
| 2041s 245 | 0.991 | 0.988 | 0.961 |
| 2041s 585 | 0.991 | 0.987 | 0.960 |
| 2081s 126 | 0.991 | 0.989 | 0.964 |
| 2081s 245 | 0.990 | 0.989 | 0.968 |
| 2081s 585 | 0.991 | 0.989 | 0.962 |
| Highly Suitable Habitat | Low Suitable Habitat | Unsuitable Habitat | |
|---|---|---|---|
| 2041s SSP126 | 2.51 | 45.03 | 913.53 |
| 2081s SSP126 | 2.76 | 55.44 | 905.38 |
| 2041s SSP245 | 3.89 | 51.80 | 902.39 |
| 2081s SSP245 | 12.61 | 48.49 | 902.87 |
| 2041s SSP585 | 5.95 | 52.73 | 899.97 |
| 2081s SSP585 | 29.58 | 46.14 | 885.34 |
| Current | 2041s126 | 2081s126 | 2041s245 | 2081s245 | 2041s585 | 2081s585 | |
|---|---|---|---|---|---|---|---|
| current | 1 | 0.97 | 0.97 | 0.98 | 0.96 | 0.97 | 0.95 |
| 2041s126 | 0.89 | 1 | 0.96 | 0.97 | 0.93 | 0.95 | 0.92 |
| 2081s126 | 0.85 | 0.86 | 1 | 0.97 | 0.96 | 0.97 | 0.96 |
| 2041s245 | 0.84 | 0.85 | 0.82 | 1 | 0.96 | 0.97 | 0.97 |
| 2081s245 | 0.89 | 0.90 | 0.86 | 0.86 | 1 | 0.96 | 0.97 |
| 2041s585 | 0.87 | 0.88 | 0.83 | 0.84 | 0.84 | 1 | 0.95 |
| 2081s585 | 0.94 | 0.95 | 0.93 | 0.98 | 0.95 | 0.94 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zhang, Y.; Su, Q.; Gan, Q.; Zhou, Q.; Guo, Y.; Liu, Z.; Zhang, Y.; Zhou, B.; Asseri, T.A.Y.; et al. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Camellia oleifera Abel Under Climate Change. Forests 2025, 16, 1026. https://doi.org/10.3390/f16061026
Jiang Z, Zhang Y, Su Q, Gan Q, Zhou Q, Guo Y, Liu Z, Zhang Y, Zhou B, Asseri TAY, et al. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Camellia oleifera Abel Under Climate Change. Forests. 2025; 16(6):1026. https://doi.org/10.3390/f16061026
Chicago/Turabian StyleJiang, Zhiyin, Yuxin Zhang, Qitao Su, Qing Gan, Qin Zhou, Yiliu Guo, Zhao Liu, Yanping Zhang, Bing Zhou, Tahani A. Y. Asseri, and et al. 2025. "MaxEnt Modeling for Predicting the Potential Geographical Distribution of Camellia oleifera Abel Under Climate Change" Forests 16, no. 6: 1026. https://doi.org/10.3390/f16061026
APA StyleJiang, Z., Zhang, Y., Su, Q., Gan, Q., Zhou, Q., Guo, Y., Liu, Z., Zhang, Y., Zhou, B., Asseri, T. A. Y., & Hassan, M. U. (2025). MaxEnt Modeling for Predicting the Potential Geographical Distribution of Camellia oleifera Abel Under Climate Change. Forests, 16(6), 1026. https://doi.org/10.3390/f16061026







