Biodiversity and Soil Jointly Drive Ecosystem Multifunctionality in Larch Forests
Abstract
1. Introduction
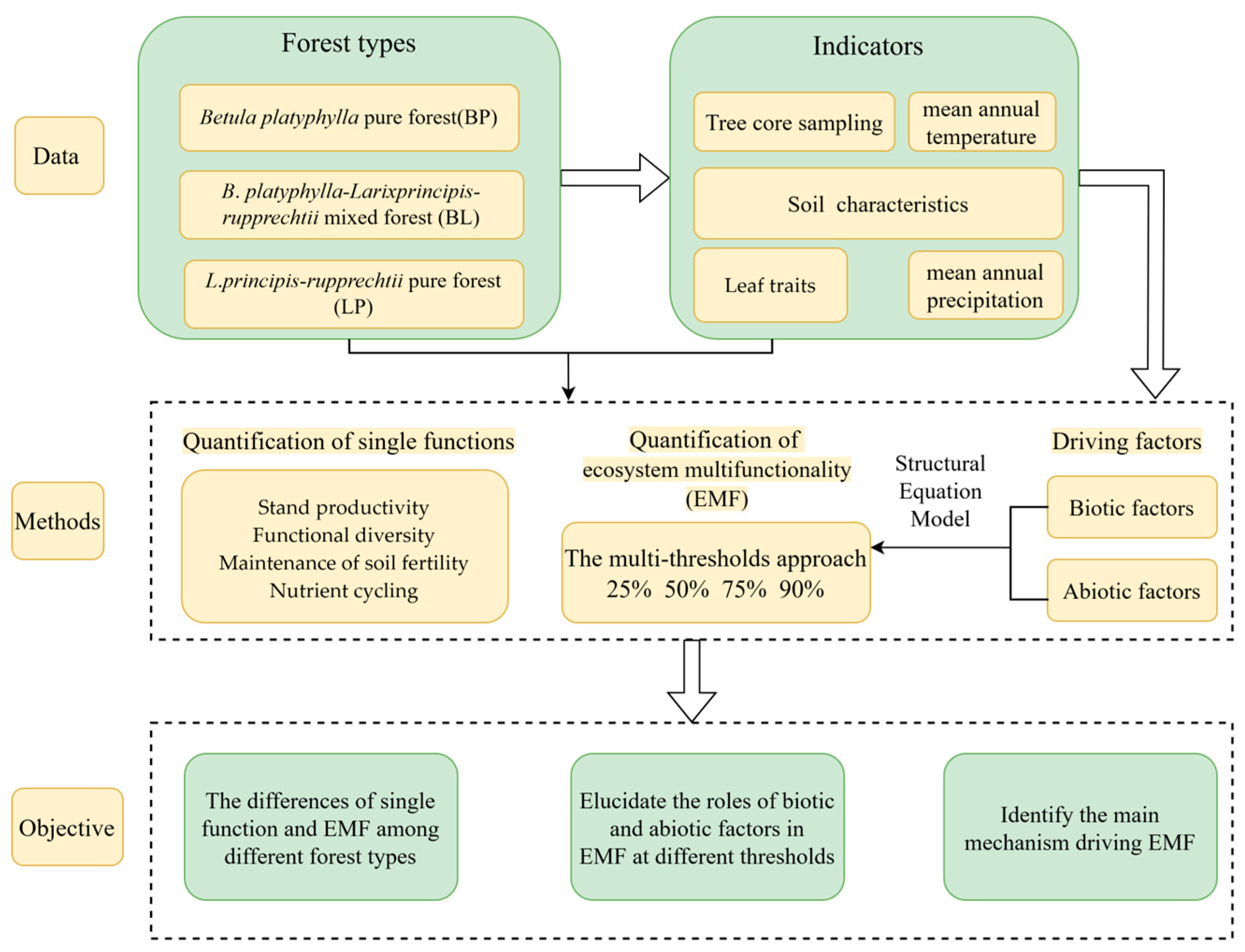
2. Materials and Methods
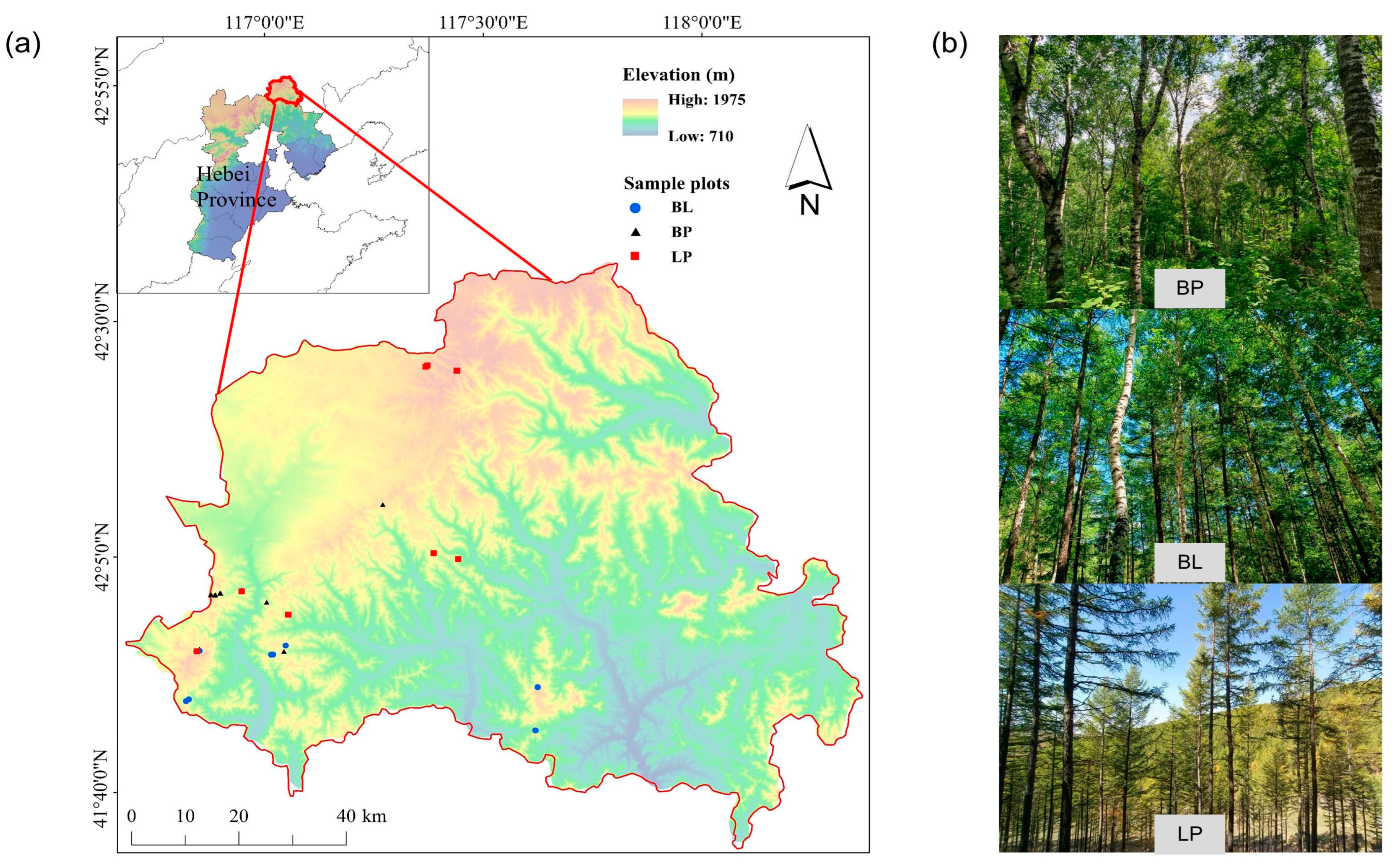
2.1. Study Area and Sample Plots
2.2. Quantification of Single Functions
2.2.1. Stand Productivity
2.2.2. Functional Diversity
2.2.3. Maintenance of Soil Fertility
2.2.4. Nutrient Cycling
2.3. Quantifying Ecosystem Multifunctionality
2.4. Selection of Driving Factors of EMF
2.4.1. Biotic Factors
2.4.2. Abiotic Factors
2.5. Statistical Analysis
3. Results
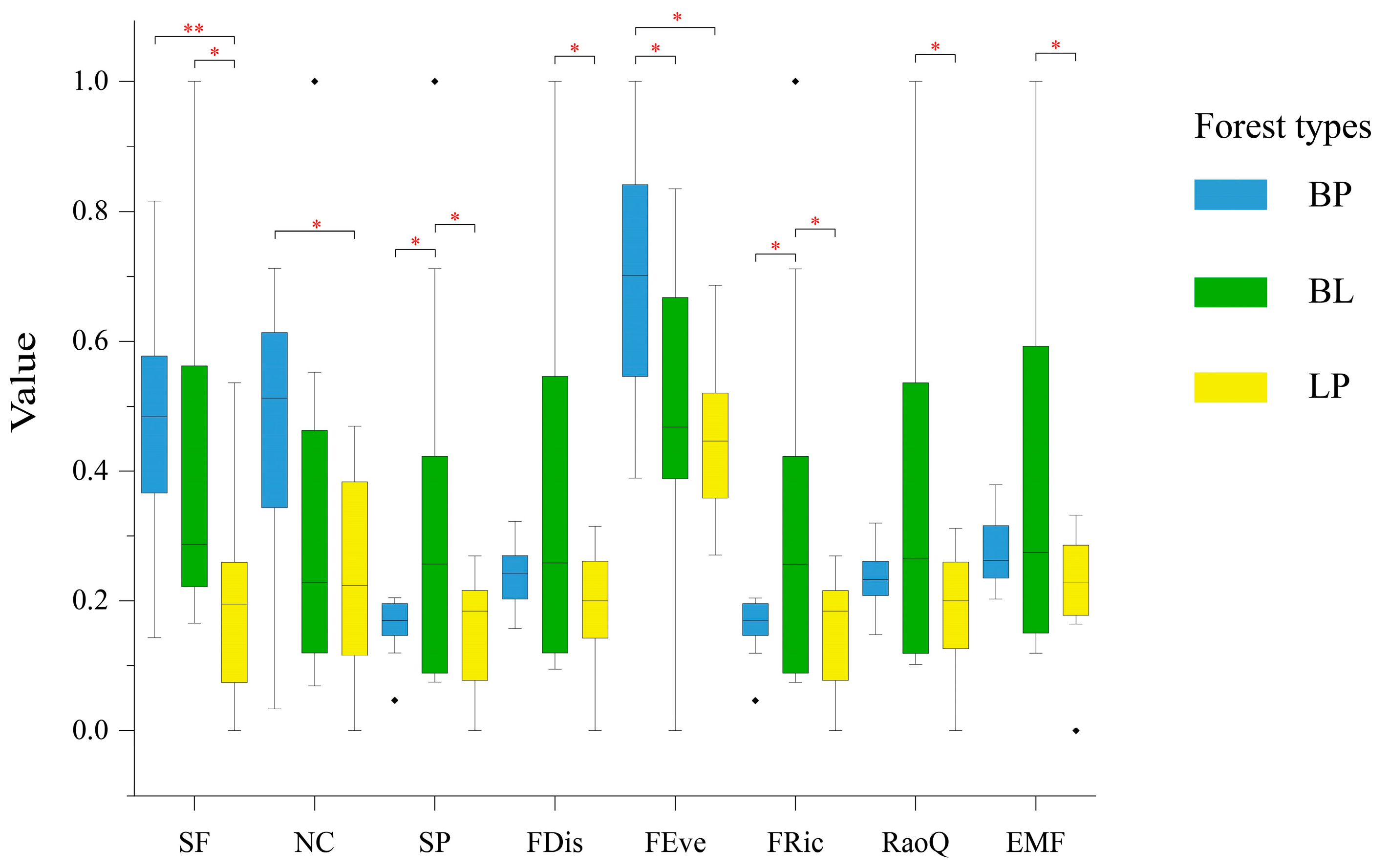
3.1. Comparisons of Single Function and EMF Across Forest Types
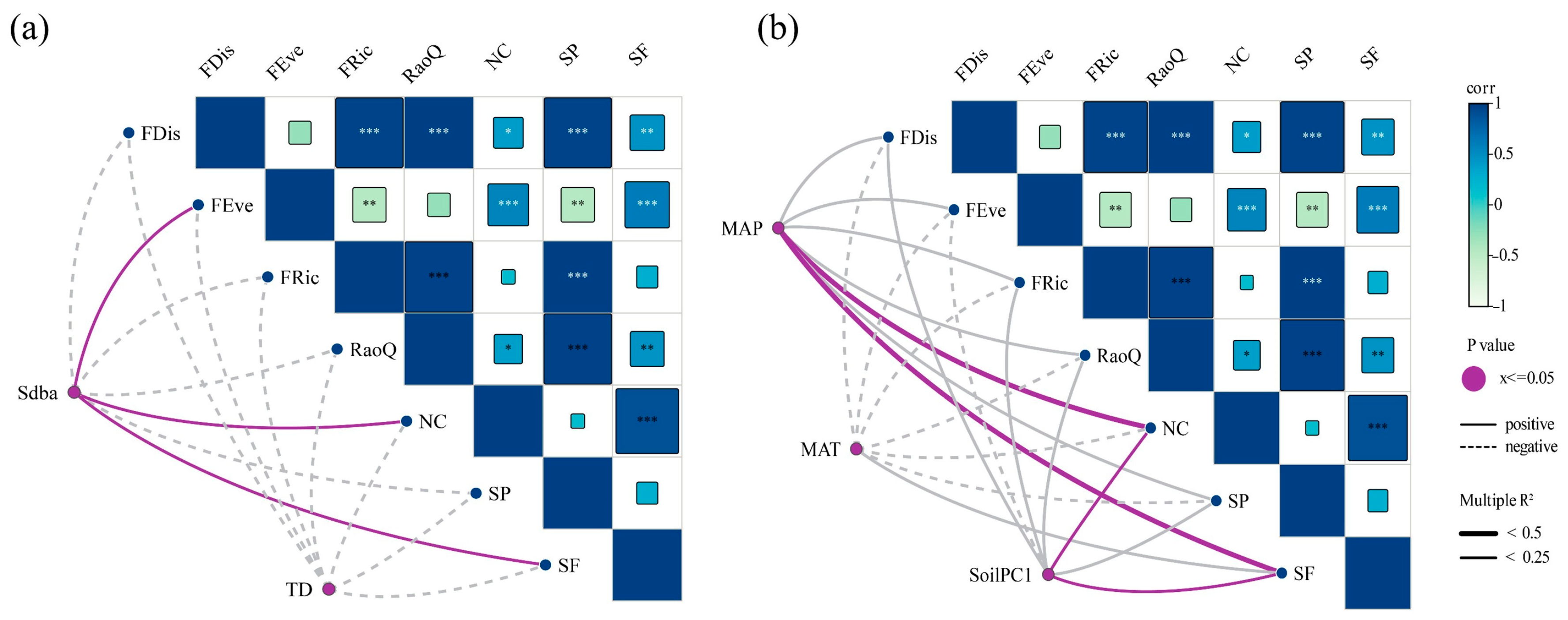
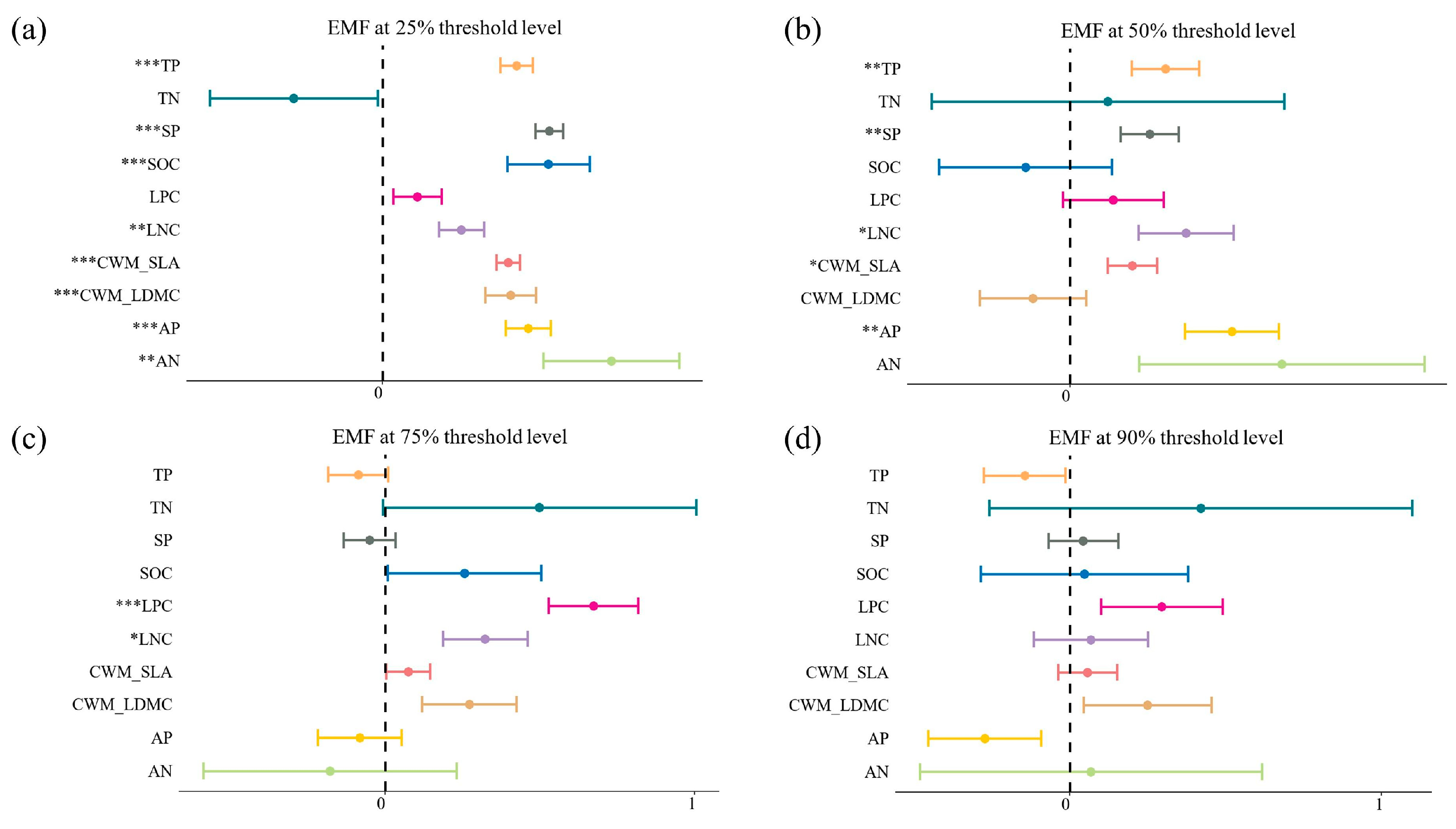
3.2. Effects of Biotic and Abiotic Factors on Single Function
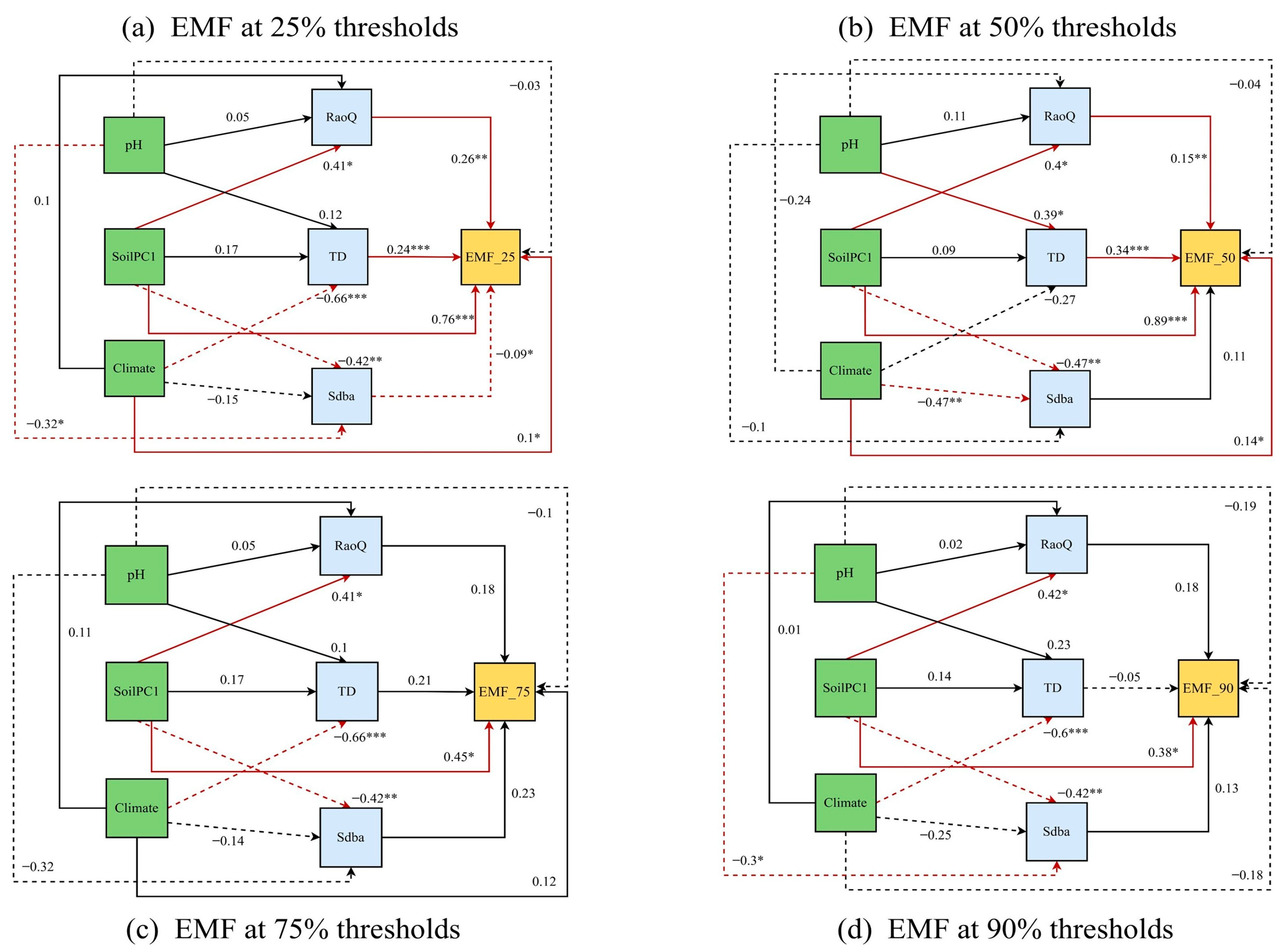
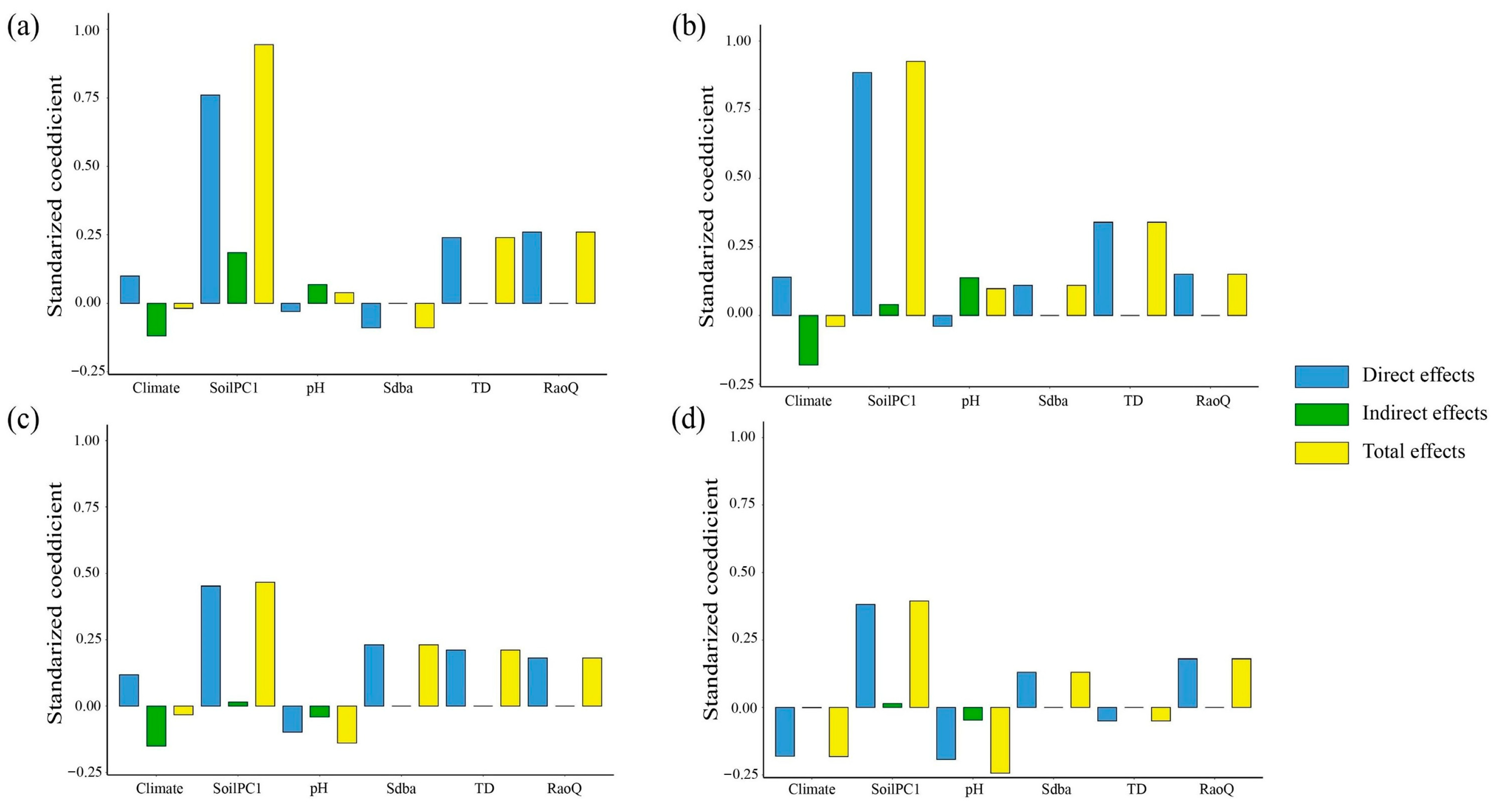
3.3. Effects of Biotic and Abiotic Factors on EMF
4. Discussion
4.1. Comparisons of Single Function and EMF Across Forest Types
4.2. Effects of Biological Factors on EMF
4.3. Effects of Abiotic Factors on EMF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lan, J.; Lei, X.; He, X.; Gao, W.; Guo, H. Multiple mechanisms drive biodiversity-ecosystem service multifunctionality but the dominant one depends on the level of multifunctionality for natural forests in northeast China. For. Ecol. Manag. 2023, 542, 121101. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T.; Brezzi, M.; Buscot, F.; Eichenberg, D.; Gutknecht, J.; Härdtle, W.; He, J.-S.; Klein, A.-M.; Kühn, P.; et al. Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 2018, 9, 2989. [Google Scholar] [CrossRef]
- Du, X.; Lei, X.; He, X.; Lan, J.; Guo, H.; Xu, Q. Ecosystem service multifunctionality of mixed conifer-broad-leaved forests under climate change and forest management based on matrix growth modelling. For. Ecosyst. 2024, 11, 100231. [Google Scholar] [CrossRef]
- Caicoya, A.T.; Vergarechea, M.; Blattert, C.; Klein, J.; Eyvindson, K.; Burgas, D.; Snäll, T.; Mönkkönen, M.; Astrup, R.; Di Fulvio, F.; et al. What drives forest multifunctionality in central and northern Europe? Exploring the interplay of management, climate, and policies. Ecosyst. Serv. 2023, 64, 101575. [Google Scholar] [CrossRef]
