Abstract
Taxodium ‘Zhongshanshan’ serves as a primary afforestation species in coastal saline–alkali soils, yet its healthy growth is significantly constrained by excessive soil salinity and nutrient deficiencies. This study investigated the synergistic effects of arbuscular mycorrhizal fungi (AMF) with organic amendments (biochar/straw) on ameliorating soil amelioration and plant adaptation. Six treatments were implemented: Control (CK), Biochar (B), Straw (S), AMF (M), AMF+Biochar (M+B), and AMF+Straw (M+S), with physiological and edaphic parameters monitored over two growth cycles. The results revealed that the M+B treatment demonstrated superior performance, achieving the lowest soil pH (8.06) and electrical conductivity (0.25 mS/cm) alongside reduced Na+ accumulation in plant tissues (0.28–0.88 mg/g). Synergistic effects were evident in enhanced chlorophyll synthesis, soluble protein production, and antioxidant enzyme activation. Partial Least Squares Path Modeling (PLS-PM) analysis revealed that soil nitrogen availability indirectly stimulated growth through upregulation of soluble proteins (path coefficient: 0.54) and antioxidant activity (0.22), with cumulative indirect effects (0.88) outweighing direct inhibition (−0.36). These finding provide actionable insights for coastal afforestation strategies using microbial–organic material co-application.
1. Introduction
Soil salinization poses a global environmental threat, necessitating comprehensive prevention and remediation strategies for coastal saline–alkali soils to mitigate its impact on China []. Coastal saline–alkali soils, spanning >8000 km2 across the northern Jiangsu and Bohai Rim regions [], represent a critical yet underutilized region for afforestation. However, their hyper-saline conditions (soil salt content > 5‰), nutrient deficiency, and compacted texture [] severely constrain tree establishment and carbon sequestration potential. Taxodium ‘Zhongshanshan’, a nationally prioritized species with exceptional salt tolerance (surviving 5‰ salinity) and rapid biomass accumulation [], offers a viable solution for coastal silviculture.
Soil salinity mainly inhibits plant growth through osmotic stress, oxidative damage, and ionic toxicity. Concomitant oxidative damage disrupts cellular architecture through deleterious effects on biomembranes, mitochondria, and chloroplasts []. Excessive Na+ and Cl− uptake further compromises enzymatic functionality and ionic homeostasis, ultimately suppressing protein biosynthesis []. Traditional saline–alkali soil remediation employs chemical, physical, and biological interventions with limited success [,,]. Organic amendments like straw improve soil structure and hydraulic conductivity while suppressing salt upward movement through capillary disruption [,]. Microbial decomposition of straw releases humic substances that neutralize alkalinity []. Biochar, with its microporous architecture (Brunauer–Emmett–Teller surface area > 200 m2/g), enhances salt adsorption capacity and soil aeration [,] while delivering stable organic carbon to stimulate microbial biomass [,]. Microbial inoculants, particularly arbuscular mycorrhizal fungi (AMF), synergize with these organic amendments by enhancing nutrient acquisition and ionic homeostasis through glomalin-mediated soil aggregation []. Previous studies demonstrate that AMF mediate sodium homeostasis by reducing Na+ accumulation and maintaining favorable K+/Na+ homeostasis while coordinately upregulating key antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) [].
Despite these advances, knowledge gaps persist regarding field-scale interactions between AMF and organic matrices in coastal ecosystems. Biochar-straw substrates may serve as microbial scaffolds, amplifying AMF colonization (hyphal density +60%) and metabolic activity through enhanced carbon provisioning [,]. However, the tripartite interplay of (1) soil physicochemical modulation, (2) microbial community dynamics, and (3) plant physiological responses remain unquantified for saline–alkali afforestation systems.
This study evaluates six amendment strategies (Control, Biochar, Straw, AMF, AMF+Biochar, AMF+Straw) in a Taxodium ‘Zhongshanshan’ plantation, addressing two critical questions: (1) How do AMF–organic combinations differentially influence soil biogeochemistry and tree ion homeostasis? (2) What mechanistic pathways connect soil amelioration to enhance the growth-promoting effects? By integrating physicochemical analyses with PLS-PM modeling, this study aims to establish an optimized protocol for coastal saline soil rehabilitation, providing actionable insights for China’s nature-based climate solutions.
2. Materials and Methods
2.1. Study Area
The study region is located in the Yancheng Dafeng District, Jiangsu, China, spanning the transitional ecotone between subtropical and warm temperate climatic zones. Characterized by a distinct monsoon-influenced climate pattern, the region demonstrates marked seasonal variations. Meteorological records indicate a mean annual temperature of 14.1 °C coupled with an average yearly precipitation of 1042.2 mm. The soil salt content is about 7‰, the water content is 20.32%, the bulk density is 1.53 g/cm3, and the pH value is 8.6. Electrical conductivity (EC) measurements range from 1.6 to 5 mS/cm. The soil is characterized as saline loam with high salinity and low fertility. The primary afforestation species in the coastal region include Taxodium ‘Zhongshanshan’, Populus spp., Zelkova serrata, and Carya illinoinensis. Previous studies have demonstrated a rich diversity of AMF in the rhizospheric soils of these dominant tree species. Specifically, the genus Glomus was identified as the predominant AMF group in the rhizosphere of Taxodium ‘Zhongshanshan’, with a relative abundance reaching 74%.
