Variation in Functional Traits of Woody Plants Across Successional Stages in Subtropical Forests
Abstract
1. Introduction
2. Methods
2.1. Study Sites
2.2. Functional Trait Measurements
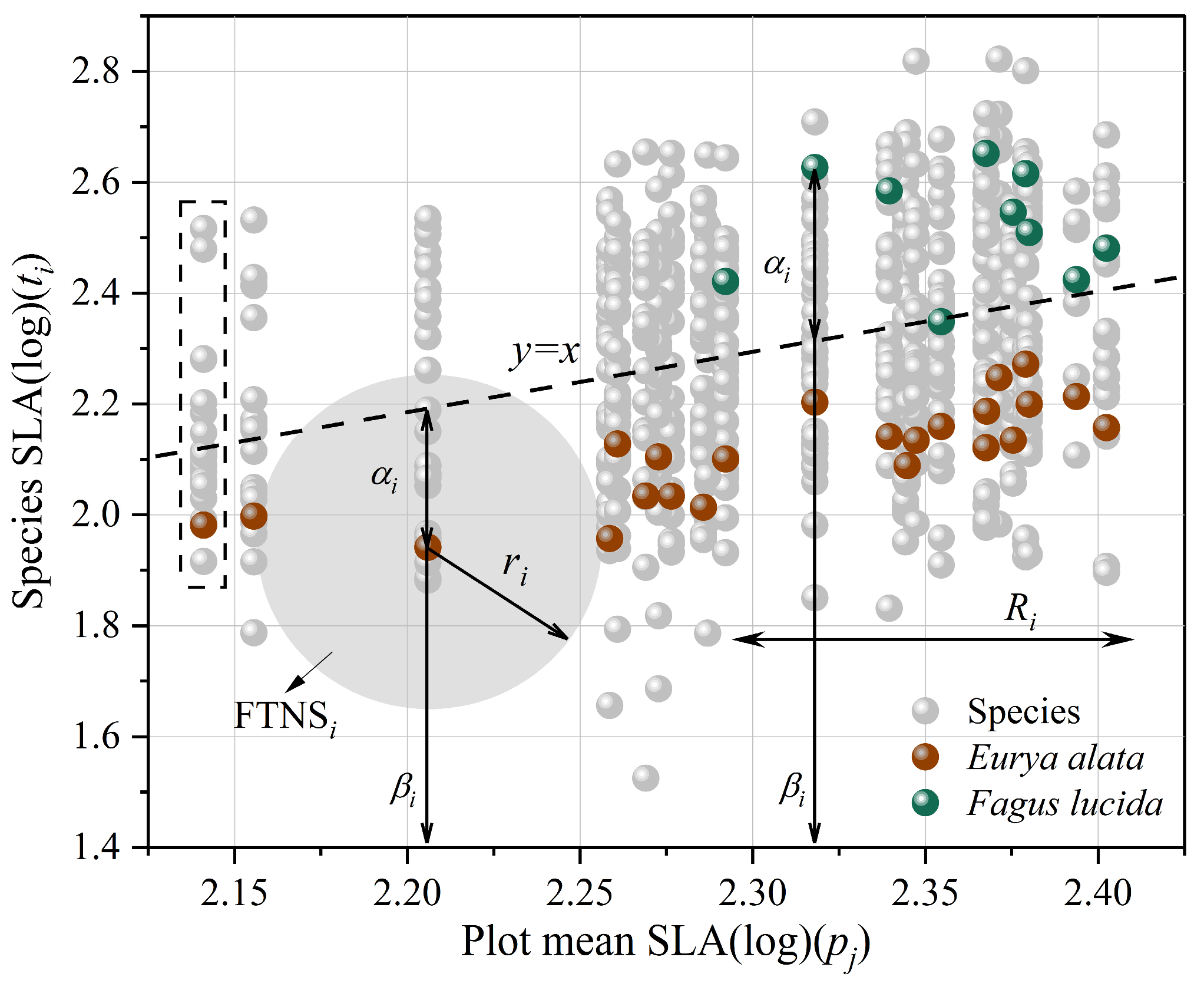
2.3. Trait Gradient Analysis
2.4. Ecological Constraint Index
2.5. Data Analysis
3. Results
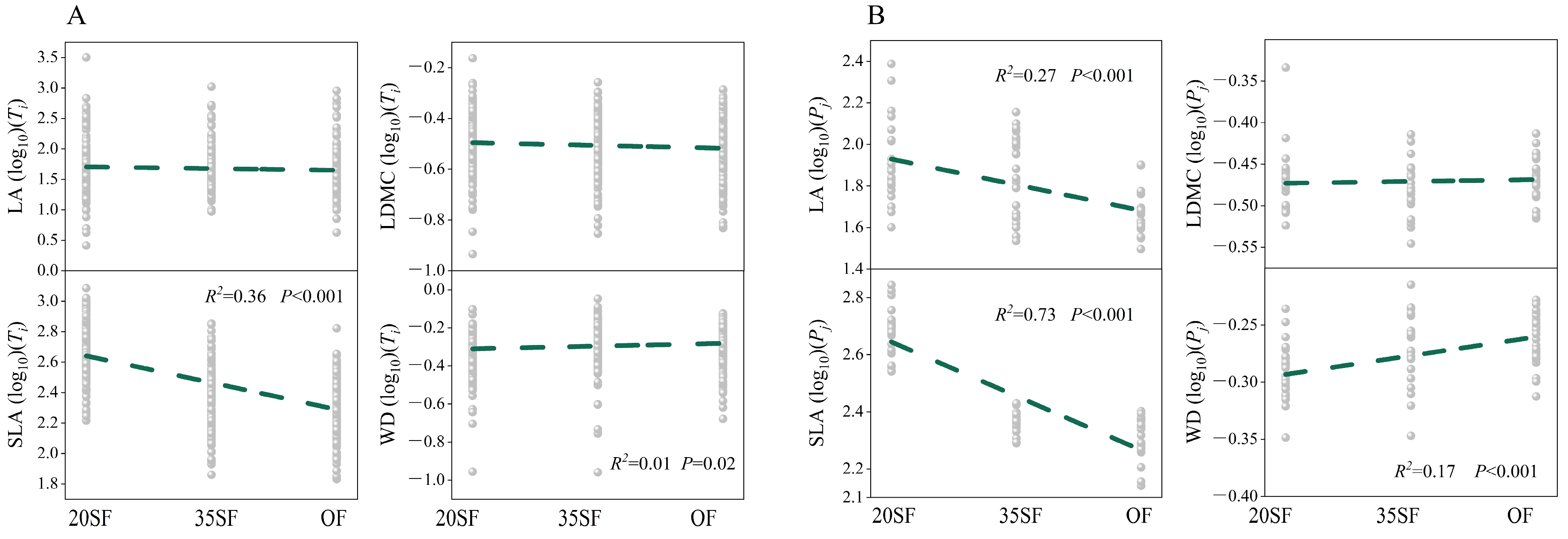
3.1. Functional Trait Variation Among Forests of Different Stand Ages
3.2. Correlations for Pairwise Combinations of Functional Traits and Distribution Characteristics of Alpha (α) and Beta (β) Components
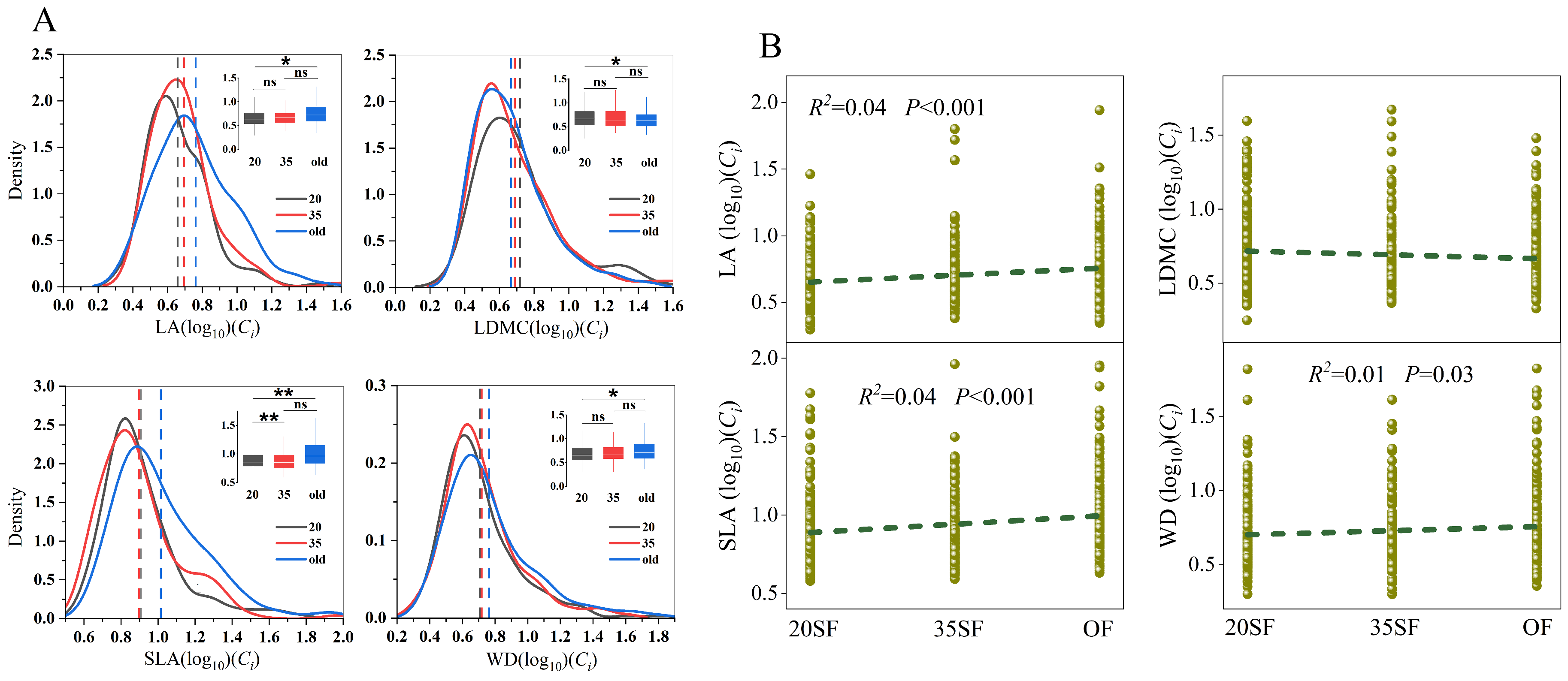
3.3. Distribution Characteristics of the Ecological Constraint Index (Ci)
4. Discussion
4.1. Variation in Functional Traits at the Species and Community Levels Across Different Stand Ages
4.2. Relationships for Pairwise Combinations of Functional Traits at Different Stand Ages
4.3. Ecological Constraints on Functional Trait Expression Across Different Stand Ages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. eLS 2016, 1, 1–8. [Google Scholar]
- Laurans, M.; Munoz, F.; Charles-Dominique, T.; Heuret, P.; Fortunel, C.; Isnard, S.; Sabatier, S.-A.; Caraglio, Y.; Violle, C. Why incorporate plant architecture into trait-based ecology? Trends Ecol. Evol. 2024, 39, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, D.C. Unifying functional and population ecology to test the adaptive value of traits. Biol. Rev. 2024, 99, 1976–1991. [Google Scholar] [CrossRef]
- Roscher, C.; Gubsch, M.; Lipowsky, A.; Schumacher, J.; Weigelt, A.; Buchmann, N.; Schulze, E.-D.; Schmid, B. Trait means, trait plasticity and trait differences to other species jointly explain species performances in grasslands of varying diversity. Oikos 2018, 127, 865. [Google Scholar] [CrossRef]
- Vandewalle, M.; de Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef]
- Liu, X.; Ma, K. Plant Functional Traits—Concepts, Applications and Future Directions. Sci. Sin. Vitae 2015, 45, 325–339. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Hallett, L.M.; Valencia, E.; Garnier, E.; Roscher, C.; Conti, L.; Galland, T.; Goberna, M.; Májeková, M.; et al. Functional trait effects on ecosystem stability: Assembling the jigsaw puzzle. Trends Ecol. Evol. 2021, 36, 822–836. [Google Scholar] [CrossRef]