- Carrasco, G.; Almeida, A.C.; Falvey, M.; Olmedo, G.F.; Taylor, P.; Santibañez, F.; Coops, N.C. Effects of climate change on forest plantation productivity in Chile. Global Change Biol. 2022, 28, 7391–7409. [Google Scholar] [CrossRef]
- Yuan, Z.; Ali, A.; Ruiz-Benito, P.; Jucker, T.; Mori, A.S.; Wang, S.; Zhang, X.; Li, H.; Hao, Z.; Wang, X.; et al. Above- and below-ground biodiversity jointly regulate temperate forest multifunctionality along a local-scale environmental gradient. J. Ecol. 2020, 108, 2012–2024. [Google Scholar] [CrossRef]
- Albrecht, J.; Classen, A.; Vollstädt, M.G.R.; Mayr, A.; Mollel, N.P.; Schellenberger Costa, D.; Dulle, H.I.; Fischer, M.; Hemp, A.; Howell, K.M.; et al. Plant and animal functional diversity drive mutualistic network assembly across an elevational gradient. Nat. Commun. 2018, 9, 3177. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ni, T.; Miao, Z.; Hao, Y.; Ma, A.; Dong, L.; Li, F. Effects of biotic and abiotic factors on ecosystem multifunctionality of plantations. Ecol. Process. 2024, 13, 44. [Google Scholar] [CrossRef]
- Mensah, S.; Salako, K.V.; Assogbadjo, A.; Glèlè Kakaï, R.; Sinsin, B.; Seifert, T. Functional trait diversity is a stronger predictor of multifunctionality than dominance: Evidence from an Afromontane forest in South Africa. Ecol. Indic. 2020, 115, 106415. [Google Scholar] [CrossRef]
- Del Río, M.; Löf, M.; Bravo-Oviedo, A.; Jactel, H. Understanding the complexity of mixed forest functioning and management: Advances and perspectives. For. Ecol. Manag. 2021, 489, 119138. [Google Scholar] [CrossRef]
- Jactel, H.; Moreira, X.; Castagneyrol, B. Tree diversity and forest resistance to insect pests: Patterns, mechanisms, and prospects. Annu. Rev. Entomol. 2021, 66, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Steckel, M.; Heym, M.; Biber, P.; Ammer, C.; Ehbrecht, M.; Bielak, K.; Bravo, F.; Ordóñez, C.; Collet, C.; et al. Stand growth and structure of mixed-species and monospecific stands of Scots pine (Pinus sylvestris L.) and oak (Q. robur L., Quercus petraea (Matt.) Liebl.) analysed along a productivity gradient through Europe. Eur. J. Forest Res. 2020, 139, 349–367. [Google Scholar] [CrossRef]
- Van Der Plas, F.; Manning, P.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Hector, A.; Ampoorter, E.; Baeten, L.; et al. Jack-of-all-trades effects drive biodiversity-ecosystem multifunctionality relationships in European forests. Nat. Commun. 2016, 7, 11109. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Bongalov, B.; Burslem, D.F.R.P.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef]