2.2. Experimental Design
A randomized block design with six treatments was implemented in April 2018 using 108 two-year-old Taxodium ‘Zhongshanshan’ saplings (mean height: 2.06 ± 0.15 m; basal diameter: 2.90 ± 0.21 cm). The treatments comprised the following: Control (CK), Biochar (B), Straw (S), AMF (M), AMF+Biochar (M+B), and AMF+Straw (M+S), each containing 18 plants arranged in 6 × 3 grids with 3 m interplant spacing.
Rice husk biochar (particle size < 2 mm) was produced through optimized pyrolysis: (1) feedstock pre-drying (<10% moisture) and (2) pyrolysis at 800 °C for <30 min under oxygen-limited conditions (H/C ratio: 0.42 ± 0.05), following established protocols []. This biochar was spread around the seedlings and then thoroughly incorporated into the soil by deep plowing. Chopped rice straw (5 cm segments) was buried at 40 cm depth to disrupt capillary salt transport, with an equivalent application rate (0.5 kg/plant).
Based on previous investigations of AMF resources in the rhizospheric soil of Taxodium ‘Zhongshanshan’ in coastal regions, Glomus mosseae was selected as the microbial inoculant for this study. This strain was sourced from the Institute of Plant Nutrition and Resources at the Beijing Academy of Agriculture and Forestry Sciences []. G. mosseae was propagated through trap culture with Trifolium repens in sterilized yellow sand (121 °C, 0.14 MPa, 2 h). After 12-week colonization, the inoculum (hyphae-sand-root fragments mixture, 0.5 kg/plant) was applied to rhizosphere zones.
In October 2019, after two full growth seasons, sampling was carried out. From each treatment plot, three Taxodium ‘Zhongshanshan’ with consistent and stable growth were carefully selected for sampling purposes. Separate samples were meticulously collected from different components: the soil within a 0–20 cm radius around the root zone, branches, leaves, and roots. The plant samples were promptly placed into small, portable refrigerators and maintained at a temperature of 4 °C to preserve their integrity.
Soil samples underwent sequential processing for physicochemical characterization. For chemical analysis, visible roots and particulate debris (>2 mm) were manually excised prior to air-drying at ambient temperature. Physical property determination employed standardized cutting ring methodology []: (1) Site preparation: clear surface debris within 20 cm diameter. (2) Core extraction: align 100 cm3 stainless steel cutting ring vertically and apply gradual penetration via calibrated impact hammer. (3) Sample refinement: trim end surfaces using tungsten carbide blade to preserve structural integrity. (4) Hermetic sealing: double-layer polyethylene packaging with inert gas displacement, labeled with GPS coordinates and sampling depth.
2.3. Samples Determination
Sapling height was measured at planting (April 2018) and harvest (October 2019) using a measuring tape. Height growth rate is calculated based on these measured values []. Soil bulk density and total porosity were quantified through the standardized cutting ring methodology using undisturbed cores oven-dried at 105 °C until reaching constant mass []. Soil pH and electrical conductivity (EC) were measured in 1:5 (w/v) soil–water suspensions at 25 ± 0.5 °C using calibrated PHS-3C pH and DDSJ-318L EC meters. Soil alkaline-hydrolyzable nitrogen was determined by zinc-sulfuric acid reduction method with liberated NH3 absorbed in boric acid and titrated using 0.01 mol/L HCl (mg/kg) []. Soil available phosphorus was extracted with 0.5 mol/L NaHCO3 (pH 8.5) and quantified via molybdenum blue colorimetry at 880 nm [].
Chlorophyll content was quantified via dual-solvent extraction (acetone:ethanol = 1:1, v/v) followed by spectrophotometric determination at 645 and 663 nm. SOD was assayed by nitroblue tetrazolium (NBT) photoreduction inhibition at 560 nm []. POD was measured via guaiacol oxidation kinetics at 470 nm []. CAT was determined by H2O2 decomposition rate at 240 nm []. The mass fraction of malondialdehyde (MDA) was quantified as thiobarbituric acid reactive substances (TBARS) at 532 nm []. Root vitality was assessed through riphenyltetrazolium chloride (TTC) reduction to triphenylformazan at 485 nm after 2 h incubation with 0.4% TTC (37 °C), expressed as μg TTF/g·h []. The soluble sugar mass fraction was estimated by the anthrone colorimetric method at 625 nm using a glucose standard curve. The soluble protein mass fraction was quantified by the Coomassie Brilliant Blue colorimetric method at 595 nm. Cations (K+, Ca2+, Na+, Mg2+) were quantified via atomic absorption spectroscopy (PerkinElmer PinAAcle 900T) after HNO3-HClO4 digestion. Total phosphorus was measured via vanadomolybdate colorimetry at 440 nm.
