Ecosystem Service Synergies and Trade-Offs in Poplar–Birch Mixed Natural Forests Across Different Developmental Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Plots
2.2. Data Sources and Processing
2.3. Calculating Ecosystem Services
2.3.1. Quantifying Carbon Storage
| Tree Species | Biomass Model | Carbon Stock Conversion Factor | References |
|---|---|---|---|
| Betula platyphylla | W = H0.5202 | 0.487 | [28] |
| Populus davidiana | W = H0.5916 | 0.471 | |
| Larix principis-rupprechtii | W = H0.5915 | 0.489 | |
| Picea asperata | W = H0.2566 | 0.490 | |
| Betula pendula | W = H0.5202 | 0.487 | |
| Tilia mongolica | W = H0.61117 | 0.475 | |
| Ulmus pumila | W = H0.4907 | 0.450 | |
| Pinus sylvestris var. mongolica | W = H0.3476 | 0.484 | |
| Quercus mongolica | W = H | 0.500 | [29] |
| Carbon Density | Formula | Interpretation | References |
|---|---|---|---|
| Belowground | Cbelow is the below-ground carbon density (kg/km2); Cabove is the above-ground carbon density (kg/km2); and b is the ratio of below-ground biomass to above-ground biomass. The b value of forest land was set at 0.36 in this study. | [30] | |
| Dead organic matter | Cdead is dead organic carbon density (kg/hm2); TOC is organic carbon content (g/kg); G is the total fresh weight of the sample in a 1 m × 1 m sample plot (g); W is the water content of the sample (%) | [31] | |
| Soil organic matter | Csoil is soil organic carbon density (kg/hm2); TOC is organic carbon content(g/kg); y is soil density (g/cm3); H is average soil thickness (cm) | [32] |
2.3.2. Quantify Productivity
2.3.3. Quantifying Water Conservation
2.4. Validation for InVEST Model
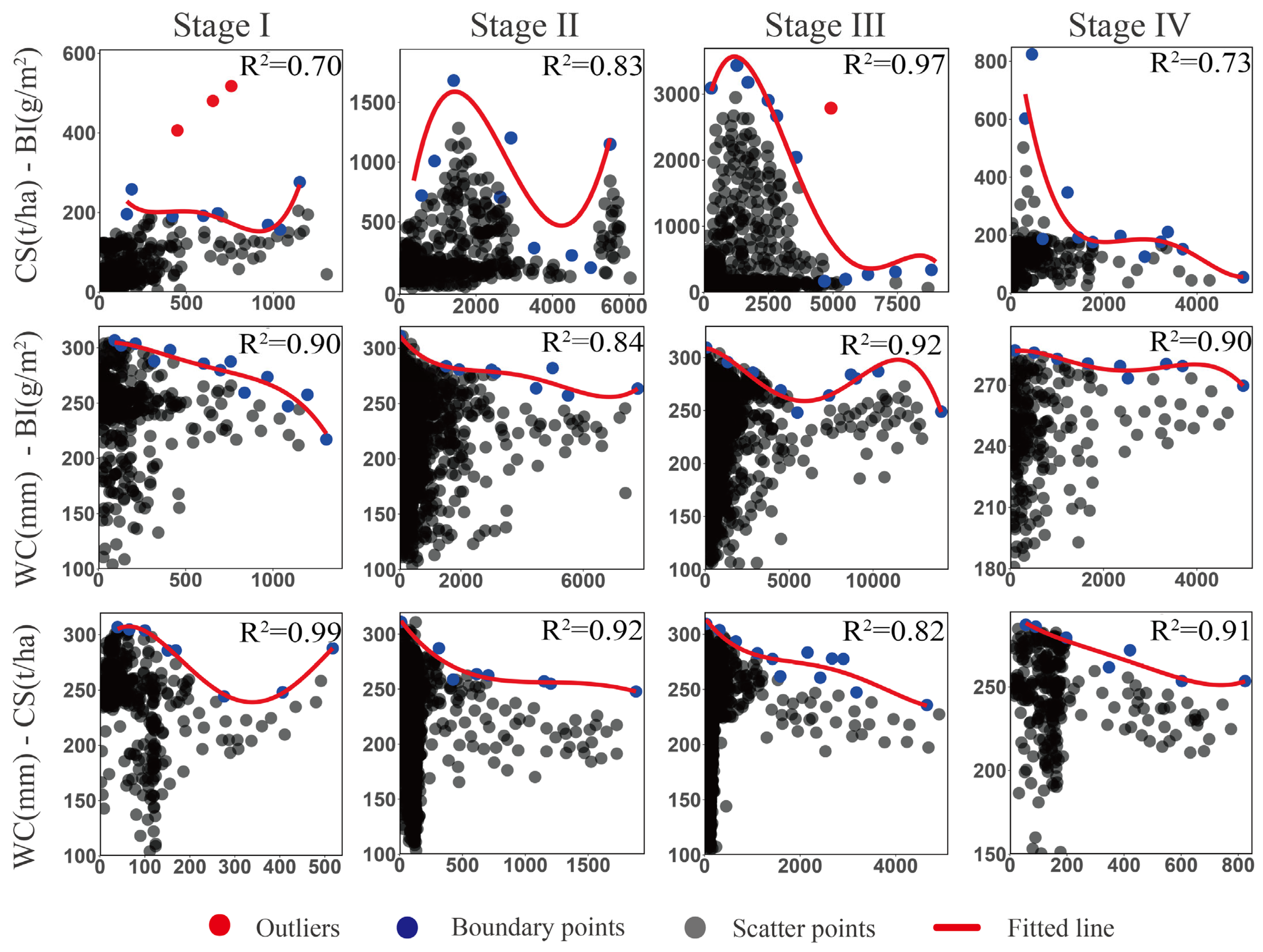
2.5. Ecosystem Service Trade-Offs/Synergies
3. Results
3.1. Spatial Distribution of Ecosystem Services
3.2. Changes in Ecosystem Services with Developmental Stage
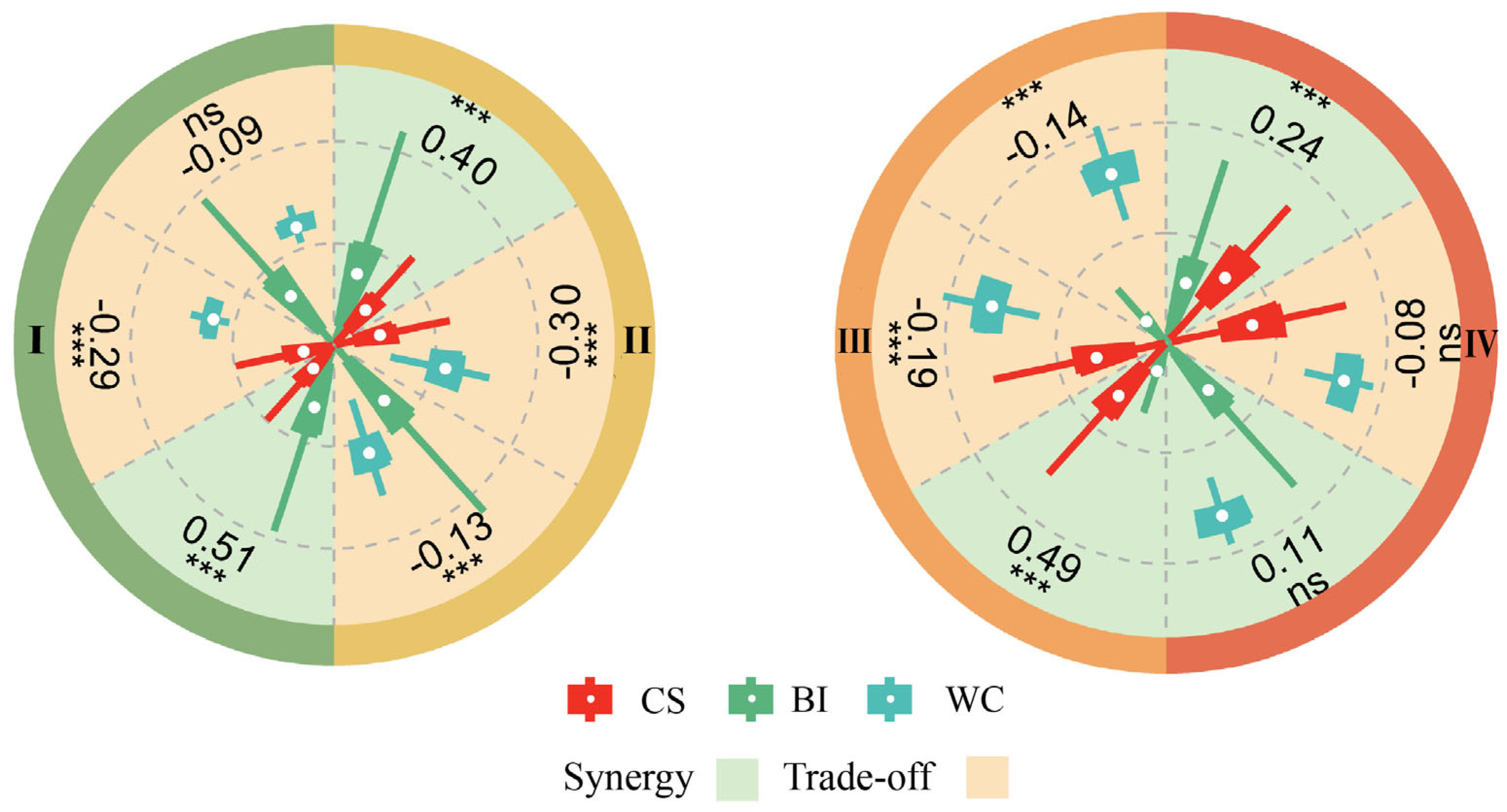
3.3. Trade-Offs/Synergies in Ecosystem Services
4. Discussion
4.1. Changes in Ecosystem Services with Developmental Stage
4.2. Trade-Offs/Synergies Between BI and CS
4.3. Trade-Offs/Synergies Between WC and BI
4.4. Trade-Offs/Synergies Between WC and CS
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Data | Source | Resolution (m) | Pre-Processing Methods | Format |
|---|---|---|---|---|
| Climate data | The high-resolution climate model ClimateAP [70] | 50 | Firstly, bilinear interpolation and dynamic local regression were used to downscale monthly data from the ClimateAP model to scale-free point values. Then, the Barrier Spline Function Interpolation Tool in ArcGIS was used to create a rasterized climate dataset of the study area. | Raster |
| Topographic data | Geospatial Data Cloud (https://www.gscloud.cn) (accessed on 5 December 2023) | 50 | Using ArcGIS 10.8, the original projection was converted into WGS_1984_UTM_Zone_50N through the “Projection” tool. Subsequently, the corresponding data were extracted based on the vector of the study area by means of the “Extract by Mask” tool or the “Clip” tool. | Raster |
| Soil data | Mapping high resolution National Soil Information Grids of China [71] | 90 | Raster | |
| Root restricting layer depth | Depth-to-bedrock map of China [72] | 100 | Raster | |