- Soliveres, S.; Van Der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef]
- Wang, S.; Isbell, F.; Deng, W.; Hong, P.; Dee, L.E.; Thompson, P.; Loreau, M. How complementarity and selection affect the relationship between ecosystem functioning and stability. Ecology 2021, 102, e03347. [Google Scholar] [CrossRef]
- Kou, X.; Liu, H.; Chen, H.; Xu, Z.; Yu, X.; Cao, X.; Liu, D.; Wen, L.; Zhuo, Y.; Wang, L. Multifunctionality and maintenance mechanism of wetland ecosystems in the littoral zone of the northern semi-arid region lake driven by environmental factors. Sci. Total. Environ. 2023, 870, 161956. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Yang, Q.; Shen, G.; Zhu, X.; Xu, Y.; Wang, X. Topography and structural diversity regulate ecosystem multifunctionality in a subtropical evergreen broad-leaved forest. Front. For. Glob. Change 2023, 6, 1309660. [Google Scholar] [CrossRef]
- Lutz, J.A.; Furniss, T.J.; Johnson, D.J.; Davies, S.J.; Allen, D.; Alonso, A.; Anderson-Teixeira, K.J.; Andrade, A.; Baltzer, J.; Becker, K.M.L.; et al. Global importance of large-diameter trees. Global Ecol. Biogeogr. 2018, 27, 849–864. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Lang, X.; Huang, X.; Su, J. Species richness, not abundance, drives ecosystem multifunctionality in a subtropical coniferous forest. Ecol. Indic. 2021, 120, 106911. [Google Scholar] [CrossRef]
- Yue, Q.; Hao, M.; Geng, Y.; Wang, X.; Von Gadow, K.; Zhang, C.; Zhao, X.; Gao, L. Evaluating alternative hypotheses behind biodiversity and multifunctionality relationships in the forests of Northeastern China. For. Ecosyst. 2022, 9, 100027. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The links between ecosystem multifunctionality and above- and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Su, J. Tree species diversity and stand attributes differently influence the ecosystem functions of Pinus yunnanensis secondary forests under the climate context. Sustainability 2022, 14, 8332. [Google Scholar] [CrossRef]
- Zhao, F.; Hao, M.; Yue, Q.; Lin, S.; Zhao, X.; Zhang, C.; Fan, X.; Von Gadow, K. Community diversity and composition affect ecosystem multifunctionality across environmental gradients in boreal and temperate forests. Ecol. Indic. 2024, 159, 111692. [Google Scholar] [CrossRef]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.; Zhu, J.; Hong, P.; Ji, C.; et al. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef]