2.4. Statistical Analyses
Data were organized using Excel 2019. One-way ANOVA in SPSS 16.0 was applied to analyze seedling height growth rate, soil physical and chemical properties, and physiological characteristics of Taxodium ‘Zhongshanshan’. Two-way ANOVA in SPSS 16.0 was used to examine the element content in plant organs. Duncan’s multiple range test was performed to compare the significance of mean differences (p < 0.05) among different soil improvement measures and plant organs. Pearson correlation analysis and image production were performed using Origin 2024. Using models created with R4.4.1 software, partial least squares path modeling (PLS-PM) was used to evaluate the direct and indirect effects of soil physicochemical qualities, ion concentration in forest tree organs, and physiological traits on forest tree growth.
3. Results
3.1. The Influence of AMF Inoculant and Organic Amendments on the Physicochemical Properties of Soil in Taxodium ‘Zhongshanshan’ Plantations
AMF inoculant with organic amendments exerted no significant influence on the soil bulk density, capillary porosity, or moisture content (p > 0.05; Figure 1A–C). Conversely, it notably increased the contents of soil alkaline-hydrolyzed nitrogen and available phosphorus (p < 0.05; Figure 1F,G) and reduced the soil electrical conductivity (p < 0.05; Figure 1E). Among the treatments, Biochar (p = 0.108) and AMF (p = 0.537) treatments had no significant impact on soil pH (Figure 1D). The AMF+Biochar treatment exhibited the lowest values for soil pH and electrical conductivity, registering at 8.06 and 0.25 ms/cm, respectively (Table S3). Furthermore, the AMF inoculant, organic amendments, and their combined treatments significantly increased soil alkaline-hydrolyzed nitrogen and the soil available phosphorus content (p < 0.05, Figure 1F,G). However, no significant differences were found among these improvement treatments (p > 0.05).
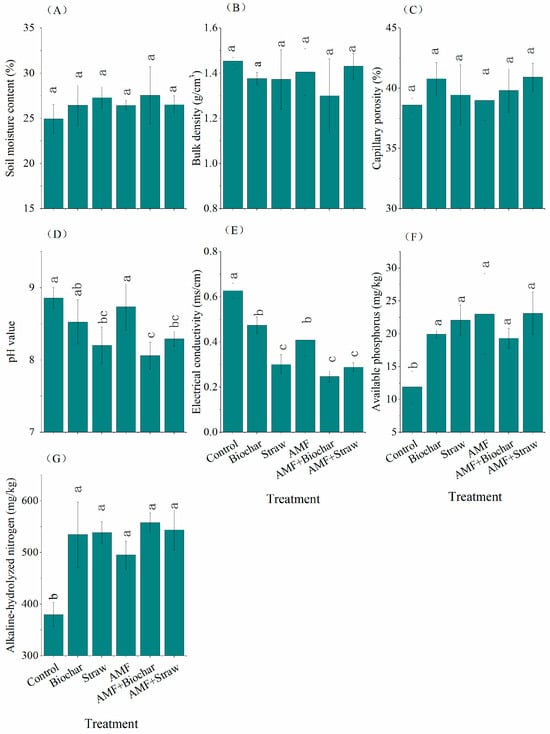
Figure 1.
Soil physical and chemical properties. Note: Different lowercase letters represent significant difference between different treatments (p < 0.05). (A) soil moisture content; (B) bulk density; (C) capillary porosity; (D) pH; (E) electrical conductivity; (F) available phosphorus; (G) alkaline-hydrolyzed nitrogen.
3.2. The Influence of AMF Inoculant and Organic Amendments on the Growth of Taxodium ‘Zhongshanshan’
The application of Biochar (p = 0.004), AMF+Biochar (p = 0.00002), or AMF+Straw (p = 0.005) treatments significantly enhanced the height growth of Taxodium ‘Zhongshanshan’ seedlings compared to CK treatment (Figure 2A). Among the treatments, the AMF+Biochar treatment demonstrated superior efficacy (21.50%), achieving a 103.76% greater height growth rate than CK treatment (10.55%) (Table S1). Regarding mineral nutrition, both treatment type and plant organ significantly influenced phosphorus and cation concentrations (Figure 2B–F), though no treatment–organ interactions were observed for potassium and sodium (p > 0.05, Figure 2C,E). Foliar tissues consistently showed higher total phosphorus, potassium, and magnesium concentrations than branches and roots across all treatments, with maximum values recorded in the AMF+Biochar treatment (Figure 2B,C,F). Notably, calcium distribution patterns differed from other elements, as both the Straw and AMF+Biochar treatments significantly reduced branch calcium content compared to other organs (Figure 2D). Sodium accumulation exhibited treatment-specific patterns, with the AMF+Biochar treatment showing the lowest values across all organs (leaf: 0.88 mg/g, branch: 0.28 mg/g, root: 0.82 mg/g) (Tables S4–S6). However, only root sodium content in this treatment showed statistically significant reduction relative to the control (p < 0.05).