| Watershed data | China metropolis group of basic geographic data (1951–2023) [73] | - | vector | |
| Land Use/Land Cover data | Forest inventory data | 50 | Based on the screening principles, ArcGIS 10.8 was used to precisely extract the distribution information of different developmental stages of poplar (Populus davidiana)–birch (Betula platyphylla) mixed natural secondary forests (MPB) from the forest resource inventory data, and then the distribution ranges of MPB were determined. | Raster |
References
- Guo, Z.L.; Liu, Y.N.; Zhang, L.; Feng, C.Y.; Chen, Y.M. Research progress and prospect of ecosystem services. J. Environ. Eng. Technol. 2022, 12, 928–936. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. Forest contribution to climate change mitigation: Management oriented to carbon capture and storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef]
- Jin, J.; Xiang, W.H.; Zeng, Y.L.; Ouyang, S.; Zhou, X.L.; Hu, Y.T.; Zhao, Z.H.; Chen, L.; Lei, P.F.; Deng, X.W.; et al. Stand carbon storage and net primary production in China’s subtropical secondary forests are predicted to increase by 2060. Carbon Balance Manag. 2022, 17, 6–14. [Google Scholar] [CrossRef]
- Biber, P.; Felton, A.; Nieuwenhuis, M.; Lindbladh, M.; Black, K.; Bahýl, J.; Bingöl, Ö.; Borges, J.G.; Botequim, B.; Brukas, V.; et al. Forest biodiversity, carbon sequestration, and wood production: Modeling synergies and trade-offs for ten forest landscapes across Europe. Front. Ecol. Evol. 2020, 8, 547696. [Google Scholar] [CrossRef]
- Yan, X.; Cao, G.C.; Cao, S.K.; Yuan, J.; Zhao, M.L.; Tong, S.; Li, H.D. Spatiotemporal variations of water conservation and its influencing factors in the Qinghai Plateau, China. Ecol. Indic. 2023, 155, 111047. [Google Scholar] [CrossRef]
- Sandifer, P.A.; Sutton-Grier, A.E.; Ward, B.P. Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosyst. Serv. 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Darvishi, A.; Yousefi, M.; Dinan, N.M.; Angelstam, P. Assessing levels, trade-offs and synergies of landscape services in the Iranian province of Qazvin: Towards sustainable landscapes. Landscape Ecol. 2022, 37, 305–327. [Google Scholar] [CrossRef]
- Mazziotta, A.; Lundström, J.; Forsell, N.; Moor, H.; Eggers, J.; Subramanian, N.; Aquilué, N.; Morán-Ordóñez, A.; Brotons, L.; Snäll, T. More future synergies and less trade-offs between forest ecosystem services with natural climate solutions instead of bioeconomy solutions. Glob. Change Biol. 2022, 28, 6333–6348. [Google Scholar] [CrossRef]
- Gutsch, M.; Lasch-Born, P.; Kollas, C.; Suckow, F.; Reyer, C.P. Balancing trade-offs between ecosystem services in Germany’s forests under climate change. Environ. Res. Lett. 2018, 13, 045012. [Google Scholar] [CrossRef]
- Soimakallio, S.; Kalliokoski, T.; Lehtonen, A.; Salminen, O. On the trade-offs and synergies between forest carbon sequestration and substitution. Mitig. Adapt. Strat. Gl. 2021, 26, 4. [Google Scholar] [CrossRef]
- Morán-Ordóñez, A.; Ameztegui, A.; De Cáceres, M.; de-Miguel, S.; Lefèvre, F.; Brotons, L.; Coll, L. Future trade-offs and synergies among ecosystem services in Mediterranean forests under global change scenarios. Ecosyst. Serv. 2020, 45, 101174. [Google Scholar] [CrossRef]