- Kooyman, R.; Cornwell, W.; Westoby, M. Plant functional traits in Australian subtropical rain forest: Partitioning within-community from cross-landscape variation. J. Ecol. 2010, 98, 517–525. [Google Scholar] [CrossRef]
- Dong, L.-W.; Ren, Z.-W.; Zhang, R.; Xie, C.-D.; Zhou, X.-L. Functional diversity rather than species diversity can explain community biomass variation following short-term nitrogen addition in an alpine grassland. Chin. J. Plant Ecol. 2022, 46, 871–881. [Google Scholar] [CrossRef]
- He, N.; Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, J.; Tang, Z.; Han, X.; Ye, Q.; et al. Plant Trait Networks: Improved Resolution of the Dimensionality of Adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef]
- Ramachandran, A.; Dee, L.; Hayden, M.; Suding, K. Leveraging plant functional traits in the design of nature-based solutions: A research agenda. J. Ecol. 2024, 112, 2492–2501. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Cornwell, W.K. A trait-based approach to community assembly: Partitioning of species trait values into within- and among-community components. Ecol. Lett. 2007, 10, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Westerband, A.C.; Knight, T.M.; Barton, K.E. Scale-dependent variation in leaf functional traits clarifies mechanisms of invasion. Ecography 2025, e07539. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Cadotte, M.W. Assessing the importance of species and their assemblages for the biodiversity-ecosystem multifunctionality relationship. Ecology 2023, 104, e4104. [Google Scholar] [CrossRef]
- He, Y.-Y.; Guo, S.-L.; Wang, Z. Research progress of trade-off relationships of plant functional traits. Chin. J. Plant Ecol. 2019, 43, 1021–1035. [Google Scholar] [CrossRef]
- Stubbs, W.J.; Wilson, J.B. Evidence for Limiting Similarity in a Sand Dune Community. J. Ecol. 2004, 92, 557–567. [Google Scholar] [CrossRef]
- Freschet, G.T.; Dias, A.T.C.; Ackerly, D.D.; Aerts, R.; van Bodegom, P.M.; Cornwell, W.K.; Dong, M.; Kurokawa, H.; Liu, G.; Onipchenko, V.G.; et al. Global to community scale differences in the prevalence of convergent over divergent leaf trait distributions in plant assemblages. Glob. Ecol. Biogeogr. 2011, 20, 755–765. [Google Scholar] [CrossRef]