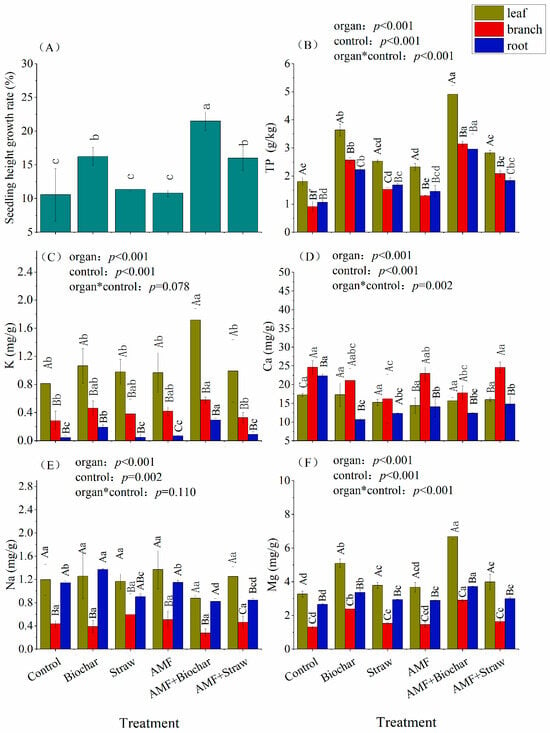
Figure 2.
Height growth of Taxodium ‘Zhongshanshan’ and element content in different organs. Note: Lowercase letters denote statistically significant differences (p < 0.05) among treatment groups within homologous plant organs, and different uppercase letters represent significant difference between different plant organs of the same treatment (p < 0.05). (A) seeding height growth rate; (B) TP, total phosphorus; (C) K; (D) Ca; (E) Na; (F) Mg. The organ represents the significance of different plant organs. The control represents the significance of different treatments. The organ*control represents the interactive effects between different treatment and plant organ.
The combined application of AMF inoculant with organic amendments significantly enhanced leaf physiological performance in Taxodium ‘Zhongshanshan’, notably reducing malondialdehyde content (Figure 3E) while increasing chlorophyll levels (Figure 3A) and antioxidant enzyme activities, including superoxide dismutase (SOD, Figure 3B) and catalase (CAT, Figure 3C). Peroxidase (POD, Figure 3D) and soluble protein content (Figure 3G) also rose significantly across treatments, with the exception of sole AMF inoculant application. Among these interventions, the AMF+Biochar showed the most pronounced enhancement effects. Notably, biochar alone achieved maximal values for leaf soluble sugars (2.66%) and root vitality (10.61 μg/g/h) (Figure 3F,G) (Table S2).
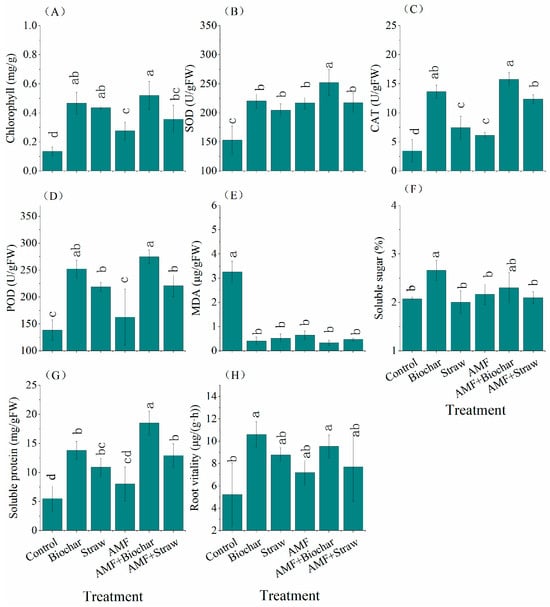
Figure 3.
Physiological characteristics of Taxodium ‘Zhongshanshan’. Note: Different lowercase letters represent significant difference between different treatments (p < 0.05). (A) chlorophyll; (B) SOD, superoxide dismutase; (C) CAT, catalase; (D) POD, peroxidase; (E) MDA, malondialdehyde; (F) soluble sugar; (G) soluble protein; (H) root vitality.