- Carmona-Yáñez, M.D.; Lucas-Borja, M.E.; Zema, D.A.; Jing, X.; Kooch, Y.; Garrido Gallego, P.; Plaza-Alvarez, P.A.; Zhou, G.; Delgado-Baquerizo, M. Influence of management and stand composition on ecosystem multifunctionality of Mediterranean tree forests. Trees 2023, 37, 1801–1816. [Google Scholar] [CrossRef]
- Lan, J.; Lei, X.; He, X.; Gao, W.; Guo, H. Stand density, climate and biodiversity jointly regulate the multifunctionality of natural forest ecosystems in northeast China. Eur. J. Forest Res. 2023, 142, 493–507. [Google Scholar] [CrossRef]
- Xu, H.; Yu, M.; Cheng, X. Abundant fungal and rare bacterial taxa jointly reveal soil nutrient cycling and multifunctionality in uneven-aged mixed plantations. Ecol. Indic. 2021, 129, 107932. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, D.; Zhang, D.; Lu, W.; Li, Y.; Liu, Q. Developing the additive systems of stand basal area model for broad-leaved mixed forests. Plants 2024, 13, 1758. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Yang, X.; Fan, N.; Wang, Y.; Zhang, Z. Effects of stand density, age, and drought on the size-growth relationship in Larix principis-rupprechtii forests. Forests 2024, 15, 413. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; Ouimet, R.; Caldwell, L.; Gloor, M.; Brienen, R. Large apparent growth increases in boreal forests inferred from tree-rings are an artefact of sampling biases. Sci. Rep. 2019, 9, 6832. [Google Scholar] [CrossRef]
- Holmes; Richard, L. Computer—assisted quality control in tree -ring dating and measirement. Tree-Ring Bull 1983, 43, 51–67. [Google Scholar]
- Malhi, S.S.; Brandt, S.A.; Kutcher, H.R.; Ulrich, D. Effects of broad-leaf crop frequency and fungicide application in various rotations on nitrate nitrogen and extractable phosphorus in a dark brown soil. Commun. Soil Sci. Plan. 2011, 42, 2795–2812. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wang, D.; Zhang, Z. Soil microbial community, soil quality, and productivity along a chronosequence of Larix principis-rupprechtii forests. Plants 2023, 12, 2913. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.; Pandey, R. Associations of plant functional diversity with carbon accumulation in a temperate forest ecosystem in the Indian Himalayas. Ecol. Indic. 