- Agol, D.; Reid, H.; Crick, F.; Wendo, H. Ecosystem-based adaptation in Lake Victoria Basin; synergies and trade-offs. R. Soc. Open Sci. 2021, 8, 201847. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, L.; Wu, J.; Liao, Y.H. Past and future ecosystem service trade-offs in Poyang Lake basin under different land use policy scenarios. Arab. J. Geosci. 2020, 13, 46. [Google Scholar] [CrossRef]
- Karimi, J.D.; Corstanje, R.; Harris, J.A. Bundling ecosystem services at a high resolution in the UK: Trade-offs and synergies in urban landscapes. Landscape Ecol. 2021, 36, 1817–1835. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhang, P.; Qiu, X.C.; Xu, G.L.; Chi, J.Y. Spatial-temporal variations and trade-offs of ecosystem services in Anhui Province, China. Int. J. Env. Res. Public Health 2023, 20, 855. [Google Scholar] [CrossRef] [PubMed]
- Meraj, G.; Singh, S.K.; Kanga, S.; Islam, M.N. Modeling on comparison of ecosystem services concepts, tools, methods and their ecological—Economic implications: A review. Model. Earth Syst. Environ. 2022, 8, 15–34. [Google Scholar] [CrossRef]
- Zou, K.H.; Tuncali, K.; Silverman, S.G. Correlation and simple linear regression. Radiology 2003, 227, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.E.; Wulder, M.A.; Nelson, T.A.; Coops, N.C. Spatial data, analysis approaches, and information needs for spatial ecosystem service assessments: A review. GISci. Remote Sens. 2015, 52, 344–373. [Google Scholar] [CrossRef]
- Knoke, T.; Kindu, M.; Jarisch, I.; Gosling, E.; Friedrich, S.; Bödeker, K.; Paul, C. How considering multiple criteria, uncertainty scenarios and biological interactions may influence the optimal silvicultural strategy for a mixed forest. For. Policy Econ. 2020, 118, 102239. [Google Scholar] [CrossRef]
- Zuo, L.Y.; Gao, J.B.; Du, F.J. The pairwise interaction of environmental factors for ecosystem services relationships in karst ecological priority protection and key restoration areas. Ecol. Indic. 2021, 131, 108125. [Google Scholar] [CrossRef]
- Ding, X.M.; Jian, S.Q. Synergies and trade-offs of ecosystem services affected by land use structures of small watershed in the Loess Plateau. J. Environ. Manag. 2024, 350, 119589. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.F.; Yu, D.Y.; Wu, J.G. Relationship between paired ecosystem services in the grassland and agro—Pastoral transitional zone of China using the constraint line method. Agric. Ecosyst. Environ. 2017, 240, 171–181. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Ge, Z.X.; Zhang, Z.D. Stand spatial structure and productivity based on random structural unit in Larix principis-rupprechtii forests. Ecosphere 2024, 15, e4824. [Google Scholar] [CrossRef]
- Bohnett, E.; An, L. Special issue editorial: Biodiversity and ecosystem services in forest ecosystems. Sustainability 2023, 15, 16364. [Google Scholar] [CrossRef]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Global Environ. Change 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Clerici, N.; Cote-Navarro, F.; Escobedo, F.J.; Rubiano, K.; Villegas, J.C. Spatio—Temporal and cumulative effects of land use—Land cover and climate change on two ecosystem services in the Colombian Andes. Sci. Total Environ. 2019, 685, 1181–1192. [Google Scholar] [CrossRef]