3.3. Analysis of the Factors Influencing the Growth of Taxodium ‘Zhongshanshan’ Following the Application of AMF Inoculant and Organic Amendments
As depicted in Figure 4, the height growth of Taxodium ‘Zhongshanshan’ seedlings demonstrates statistically significant positive correlations with foliar soluble protein content, root vitality, and leaf antioxidant enzyme activity. Conversely, a pronounced negative correlation was observed with electrical conductivity and soil pH. Furthermore, soil alkaline-hydrolyzable nitrogen content and soil moisture exhibited significant positive associations with root vitality. Based on these correlation patterns, we constructed a partial least squares path model (Figure 5) to quantify the causal relationships. The model revealed direct positive effects of foliar soluble protein (path coefficient = 0.56) and antioxidant enzyme activity (0.48) on seedling height increment. In contrast, soil alkaline-hydrolyzable nitrogen (−0.36) and electrical conductivity (−0.18) showed direct inhibitory effects. Notably, alkaline-hydrolyzable nitrogen demonstrated a dual regulatory mechanism—while exerting direct negative impacts, it indirectly promoted vertical growth through mediation effects on soluble protein synthesis (0.54) and antioxidant enzyme activation (0.22). The cumulative indirect effect (0.88) substantially outweighed the direct negative effect (−0.36), indicating the predominance of its indirect regulatory pathway.
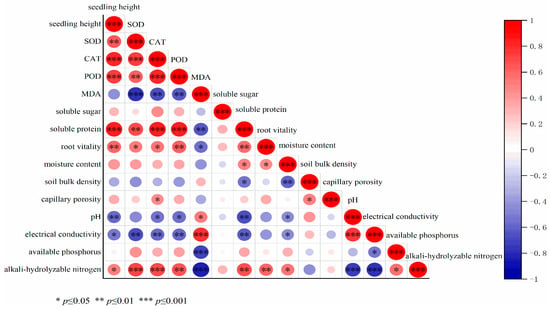
Figure 4.
Correlation analysis.
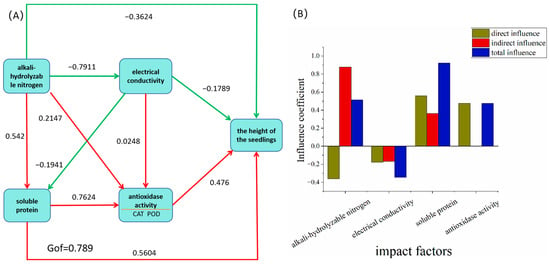
Figure 5.
Partial least squares path modeling (PLS-PM) analysis. Note: (A) partial least squares path model (PLS-PM); (B) standardization influence coefficient; CAT, catalase activity; POD, peroxidase activity; Gof, goodness-of-fit index. The red line indicates the positive effect, and the green line indicates the negative effect. The yellow underline means the CAT and POD are the latent variables of the antioxidase activity.
4. Discussion
Coastal saline–alkaline soils primarily originate from fluvial deposits, characterized by high silt content and dominance of capillary pores. These properties contribute to elevated soil bulk density, compromised aeration and permeability, and suboptimal conditions for plant growth. Biochar, with its high specific surface area and intricate porous structure [], has been shown to enhance soil porosity and reduce bulk density []. In this study, biochar application reduced soil bulk density in Taxodium ‘Zhongshanshan’ plantations from 1.45 g/cm3 to 1.38 g/cm3, though the reduction was not statistically significant compared to controls, potentially due to suboptimal application rates or a power issue. Notably, AMF+Biochar further decreased bulk density to 1.30 g/cm3, likely mediated through enhanced microbial activity and accelerated soil mineralization processes []. Straw incorporation increased soil organic binding agents (e.g., polysaccharides, proteins, and organic acids), facilitating macro-aggregate formation and bulk density reduction []. However, the observed non-significant decrease in bulk density under straw treatment may relate to application methodology; straw was exclusively embedded in the 40 cm soil layer to disrupt capillary continuity [] and inhibit upward salt migration, resulting in significantly lower electrical conductivity but negligible bulk density effects. Future saline soil afforestation strategies should consider combining surface mixing and subsurface layering for straw application.
In addition, the straw degradation process generates organic matter such as proteins and lignin while introducing substantial amounts of nitrogen, phosphorus, and potassium nutrients into the soil. This enhances the soil’s nutrient supply capacity [,] and further promotes the growth and reproduction of beneficial microorganisms. This study found that straw addition significantly increased the content of soil available phosphorus and alkali-hydrolyzable nitrogen in Taxodium ‘Zhongshansha’ plantation, consistent with findings reported by Chen et al. []. Similarly to straw treatment, biochar application also significantly enhanced soil available phosphorus and alkali-hydrolyzable nitrogen levels, primarily because biochar itself is rich in nitrogen, phosphorus, and potassium nutrients []. Furthermore, biochar can improve the aggregate stability of saline–alkali soil [], thereby enhancing its nutrient retention capacity. Moreover, the addition of arbuscular mycorrhizal fungi (AMF) inoculant significantly improved soil available nutrients. However, the combined application of AMF inoculants with organic amendments did not produce a significant synergistic enhancement effect on soil available nutrients. This phenomenon may be attributed to the enhanced microbial activity under co-application conditions, which facilitates the conversion of available nutrients into gaseous forms or less-utilizable forms (e.g., ammonia volatilization of nitrogen []) during microbial decomposition of organic materials, ultimately resulting in no significant improvement in soil available nutrients.