2019, 98, 861–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Xu, J.; Chai, Y.; Liu, P.; Quan, J.; Wu, X.; Li, C.; Yue, M. Effects of water availability on the relationships between hydraulic and economic traits in the Quercus wutaishanica forests. Front. Plant Sci. 2022, 13, 902509. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Cheng, R.; Ge, Z.; Liu, C.; Zhang, Z. Stand structure and functional traits determine productivity of Larix principis-rupprechtii forests. Forests 2024, 15, 924. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Su, J. Selective logging enhances ecosystem multifunctionality via increase of functional diversity in a Pinus yunnanensis forest in Southwest China. For. Ecosyst. 2020, 7, 55. [Google Scholar] [CrossRef]
- Huxley, J.D.; White, C.T.; Humphries, H.C.; Weber, S.E.; Spasojevic, M.J. Plant functional traits are dynamic predictors of ecosystem functioning in variable environments. J. Ecol. 2023, 111, 2597–2613. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Jiang, L.; Zayit, A.; Sattar, K.; Wang, S.; He, X.; Hu, D.; Wang, H.; Yang, J. The Influence of intraspecific trait variation on plant functional diversity and community assembly processes in an arid desert region of northwest China. Forests 2023, 14, 1536. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, M.; Miao, L.; Yang, C.; Wang, Q.; Wang, M. Plant diversity and ecosystem multifunctionality of different degraded grasslands in alpine meadows of Maqu. Ecol. Res. 2024, 39, 54–71. [Google Scholar] [CrossRef]
- Ramazanoglu, E.; Almarie, V.; Suzer, M.H.; Shan, J.; Wei, Z.; Cullu, M.A.; Bol, R.; Senbayram, M. Interactive effects of soil salinity and nitrogen fertilizer types on nitrous oxide and ammonia fluxes. Geoderma. Reg. 2024, 38, e00831. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning drives C: N: P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, G.; Yan, L. Changes in soil carbon fractions and enzyme activities under different vegetation types of the northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tsukamoto, J.; Tokumoto, Y.; Shuvo, M.A.R. The role of quantitative traits of leaf litter on decomposition and nutrient cycling of the forest ecosystems. J. For. Environ. Sci. 2013, 29, 38–48. [Google Scholar] [CrossRef]