- Huang, L.; Pan, X.; Ma, J.; Jian, R.; Zhang, H.; Bai, K.; Mo, Y.; Zhang, Q.; Yang, Z. Assembly of understory woody communities during the close-to-nature restoration of a Pinus massoniana lamb. plantation in the southern subtropical region of China: From environmental filtering to competitive exclusion. For. Ecol. Manag. 2023, 541, 121060. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M.; Fox, J. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2014, 29, 592–599. [Google Scholar] [CrossRef]
- Denelle, P.; Violle, C.; Munoz, F. Distinguishing the signatures of local environmental filtering and regional trait range limits in the study of trait–environment relationships. Oikos 2019, 128, 960–971. [Google Scholar] [CrossRef]
- Cai, L.; Kreft, H.; Denelle, P.; Taylor, A.; Craven, D.; Dawson, W.; Essl, F.; van Kleunen, M.; Pergl, J.; Pyšek, P.; et al. Environmental filtering, not dispersal history, explains global patterns of phylogenetic turnover in seed plants at deep evolutionary timescales. Nat. Ecol. Evol. 2025, 9, 314–324. [Google Scholar] [CrossRef]
- Schwilk, D.W.; Ackerly, D.D. Limiting similarity and functional diversity along environmental gradients. Ecol. Lett. 2005, 8, 272–281. [Google Scholar] [CrossRef]
- Li, Y.; Mo, Y.-X.; Cui, H.-L.; Zhang, Y.-J.; Dossa, G.G.O.; Tan, Z.-H.; Song, L. Intraspecific plasticity and co-variation of leaf traits facilitate Ficus tinctoria to acclimate hemiepiphytic and terrestrial habitats. Tree Physiol. 2024, 44, tpae007. [Google Scholar] [CrossRef]
- Cadotte, M.W. The interacting influences of competition, composition and diversity determine successional community change. J. Ecol. 2023, 111, 1670–1680. [Google Scholar] [CrossRef]
- Ottaviani, G.; Tsakalos, J.L.; Keppel, G.; Mucina, L. Quantifying the effects of ecological constraints on trait expression using novel trait-gradient analysis parameters. Ecol. Evol. 2018, 8, 435–440. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, Z.; Wang, Q.; Lu, J.; Xu, Y.; Meng, H.; Liu, H.; Zhang, J.; Bao, D.; Qiao, X.; et al. Detecting density dependence from spatial patterns in a heterogeneous subtropical forest of central China. Can. J. For. Res. 2015, 45, 710–720. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High Carbon Dioxide Uptake by Subtrop. For. Ecosyst. East Asian Monsoon Reg. 2014, 111, 4910–4915. [Google Scholar]