Notably, we observed that the combined application of organic amendments with AMF inoculant exerted substantial impacts on soil pH and electrical conductivity. Particularly, the co-application of biochar and inoculants resulted in the lowest soil pH and electrical conductivity. This outcome can be primarily explained by two mechanisms: (1) The calcium and magnesium ions abundantly present on biochar surfaces can effectively displace sodium ions in saline–alkali soil []. (2) Biochar significantly increases soil porosity and specific surface area, thereby improving soil water-holding capacity. The synergistic application with inoculant further enhances the leaching process in saline–alkali soil, ultimately leading to significant reduction in soil salinity and alkalinity [].
Excessive salt ions in coastal saline soils impair chloroplast photosystems, disrupt ion homeostasis, and induce osmotic stress, culminating in physiological drought []. Our findings demonstrate that Biochar and AMF+Straw mitigated salt inhibition by improving rhizospheric conditions. Seedling height, a sensitive salt-stress indicator, showed significant increases under Biochar and AMF–organic treatments, consistent with chlorophyll content trends and prior reports [,,]. Excessive Na+ accumulation is a major factor limiting tree growth in saline–alkali soils. Plant cells can alleviate Na+ toxicity by increasing Na+ efflux, decreasing root absorption, and closing K+ outward-transport channels while increasing intracellular levels of K+, Ca2+, and Mg2+ []. However, single AMF inoculant application has no significant effect on the Na+ content in Taxodium ‘Zhongshanshan’ organs. This may be due to the lack of carbon sources in coastal saline soils, which restricts the rapid and healthy growth of AMF and, thus, fails to show good results in the short term. In contrast, its combination with organic materials—particularly biochar—achieved pronounced Na+ mitigation. This highlights the critical role of organic carbon supplementation in optimizing AMF functionality under saline conditions [].
Enhanced synthesis of osmolytes and antioxidant enzymes constitutes a key adaptive strategy to salt stress. Osmolytes stabilize membrane integrity and cellular enzymes while maintaining low water potential to counteract dehydration []. Both soluble sugars and proteins act as osmoprotectants under salinity []. In this study, AMF–organic amendments significantly elevated foliar soluble protein levels, confirming their osmoprotective effects. Salt stress induces reactive oxygen species (ROS) overproduction in chloroplasts, mitochondria, and apoplasts [], which disrupt protein and nucleic acid metabolism []. Antioxidant enzymes (SOD, CAT, POD) mitigate ROS damage by catalyzing superoxide radical dismutation and hydrogen peroxide decomposition [,]. Our results revealed that biochar (alone or with AMF) most effectively enhanced antioxidant enzyme activities. Structural equation modeling further demonstrated that AMF–organic treatments directly modulated soil conductivity and alkali-hydrolyzable nitrogen, indirectly upregulating foliar osmolytes and antioxidant enzymes to promote height growth of Taxodium ‘Zhongshanshan’.
5. Conclusions
Arbuscular mycorrhizal fungi (AMF) inoculation combined with organic amendments effectively mitigates soil constraints in coastal saline–alkali Taxodium ‘Zhongshansha’ plantations by reducing bulk density, pH, and electrical conductivity while enhancing soil nutrient availability. The modified rhizospheric environment further increased total phosphorus and potassium concentrations across plant tissues, concurrently mitigating sodium ion toxicity. Moreover, AMF and organic amendments indirectly improve foliar chlorophyll content, soluble protein levels, and antioxidant enzyme activities through soil amelioration, thereby promoting plant growth. Integrative analysis of soil property optimization, ion homeostasis regulation, and physiological adaptation demonstrated that AMF+Biochar treatment yielded the most substantial growth enhancement. This synergy operated through three primary mechanisms: stabilizing soil structure, improving nutrient cycling efficiency, and strengthening osmotic adjustment capacity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16050828/s1, Table S1. Hight growth of Taxodium ‘zhongshanshan’; Table S2. Physiological characteristics of Taxodium ‘zhongshanshan’; Table S3. Soil physical and chemical properties; Table S4. Leaf element content; Table S5. branch element content; Table S6. root element content.