- Deng, X.; Xiao, W.; Zeng, L.; Lei, L.; Xu, J.; Xin, X.; Pei, S. Trade-off of NSC allocation in response to the phosphorus limitation caused by increased stand age in Pinus massoniana. For. Ecol. Manag. 2024, 561, 121883. [Google Scholar] [CrossRef]
- Conradi, T.; Van Meerbeek, K.; Ordonez, A.; Svenning, J. Biogeographic historical legacies in the net primary productivity of Northern Hemisphere forests. Ecol. Lett. 2020, 23, 800–810. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Ding, J.; Zhu, D.; Hu, H.-W.; Delgado-Baquerizo, M.; Ma, Y.-B.; He, J.-Z.; Zhu, Y.-G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2020, 141, 107686. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, A.; Ali, A.; Yuan, Z.; Liu, S.; Lin, F.; Fang, S.; Ye, J.; Hao, Z.; Loreau, M.; Bai, E.; et al. Context-dependency of tree species diversity, trait composition and stand structural attributes regulate temperate forest multifunctionality. Sci. Total. Environ. 2021, 757, 143724. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Jin, G. Plant-soil microbial diversity and structural attributes jointly dominate the multifunctionality of the temperate forest. Ecol. Indic. 2024, 166, 112282. [Google Scholar] [CrossRef]
- Manning, P.; Van Der Plas, F.; Soliveres, S.; Allan, E.; Maestre, F.T.; Mace, G.; Whittingham, M.J.; Fischer, M. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Manzanedo, R.D.; D’Orangeville, L.; Lv, P.; Wang, C.; Xu, C.; Hou, M.; Huang, X.; Rademacher, T. High risk of growth cessation of planted larch under extreme drought. Environ. Res. Lett. 2021, 16, 014040. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Innes, J.L.; Seely, B.; Chen, B. ClimateAP: An application for dynamic local downscaling of historical and future climate data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, B.; Zhang, F.; Zhao, T.; Wang, Q.; Han, G.; Zhao, M. Effects of fencing on near-term ecosystem multifunctionality in a typical steppe in Inner Mongolia. Agric. Ecosyst. Environ. 2023, 342, 108238. [Google Scholar] [CrossRef]