- Cheng, K.; Zhang, Y.; Yang, H.; Ren, Y.; Xiang, T.; Chen, Y.; Yang, Z.; Chen, M.; Xu, J.; Huang, G.; et al. China’s naturally regenerated forests currently have greater aboveground carbon accumulation rates than newly planted forests. Commun. Earth Environ. 2025, 6, 345. [Google Scholar] [CrossRef]
- Yao, L.; Ding, Y.; Yao, L.; Ai, X.; Zang, R. Trait Gradient Analysis for Evergreen and Deciduous Species in a Subtropical Forest. Forests 2020, 11, 364. [Google Scholar] [CrossRef]
- Xi, X.-Q.; Zhao, Y.-J.; Liu, Y.-G.; Wang, X.; Gao, X.-M. Variation and correlation of plant functional traits in karst area of central Guizhou Province, China. Chin. J. Plant Ecol. 2011, 35, 1000–1008. [Google Scholar] [CrossRef]
- Guang, F.; Jun-Qing, L.; Run-Guo, Z.; Yi, D.; Xun-Ru, A.; Lan, Y. Variation in three community features across habitat types and scales within a 15-ha subtropical evergreen-deciduous broadleaved mixed forest dynamics plot in China. Ecol. Evol. 2018, 8, 11987–11998. [Google Scholar]
- Hulshof, C.M.; Violle, C.; Spasojevic, M.J.; McGill, B.; Damschen, E.; Harrison, S.; Enquist, B.J. Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. J. Veg. Sci. 2013, 24, 921–931. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A Standardized Protocol for the Determination of Specific Leaf Area and Leaf Dry Matter Content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Ryser, P.; Urbas, P. Ecological Significance of Leaf Life Span among Central European Grass Species. Oikos 2000, 91, 41–50. [Google Scholar] [CrossRef]
- Cornelissen, J.H.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Carboni, M.; Calderon-Sanou, I.; Pollock, L.; Violle, C.; DivGrass, C.; Thuiller, W. Functional traits modulate the response of alien plants along abiotic and biotic gradients. Glob. Ecol. Biogeogr. 2018, 27, 1173–1185. [Google Scholar] [CrossRef]
- Tilman, D. Competition and biodiversity in spatially structured habitats. Ecology 1994, 75, 2–16. [Google Scholar] [CrossRef]
- Hu, Y.K.; Pan, X.; Yang, X.J.; Liu, G.F.; Liu, X.Y.; Song, Y.B.; Zhang, M.Y.; Cui, L.J.; Dong, M. Is there coordination of leaf and fine root traits at local scales? A test in temperate forest swamps. Ecol. Evol. 2019, 9, 8714–8723. [Google Scholar] [CrossRef] [PubMed]
- Gamier, E.; Navas, M.-L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar]
- Anderegg, L.D.L. Why can’t we predict traits from the environment? New Phytol. 2023, 237, 1998–2004. [Google Scholar] [CrossRef]