Author Contributions
Conceptualization, X.L. and J.Z. (Jinchi Zhang); Data curation, X.L. and J.M.; Formal analysis, X.P.; Funding acquisition, J.L. and J.Z.(Jinchi Zhang); Investigation, X.P., J.M., Q.C., and J.Z. (Jingyi Zeng); Methodology, X.L.; Project administration, X.L. and J.Z.(Jinchi Zhang); Resources, X.L.; Software, X.P. and J.M.; Supervision, X.L.; Validation, X.L.; Visualization, X.P.; Writing—original draft, X.P.; Writing—review and editing, X.L. All authors have read and agreed to the published version of the manuscript. All authers have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiangsu Science and Technology Plan Project (BE2022420) and Jiangsu Province College Students’ Innovation and Entrepreneurship Training Program (202210298075Y).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhang, Y.; Xu, X.; Wang, L.; Ouyang, Z. Evaluation of coastal farming under salinization and optimized fertilization strategies in China. Sci. Total Environ. 2021, 797, 149038. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, Y.; Zhang, B.; Zhai, L.; Liu, X.; Zhang, J. Determinants of rhizospheric organic carbon fractions and accumulation in four different vegetations of coastal saline-alkali soils. Catena 2024, 246, 108454. [Google Scholar] [CrossRef]
- Yu, C.; Xu, S.; Yin, Y. Transcriptome analysis of the Taxodium ‘Zhongshanshan 405’ roots in response to salinity stress. Plant Physiol. Biochem. 2016, 100, 156–165. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, L.; Ma, J.; Zhang, J.; Wang, G.G.; Liu, X.; Zhang, S.; Song, J.; Wu, Y. Comparative physiological mechanisms of arbuscular mycorrhizal fungi mitigate salt-induced adverse effects in leaves and roots of Zelkova serrata. Mycorrhiza 2020, 30, 341–355. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, L.; Zhang, P.; Liu, F.D.; Xiao, H.; Dong, Y.C.; Sun, H.W. Research and Application Progress of Biochar in Amelioration of Saline-Alkali Soil. Huan Jing Ke Xue 2024, 45, 940–951. [Google Scholar]
- Zhang, K.; Chang, L.; Li, G.; Li, Y. Advances and future research in ecological stoichiometry under saline-alkali stress. Environ. Sci. Pollut. Res. 2023, 30, 5475–5486. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, Q.; Li, Y.; Zhuo, Y.Q.; Xu, L.Z. Research on saline-alkali soil amelioration with FGD gypsum. Resour. Conserv. Recycl. 2017, 121, 89–92. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, B.; Liu, B.; Wang, J.; Zhang, H. Effects of Straw Return Rate on Soil Physicochemical Properties and Yield in Paddy Fields. Agronomy 2024, 14, 1668. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Zheng, S.; Dou, S.; Duan, H.M. Effects of straw enrichment and deep incorporation on humus composition and humic acid structure of black soil profile in Northeast China. Appl. Ecol. Environ. Res. 2022, 20, 1051–1063. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Li, M.; Li, P. The presence of the biochar interlayer effectively inhibits soil water evaporation and salt migration to the soil surface. Agriculture 2023, 13, 638. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.X.; Wang, Z.F.; Gao, M.; Tian, D.; Huang, R.; Liu, J.; Li, J.C. Effects of straw and biochar return in soil on soil aggregate and carbon sequestration. Huan Jing Ke Xue 2018, 39, 355–362. [Google Scholar]
- Wu, F.; Zheng, X.; Cao, M.; Guan, X.; Jiang, J. Nitrogen Addition Does Not Change AMF Colonization but Alters AMF Composition in a Chinese Fir (Cunninghamia lanceolata) Plantation. Forests 2022, 13, 979. [Google Scholar] [CrossRef]
- Duan, S.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.; Zhang, L. Cross-kingdom nutrient exchange in the plant–arbuscular mycorrhizal fungus–bacterium continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Stefanova, K.; Solaiman, Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 2016, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Nath, H.; Sarkar, B.; Mitra, S.; Bhaladhare, S. Biochar from biomass: A review on biochar preparation its modification and impact on soil including soil microbiology. Geomicrobiol. J. 2022, 39, 373–388. [Google Scholar] [CrossRef]
- Zeng, J.; Ma, S.; Liu, J.; Qin, S.; Liu, X.; Li, T.; Liao, Y.; Shi, Y.; Zhang, J. Organic Materials and AMF Addition Promote Growth of Taxodium ‘zhongshanshan’ by Improving Soil Structure. Forests 2023, 14, 731. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.J.; Fan, J.; Fu, W.; Du, M.G. Rapid in-situ measurement of soil evaporation using the cutting ring method. Chin. J. Soil Sci. 2021, 52, 55–61. [Google Scholar]
- O’Kelly, B.C. Accurate Determination of Moisture Content of Organic Soils Using the Oven Drying Method. Dry. Technol. 2004, 22, 1767–1776. [Google Scholar] [CrossRef]