- Grenié, M.; Gruson, H. fundiversity: Easy Computation of Functional Diversity Indices. 2024. Available online: https://funecology.github.io/fundiversity/ (accessed on 23 April 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lefcheck, J.S. PiecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Brunner, A.; Forrester, D.I. Tree species mixture effects on stem growth vary with stand density—An analysis based on individual tree responses. For. Ecol. Manag. 2020, 473, 118334. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Luan, J.; Chang, S.X.; Gao, B.; Wang, Y.; Liu, S. Functional diversity dominates positive species mixture effects on ecosystem multifunctionality in subtropical plantations. For. Ecosyst. 2022, 9, 100039. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, X.; Hua, H.; Liu, X.; Chang, Q.; Xu, R.; Li, M.; Mu, L. Soil aggregate stability and organic carbon content among different forest types in temperate ecosystems in northeastern China. Forests 2024, 15, 279. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Y.; Sun, G.; Zhang, Q.; Wang, Y.; Zeng, H.; Simpson, M.J.; Wang, J. Aridity affects soil organic carbon concentration and chemical stability by different forest types and soil processes across Chinese natural forests. Sci. Total. Environ. 2024, 944, 174002. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; McCormack, M.L.; Liu, X.; Hu, H.; Feng, D.F.; Bao, W.K. Vertical fine-root distributions in five subalpine forest types shifts with soil properties across environmental gradients. Plant Soil 2020, 456, 129–143. [Google Scholar] [CrossRef]
- Luo, Y.; Cadotte, M.W.; Liu, J.; Burgess, K.S.; Tan, S.; Ye, L.; Zou, J.; Chen, Z.; Jiang, X.; Li, J.; et al. Multitrophic diversity and biotic associations influence subalpine forest ecosystem multifunctionality. Ecology 2022, 103, e3745. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Li, J.; Hao, M.-H.; Fan, C.-Y.; Zhang, C.-Y.; Zhao, X.-H. Effect of tree species and functional diversity on ecosystem multifunctionality in temperate forests of northeast China. Chin. J. Plant Ecol. 2023, 47, 1507–1522. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Baruffol, M.; He, J.; Ma, K.; Schmid, B. Can niche plasticity promote biodiversity-productivity relationships through increased complementarity? Ecology 2017, 98, 1104–1116. [Google Scholar] [CrossRef]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 0132. [Google Scholar] [CrossRef]
- Ivanova, N. Global overview of the application of the braun-blanquet approach in research. Forests 2024, 15, 937. [Google Scholar] [CrossRef]