- Esri. ArcGIS Desktop: Release 10.8. Redlands, CA: Environmental Systems Research Institute. 2020. Available online: https://desktop.arcgis.com/ (accessed on 10 December 2023).
- GB/T 43648-2024; Tree Biomass Models and Related Parameters to Carbon Accounting for Major Tree Species. Standards Press of China: Beijing, China, 2024.
- Sun, H.L. Estimation of biomass in Mongolian oak forests of the northern Hebei Mountain region. Shandong For. Sci. Technol. 2018, 48, 44–45. [Google Scholar]
- Rajashekar, G.; Fararoda, R.; Reddy, R.S.; Jha, C.S.; Ganeshaiah, K.N.; Singh, J.S.; Dadhwal, V.K. Spatial distribution of forest biomass carbon (above and below ground) in Indian forests. Ecol. Indic. 2018, 85, 742–752. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhang, J.M.; Zhou, Y.N.; Guan, Y.H.; Zhou, J.X. Analyzing spatial distribution of carbon storage and hot spots in Xiaoluan river basin in Weichang County based on InVEST model. Chin. J. Ecol. 2023, 42, 2536. [Google Scholar]
- Rong, Y.J.; Zhang, H.; Zhao, X.F. Carbon stock functions under land use change in the Lake Tai basin in the last 10 years based on InVEST modeling. Jiangsu Agric. Sci. 2016, 5, 447–451. [Google Scholar]
- Djomo, A.N.; Knohl, A.; Gravenhorst, G. Estimations of total ecosystem carbon pools distribution and carbon biomass current annual increment of a moist tropical forest. For. Ecol. Manag. 2011, 261, 1448–1459. [Google Scholar] [CrossRef]
- He, X.; Lei, X.D.; Dong, L.H. How large is the difference in large-scale forest biomass estimations based on new climate-modified stand biomass models? Ecol. Indic. 2021, 126, 107569. [Google Scholar] [CrossRef]
- Zhang, L.; Hickel, K.; Dawes, W.R.; Chiew, F.H.; Western, A.W.; Briggs, P.R. A rational function approach for estimating mean annual evapotranspiration. Water Resour. Res. 2004, 40, W02502. [Google Scholar] [CrossRef]
- Yang, D.; Liu, W.; Tang, L.Y.; Chen, L.; Li, X.Z.; Xu, X.L. Estimation of water provision service for monsoon catchments of South China: Applicability of the InVEST model. Landscape Urban Plan. 2019, 182, 133–143. [Google Scholar] [CrossRef]
- Sun, W.; Shao, Q.; Liu, J.; Zhai, J. Assessing the effects of land use and topography on soil erosion on the Loess Plateau in China. Catena 2014, 121, 151–163. [Google Scholar] [CrossRef]
- Maxwell, S.E.; Delaney, H.D.; Kelley, K. Designing Experiments and Analyzing Data: A Model Comparison Perspective, 3rd ed.; Routledge: New York, NY, USA, 2017; p. 1080. [Google Scholar] [CrossRef]
- Wang, S.M.; Jin, T.T.; Yan, L.L.; Gong, J. Trade—Off and synergy among ecosystem services and the influencing factors in the Ziwuling Region, Northwest China. Chin. J. Appl. Ecol. 2022, 33, 3087–3096. [Google Scholar] [CrossRef]
- Hao, R.; Yu, D.; Wu, J.; Guo, Q.; Liu, Y. Constraint line methods and the applications in ecology. Chin. J. Plant Ecol. 2016, 40, 1100–1109. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- R Core Team. R Foundation for Statistical Computing, Vienna, Austria. 2023. Available online: https://www.r-project.org/ (accessed on 18 December 2023).