- Shang, S.S.; Yu, X.F.; Qi, Y. Comparison and precautions of determination methods for hydrolysable nitrogen in soil. Agric. Technol. 2022, 42, 97–99. [Google Scholar]
- Tang, S.Y.; Zhang, L.P.; Wang, J.R.; Yuan, H.C.; He, Z.; Geng, M.M.; Chen, W. Method optimization and research for determining soil available phosphorus extracted by sodium bicarbonate using continuous flow analyzer. Soil Fertil. Sci. China 2024, 5, 232–239. [Google Scholar]
- Zhou, K.J.; Guan, C.Y.; Xiao, W.N. Effects of chemical ripeners on chlorophyll content and antioxidant enzyme activities of rapeseed pod. Yingyong Shengtai Xuebao 2009, 20, 3015–3019. [Google Scholar]
- Ruf, M.; Brunner, I. Vitality of tree fine roots: Reevaluation of the tetrazolium test. Tree Physiol. 2003, 23, 257–263. [Google Scholar] [CrossRef]
- Artiola, J.F.; Rasmussen, C.; Freitas, R. Effects of a biochar amended alkaline soil on the growth of romaine lettuce and bermudagrass. Soil Sci. 2012, 177, 561–570. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Si, B.; Wang, Y.; Chen, X.; Wang, X.; Chen, H.; Wang, H.; Zhang, F.; Bai, Y.; et al. Optimizing biochar application to improve soil physical and hydraulic properties in saline-alkali soils. Sci. Total Environ. 2021, 771, 144802. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, S.; Li, J.; Geng, J.; Wang, L. Key soil properties influencing infiltration capacity after long-term straw incorporation in a wheat (Triticum aestivum L.)–maize (Zea mays L.) rotation system. Agric. Ecosyst. Environ. 2023, 344, 108301. [Google Scholar] [CrossRef]
- Wang, J.; Jing, H.; Xu, T.; Li, C.; Zhao, C.; Feng, W. Application Effect of Straw Returning and Biochar in the Improvement of Saline-Alkali Land in Northeast China. Biol. Bull. 2023, 50, 825–836. [Google Scholar] [CrossRef]
- Chen, T.; Mei, Y.; Liu, X.; Zhao, Z.; Liang, Y. Effects of Straw at Different Fermentation Phases on Soil Nutrient Availability and Microbial Activity. Agronomy 2024, 14, 3005. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.; Aggidis, G.; Aiouache, F. Effects of wood ash-based alkaline treatment on nitrogen, carbon, and phosphorus availability in food waste and agro-industrial waste digestates. Waste Biomass Valorization 2021, 12, 3355–3370. [Google Scholar] [CrossRef]
- Jin, F.; Piao, J.; Miao, S.; Che, W.; Li, X.; Li, X.; Shiraiwa, T.; Tanaka, T.; Taniyoshi, K.; Hua, S.; et al. Long-term effects of biochar one-off application on soil physicochemical properties, salt concentration, nutrient availability, enzyme activity, and rice yield of highly saline-alkali paddy soils: Based on a 6-year field experiment. Biochar 2024, 6, 40. [Google Scholar] [CrossRef]
- Rekaby, S.A.; Awad, M.Y.M.; Hegab, S.A. Effect of some organic amendments on barley plants under saline condition. J. Plant Nutr. 2020, 43, 1840–1851. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Alaylar, B.; Kistaubayeva, A. Biochar for improving soil biological properties and mitigating salt stress in plants on saltaffected soils. Commun. Soil Sci. Plant Anal. 2021, 53, 140–152. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Q.; Wang, B.; Zhang, N.; Qiu, R.; Yuan, Y.; Yang, M.; Wang, F.; Mei, L.; Cui, G. Arbuscular mycorrhizal fungi communities and promoting the growth of alfalfa in saline ecosystems of northern China. Front. Plant Sci. 2024, 15, 1438771. [Google Scholar] [CrossRef]
- Llanes, A.; Palchetti, M.V.; Vilo, C.; Ibañez, C. Molecular control to salt tolerance mechanisms of woody plants: Recent achievements and perspectives. Ann. For. Sci. 2021, 78, 96. [Google Scholar] [CrossRef]
- Vahedi, R.; Rasouli-Sadaghiani, M.; Barin, M.; Vetukuri, R.R. Interactions between biochar and compost treatment and mycorrhizal fungi to improve the qualitative properties of a calcareous soil under rhizobox conditions. Agriculture 2021, 11, 993. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, P.; Zhang, Q.; Li, X. Advances in salt-tolerant mechanisms of trees and forestation techniques on saline-alkali land. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 96–104. [Google Scholar]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, X.; Zhao, X. Antioxidant defense response of arbuscular mycorrhizal fungi and setaria viridis. Pak. J. Bot 2023, 55, 1951–1960. [Google Scholar] [CrossRef]
- Wu, D.; Sun, P.; Lu, P.Z.; Chen, Y.Y.; Guo, J.M.; Liu, M.; Wang, L.; Zhang, C.J. Effect and approach of Enteromorpha prolifera biochar to improve coastal saline soil. Huan Jing Ke Xue 2020, 41, 1941–1949. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).