- Yin, S.; Du, H.; Mao, F.; Li, X.; Zhou, G.; Xu, C.; Sun, J. Spatiotemporal patterns of net primary productivity of subtropical forests in China and its response to drought. Sci. Total. Environ. 2024, 913, 169439. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.M.; Ali, A.; Martins, S.V.; Neto, S.N.D.; Rodrigues, A.C.; Teshome, M.; Carvalho, F.A.; Heringer, G.; Gastauer, M. Stand structural attributes and functional trait composition overrule the effects of functional divergence on aboveground biomass during Amazon forest succession. Forest Ecol. Manag. 2020, 477, 118481. [Google Scholar] [CrossRef]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Above-ground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2018, 106, 561–570. [Google Scholar] [CrossRef]


| Stand Type | SOC (g/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | pH |
|---|---|---|---|---|---|---|---|---|
| BP | 83.64 ± 7.69 | 3.84 ± 0.36 | 0.63 ± 0.06 | 17.76 ± 0.68 | 401.61 ± 34.18 | 11.06 ± 1.00 | 154.89 ± 11.81 | 6.38 ± 0.06 |
| BL | 72.28 ± 7.68 | 3.34 ± 0.35 | 0.63 ± 0.08 | 18.79 ± 0.69 | 326.84 ± 34.59 | 7.78 ± 0.63 | 163.66 ± 13.00 | 6.17 ± 0.06 |
| LP | 49.82 ± 5.14 | 2.88 ± 0.19 | 0.48 ± 0.05 | 22.16 ± 0.75 | 308.32 ± 22.08 | 7.57 ± 0.48 | 135.48 ± 7.44 | 6.26 ± 0.04 |
| Stand Type | Age (Years) | Altitude (m) | Mean DBH (cm) | Mean Height (m) | Density (Trees/ha) |
|---|---|---|---|---|---|
| BP | 34 | 1486 ± 30 | 13.52 ± 1.14 | 11.88 ± 0.73 | 906 ± 103 |
| BL | 39 | 1379 ± 36 | 17.23 ± 1.26 | 14.01 ± 0.79 | 1094 ± 233 |
| LP | 33 | 1528 ± 63 | 17.76 ± 1.46 | 15.61 ± 1.09 | 1400 ± 223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, R.; Liu, C.; Liu, Q.; Li, M.; Zhang, Z. Biodiversity and Soil Jointly Drive Ecosystem Multifunctionality in Larch Forests. Forests 2025, 16, 745. https://doi.org/10.3390/f16050745
Zhang Y, Wang R, Liu C, Liu Q, Li M, Zhang Z. Biodiversity and Soil Jointly Drive Ecosystem Multifunctionality in Larch Forests. Forests. 2025; 16(5):745. https://doi.org/10.3390/f16050745
Chicago/Turabian StyleZhang, Yang, Ruihan Wang, Chang Liu, Qiang Liu, Minghao Li, and Zhidong Zhang. 2025. "Biodiversity and Soil Jointly Drive Ecosystem Multifunctionality in Larch Forests" Forests 16, no. 5: 745. https://doi.org/10.3390/f16050745
APA StyleZhang, Y., Wang, R., Liu, C., Liu, Q., Li, M., & Zhang, Z. (2025). Biodiversity and Soil Jointly Drive Ecosystem Multifunctionality in Larch Forests. Forests, 16(5), 745. https://doi.org/10.3390/f16050745