- Hardiman, B.S.; Gough, C.M.; Halperin, A.; Hofmeister, K.L.; Nave, L.E.; Bohrer, G.; Curtis, P.S. Maintaining high rates of carbon storage in old forests: A mechanism linking canopy structure to forest function. For. Ecol. Manag. 2013, 298, 111–119. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochem. 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Molina-Valero, J.A.; Camarero, J.J.; Álvarez-González, J.G.; Cerioni, M.; Hevia, A.; Sánchez-Salguero, R.; Martin-Benito, D.; Pérez-Cruzado, C. Mature forests hold maximum live biomass stocks. For. Ecol. Manag. 2021, 480, 118635. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity—Productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.G. Plant functional traits have globally consistent effects on competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Tree species mixing can increase stand productivity, density and growth efficiency and attenuate the trade-off between density and growth throughout the whole rotation. Ann. Bot. 2021, 128, 767–786. [Google Scholar] [CrossRef] [PubMed]
- Schwärzel, K.; Zhang, L.; Montanarella, L.; Wang, Y.; Sun, G. How afforestation affects the water cycle in drylands: A process-based comparative analysis. Glob. Change Biol. 2020, 26, 944–959. [Google Scholar] [CrossRef] [PubMed]
- van Meerveld, H.J.; Jones, J.P.; Ghimire, C.P.; Zwartendijk, B.W.; Lahitiana, J.; Ravelona, M.; Mulligan, M. Forest regeneration can positively contribute to local hydrological ecosystem services: Implications for forest landscape restoration. J. Appl. Ecol. 2021, 58, 755–765. [Google Scholar] [CrossRef]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; Van Noordwijk, M.; Creed, I.F.; Pokorny, J. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Change 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Feng, S.; Liu, C.; Zhong, X.; Ren, Y.; Liu, Y.; Huang, Y.; Yang, M. The relationship between atmospheric particulate matter, leaf surface microstructure, and the phyllosphere microbial diversity of Ulmus L. BMC Plant Biol. 2024, 24, 566. [Google Scholar] [CrossRef]
- Wang, X.Z.; Wu, J.Z.; Liu, Y.L.; Hai, X.Y.; Shanguan, Z.P.; Deng, L. Driving factors of ecosystem services and their spatiotemporal change assessment based on land use types in the Loess Plateau. J. Environ. Manag. 2022, 311, 114835. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.J.J.A.; da Cruz Silva, A.; Jamme, D.; Peña-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2020, 457, 117696. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.H.; Pang, X.Y. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Başkent, E.Z.; Kašpar, J. Exploring the effects of management intensification on multiple ecosystem services in an ecosystem management context. For. Ecol. Manag. 2022, 518, 120299. [Google Scholar] [CrossRef]
- Harel, A.; Thiffault, E.; Paré, D. Ageing forests and carbon storage: A case study in boreal balsam fir stands. Forestry 2021, 94, 651–663. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Berry, Z.C.; Jones, K.W.; Aguilar, L.R.G.; Congalton, R.G.; Holwerda, F.; Kolka, R.; Looker, N.; Lopez Ramirez, S.M.; Manson, R.; Mayer, A.; et al. Evaluating ecosystem service trade-offs along a land—Use intensification gradient in central Veracruz, Mexico. Ecosyst. Serv. 2020, 45, 101181. [Google Scholar] [CrossRef]
- Farooqi, T.J.A.; Li, X.H.; Yu, Z.; Liu, S.R.; Sun, O.J. Reconciliation of research on forest carbon sequestration and water conservation. J. For. Res. 2021, 32, 7–14. [Google Scholar] [CrossRef]
- Pan, T.; Zuo, L.; Zhang, Z.; Zhao, X.; Sun, F.; Zhu, Z.; Liu, Y. Impact of land use change on water conservation: A case study of Zhangjiakou in Yongding River. Sustainability 2020, 13, 22. [Google Scholar] [CrossRef]
- Ghimire, C.P.; Bruijnzeel, L.A.; Lubczynski, M.W.; Ravelona, M.; Zwartendijk, B.W.; van Meerveld, H.J. Measurement and modeling of rainfall interception by two differently aged secondary forests in upland eastern Madagascar. J. Hydrol. 2017, 545, 212–225. [Google Scholar] [CrossRef]
- Hu, Y.T.; Zhang, F.; Luo, Z.Z.; Badreldin, N.; Benoy, G.; Xing, Z.S. Soil and water conservation effects of different types of vegetation cover on runoff and erosion driven by climate and underlying surface conditions. Catena 2023, 231, 107347. [Google Scholar] [CrossRef]
- Souza, C.R.; Maia, V.A.; de Aguiar-Campos, N.; Santos, A.B.; Rodrigues, A.F.; Farrapo, C.L.; Gianasi, F.M.; de Paula, G.G.P.; Fagundes, N.C.A.; Silva, W.B.; et al. Long-term ecological trends of small secondary forests of the Atlantic forest hotspot: A 30-year study case. For. Ecol. Manag. 2021, 489, 119043. [Google Scholar] [CrossRef]
- Asbjornsen, H.; Wang, Y.; Ellison, D.; Ashcraft, C.M.; Atallah, S.S.; Jones, K.; Mayer, A.; Altamirano, M.; Yu, P. Multi-targeted payments for the balanced management of hydrological and other forest ecosystem services. For. Ecol. Manag. 2022, 522, 120482. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, C.F.; He, X.; Zhang, X.H.; Feng, L.Y.; Zhang, H.R. The effect of stand density, biodiversity, and spatial structure on stand basal area increment in natural spruce-fir-broadleaf mixed forests. Forests 2022, 13, 162. [Google Scholar] [CrossRef]
- Kurzweil, J.R.; Metlen, K.; Abdi, R.; Strahan, R.; Hogue, T.S. Surface water runoff response to forest management: Low-intensity forest restoration does not increase surface water yields. For. Ecol. Manag. 2021, 496, 119387. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Innes, J.L.; Seely, B.; Chen, B. ClimateAP: An application for dynamic local downscaling of historical and future climate data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448–458. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H.Y.; Zhao, Y.G.; Li, D.C.; Yang, J.L.; Song, X.D.; Shi, Z.; Zhu, A.-X.; Zhang, G.L. Mapping high resolution National Soil Information Grids of China. Sci. Bull. 2021, 67, 328–340. [Google Scholar] [CrossRef]
- Yan, F.; Shangguan, W.; Zhang, J.; Hu, B. Depth-to-bedrock map of China at a spatial resolution of 100 meters. Sci. Data 2020, 7, 2. [Google Scholar] [CrossRef]
- China Metropolis Group of Basic Geographic Data (1951–2023). National Tibetan Plateau/Third Pole Environment Data Center. Available online: https://data.tpdc.ac.cn (accessed on 19 April 2023).