- Ying, L.; Qian, L.; Li, X.; Mingxu, L.; Zhi, C.; Zhaopeng, S.; Jihua, H.; Nianpeng, H. Plant community traits can explain variation in productivity of selective logging forests after different restoration times. Ecol. Indic. 2021, 131, 108181. [Google Scholar]
- Milla, R.; Reich, P.B. The scaling of leaf area and mass: The cost of light interception increases with leaf size. Proc. Biol. Sci. 2007, 274, 2109–2115. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nat. Int. Wkly. J. Sci. 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef]
- Bongers, F.J.; Schmid, B.; Sun, Z.; Li, Y.; Härdtle, W.; Oheimb, G.; Li, Y.; Li, S.; Staab, M.; Ma, K.; et al. Growth–trait relationships in subtropical forest are stronger at higher diversity. J. Ecol. 2020, 108, 256–266. [Google Scholar] [CrossRef]
- Maynard, D.S.; Bialic-Murphy, L.; Zohner, C.M.; Averill, C.; van den Hoogen, J.; Ma, H.; Mo, L.; Smith, G.R.; Acosta, A.T.; Aubin, I.; et al. Global relationships in tree functional traits. Nat. Commun. 2022, 13, 3185. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Overton, J.M.; Collins, B.; Westoby, M. Shifts in Trait-Combinations along Rainfall and Phosphorus Gradients. J. Ecol. 2000, 88, 964–977. [Google Scholar] [CrossRef]
- Shipley, B. Structured Interspecific Determinants of Specific Leaf Area in 34 Species of Herbaceous Angiosperms. Funct. Ecol. 1995, 9, 312–319. [Google Scholar] [CrossRef]
- Grubb, P.J. A reassessment of the strategies of plants which cope with shortages of resources. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 3–31. [Google Scholar] [CrossRef]
- Zhou, J.; Cieraad, E.; van Bodegom, P.M. Global analysis of trait–trait relationships within and between species. New Phytol. 2022, 233, 1643–1656. [Google Scholar] [CrossRef]
- Baraloto, C.; Timothy Paine, C.E.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.M.; Hérault, B.; Patiño, S.; Roggy, J.C.; Chave, J. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef]
- Lasky, J.R.; Uriarte, M.; Boukili, V.K.; Chazdon, R.L. Trait-mediated assembly processes predict successional changes in community diversity of tropical forests. Proc. Natl. Acad. Sci. USA 2014, 111, 5616–5621. [Google Scholar] [CrossRef]