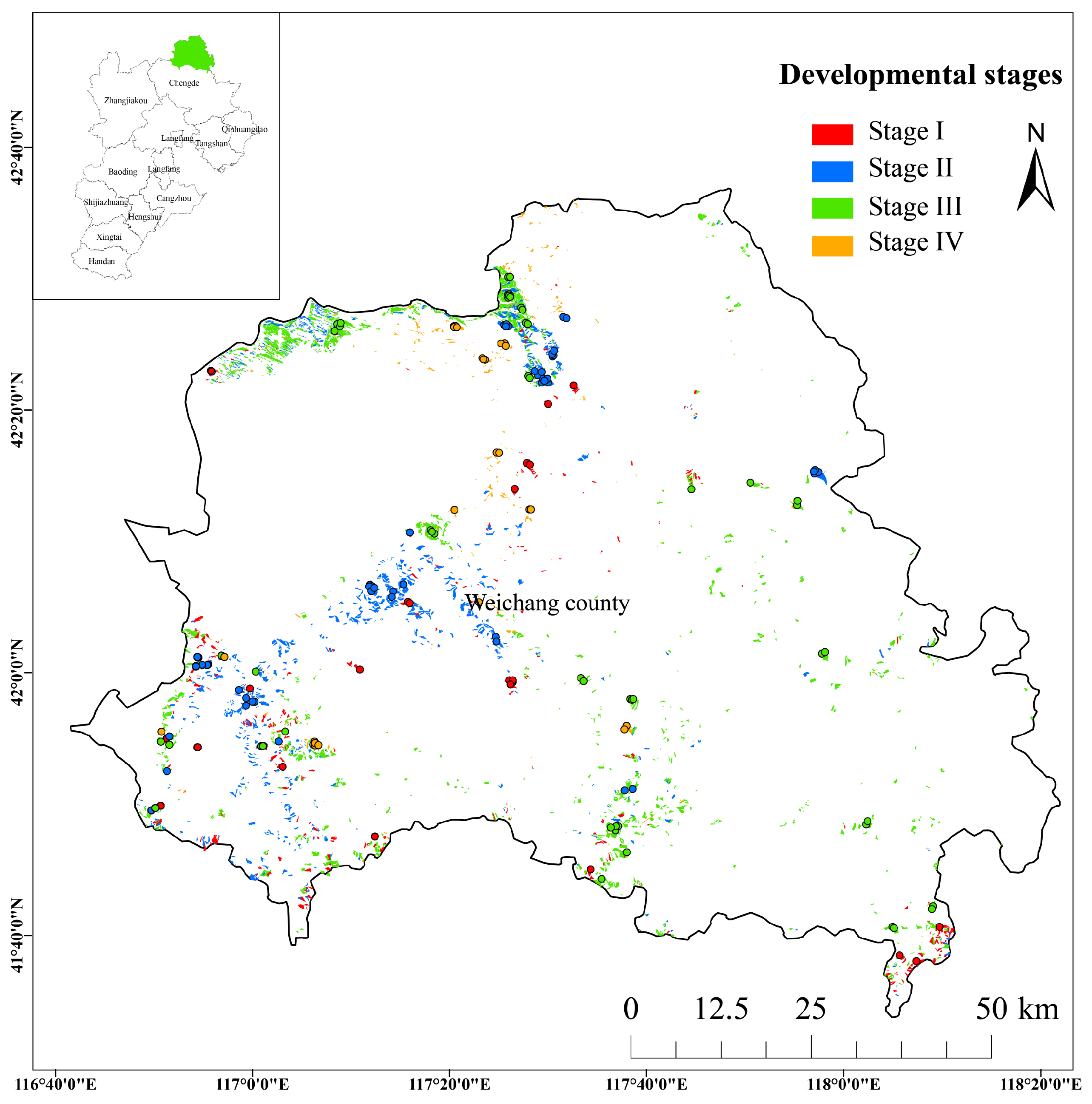
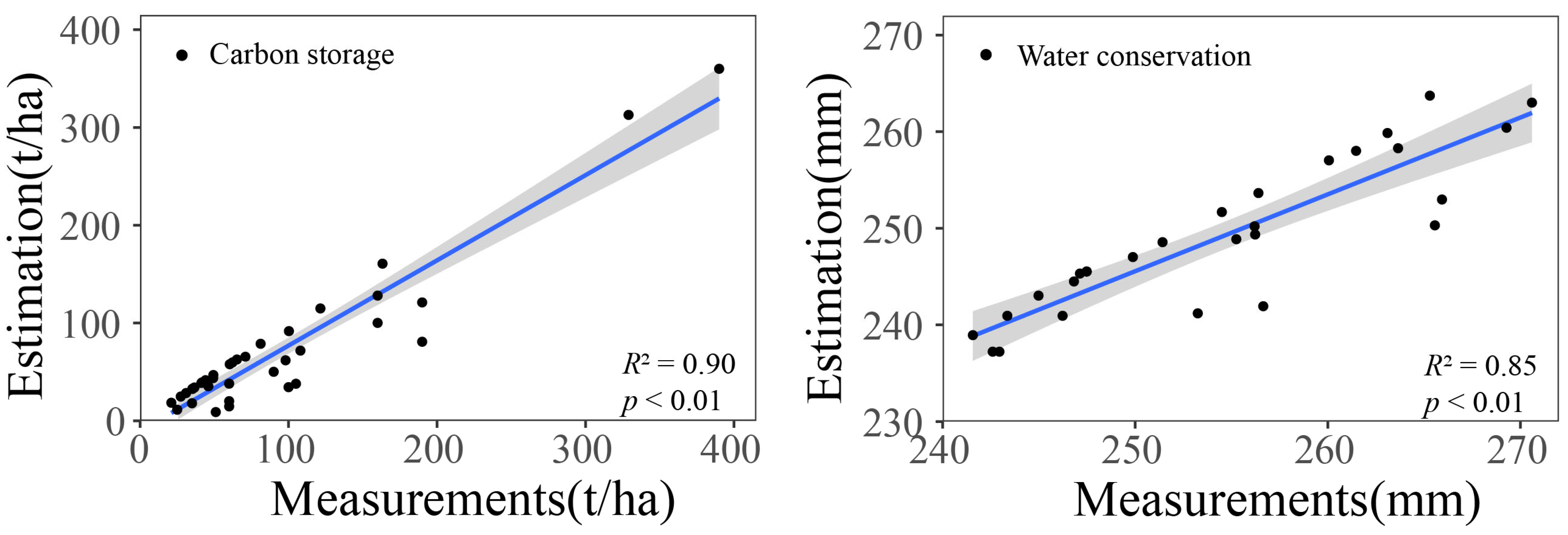

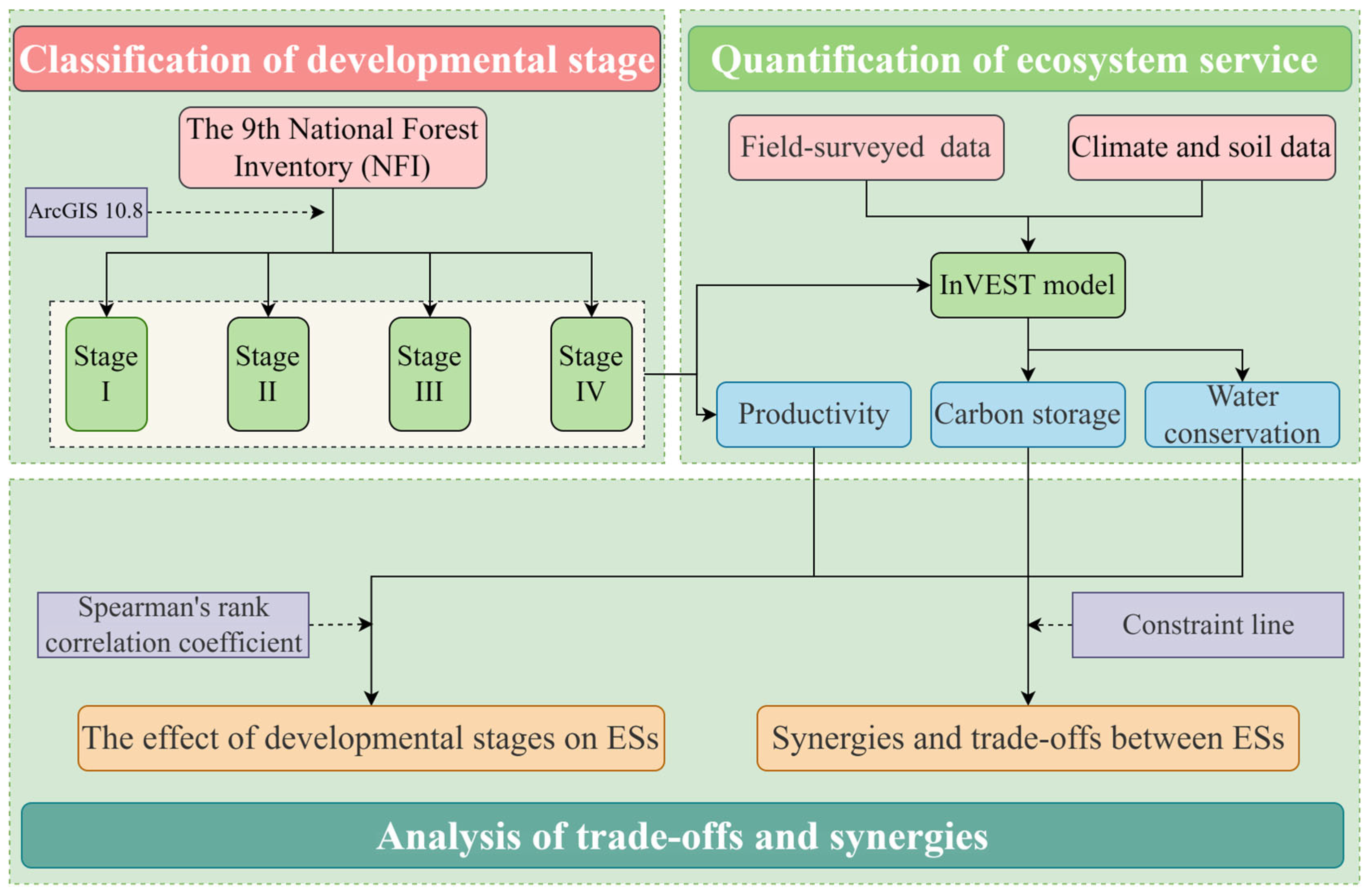
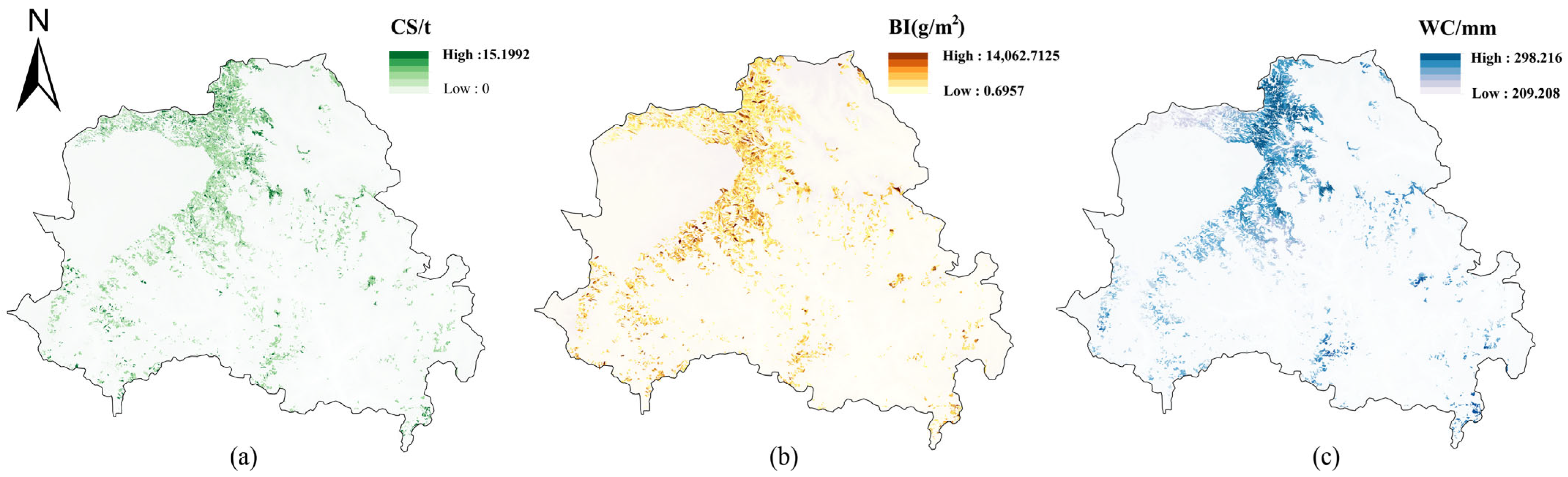

| Developmental Stage | Dominant Species | Basal Area (m2) | Mean Age (a) | Mean DBH (cm) | Mean Height (m) | Number of Plots |
|---|---|---|---|---|---|---|
| Stage I | Pd | 5.06 (33.06) | 28 | 10.7 | 11.3 | 23 |
| Bp | 6.67 (43.59) | 12.4 | 10.9 | |||
| Stage II | Pd | 9.19 (48.52) | 35 | 11.5 | 12.6 | 49 |
| Bp | 6.72 (45.33) | 14.8 | 11.9 | |||
| Stage III | Bp | 6.01 (29.55) | 51 | 15.7 | 13.8 | 52 |
| Lr | 13.08 (64.39) | 18.5 | 13.4 | |||
| Stage IV | Lr | 12.61 (54.69) | 64 | 19.6 | 15.5 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, M.; Liu, Q.; Pang, Y.; Zhang, Z. Ecosystem Service Synergies and Trade-Offs in Poplar–Birch Mixed Natural Forests Across Different Developmental Stages. Forests 2025, 16, 867. https://doi.org/10.3390/f16050867
Zhang J, Li M, Liu Q, Pang Y, Zhang Z. Ecosystem Service Synergies and Trade-Offs in Poplar–Birch Mixed Natural Forests Across Different Developmental Stages. Forests. 2025; 16(5):867. https://doi.org/10.3390/f16050867
Chicago/Turabian StyleZhang, Junfei, Minghao Li, Qiang Liu, Yue Pang, and Zhidong Zhang. 2025. "Ecosystem Service Synergies and Trade-Offs in Poplar–Birch Mixed Natural Forests Across Different Developmental Stages" Forests 16, no. 5: 867. https://doi.org/10.3390/f16050867
APA StyleZhang, J., Li, M., Liu, Q., Pang, Y., & Zhang, Z. (2025). Ecosystem Service Synergies and Trade-Offs in Poplar–Birch Mixed Natural Forests Across Different Developmental Stages. Forests, 16(5), 867. https://doi.org/10.3390/f16050867