- Slik, J.F.; Bernard, C.S.; Breman, F.C.; Van Beek, M.; Salim, A.; Sheil, D. Wood density as a conservation tool: Quantification of disturbance and identification of conservation-priority areas in tropical forests. Conserv. Biol. 2008, 22, 1299–1308. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Grman, E.; Bassett, T.; Brudvig, L.A. Landscape context explains ecosystem multifunctionality in restored grasslands better than plant diversity. Ecology 2019, 100, e02634. [Google Scholar] [CrossRef]
| Table | Age of Stand (Year) | Species Characteristics | Plot Characteristics | ||
|---|---|---|---|---|---|
| ti, Mean | ti, Min–Max | Pj, Mean | Pj, Min–Max | ||
| LA (cm2) | 20 | 96.50 ± 25.32 A | 2.59–3183.13 | 90.01 ± 47.18 A | 39.92–244.16 |
| 35 | 74.39 ± 115.26 A | 9.33–1050.05 | 76.68 ± 31.88 A | 34.26–143.01 | |
| old | 68.32 ± 106.53 A | 4.24–903.20 | 47.14 ± 11.44 B | 31.32–79.94 | |
| SLA (cm2/g) | 20 | 511.23 ± 186.45 A | 164.63–1217.88 | 500.62 ± 101.67 A | 347.64–698.65 |
| 35 | 262.79 ± 125.63 B | 72.46–714.65 | 231.50 ± 17.92 B | 194.91–269.01 | |
| old | 232.27 ± 94.48 C | 67.90–663.80 | 207.43 ± 30.93 B | 138.36–252.56 | |
| LDMC (g/g) | 20 | 0.33 ± 0.08 A | 0.12–0.69 | 0.34 ± 0.03 A | 0.30–0.46 |
| 35 | 0.32 ± 0.08 A | 0.14–0.55 | 0.33 ± 0.02 A | 0.28–0.39 | |
| old | 0.32 ± 0.08 A | 0.15–0.52 | 0.34 ± 0.02 A | 0.31–0.39 | |
| WD (g/cm3) | 20 | 0.49 ± 0.11 A | 0.11–0.79 | 0.51 ± 0.04 A | 0.37–0.58 |
| 35 | 0.53 ± 0.12 B | 0.11–0.90 | 0.54 ± 0.04 B | 0.45–0.61 | |
| old | 0.52 ± 0.11 B | 0.21–0.75 | 0.55 ± 0.02 B | 0.49–0.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Yao, J.; Huang, Y.; Zang, R. Variation in Functional Traits of Woody Plants Across Successional Stages in Subtropical Forests. Forests 2025, 16, 868. https://doi.org/10.3390/f16050868
Sun C, Yao J, Huang Y, Zang R. Variation in Functional Traits of Woody Plants Across Successional Stages in Subtropical Forests. Forests. 2025; 16(5):868. https://doi.org/10.3390/f16050868
Chicago/Turabian StyleSun, Cheng, Jie Yao, Yongtao Huang, and Runguo Zang. 2025. "Variation in Functional Traits of Woody Plants Across Successional Stages in Subtropical Forests" Forests 16, no. 5: 868. https://doi.org/10.3390/f16050868
APA StyleSun, C., Yao, J., Huang, Y., & Zang, R. (2025). Variation in Functional Traits of Woody Plants Across Successional Stages in Subtropical Forests. Forests, 16(5), 868. https://doi.org/10.3390/f16050868







