Elevational Distribution of Ants Across Seasons in a Subtropical Rainforest of Eastern Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology Overview
2.2. Ant Sampling
2.3. Litter Extraction
2.4. Bark Spray
2.5. Sorting and Identification
2.6. Data Analysis
3. Results
3.1. Overview of Ant Diversity and Habitat Distribution
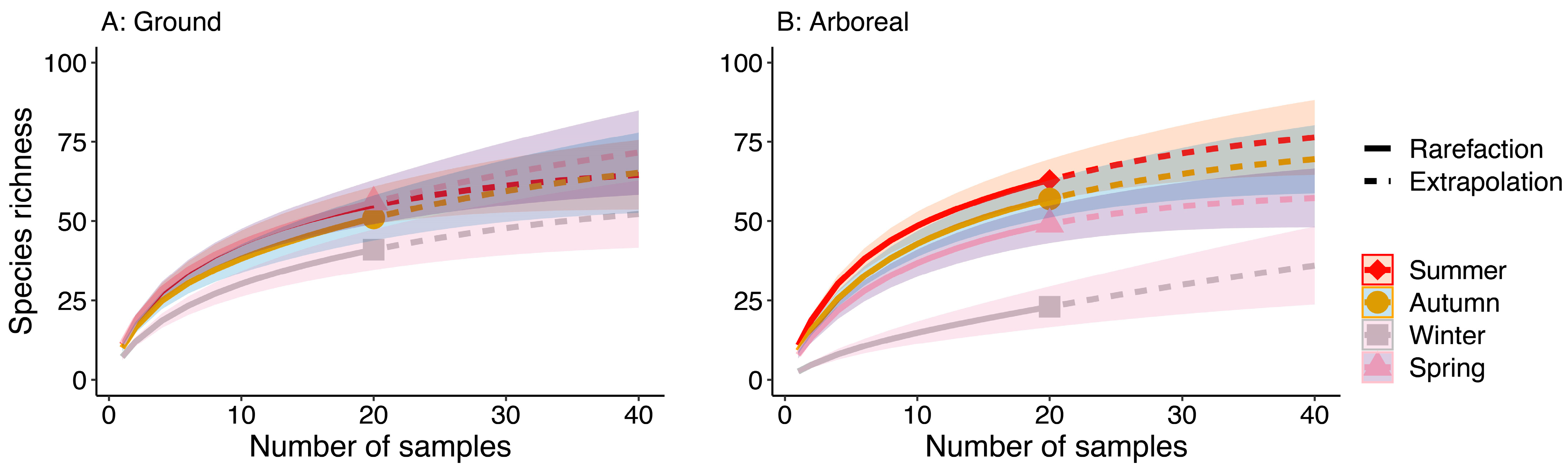
3.2. Gamma Diversity and Sampling Sufficiency
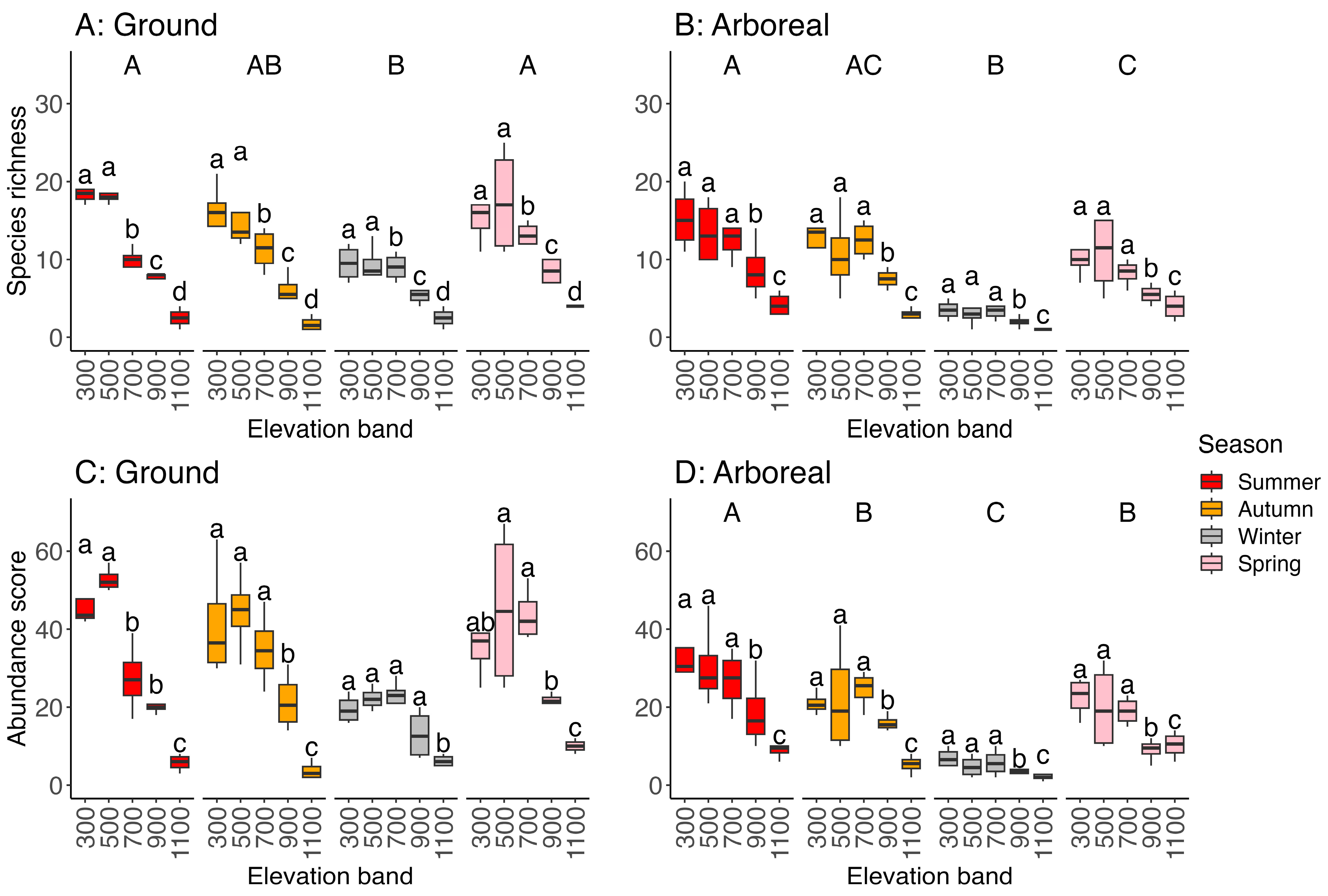
3.3. Species Richness (Alpha Diversity) and Abundance Scores
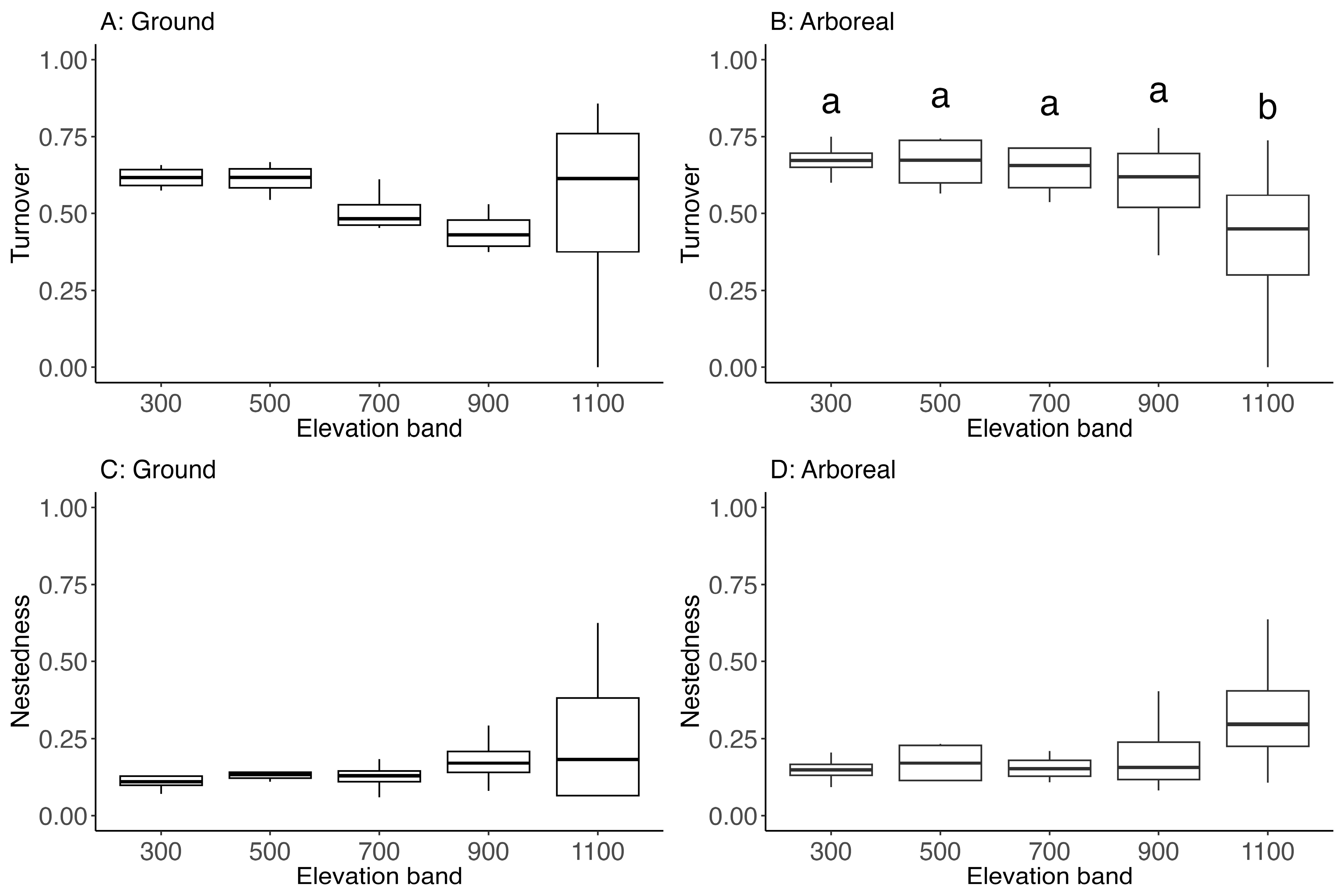
3.4. Beta Turnover and Nestedness
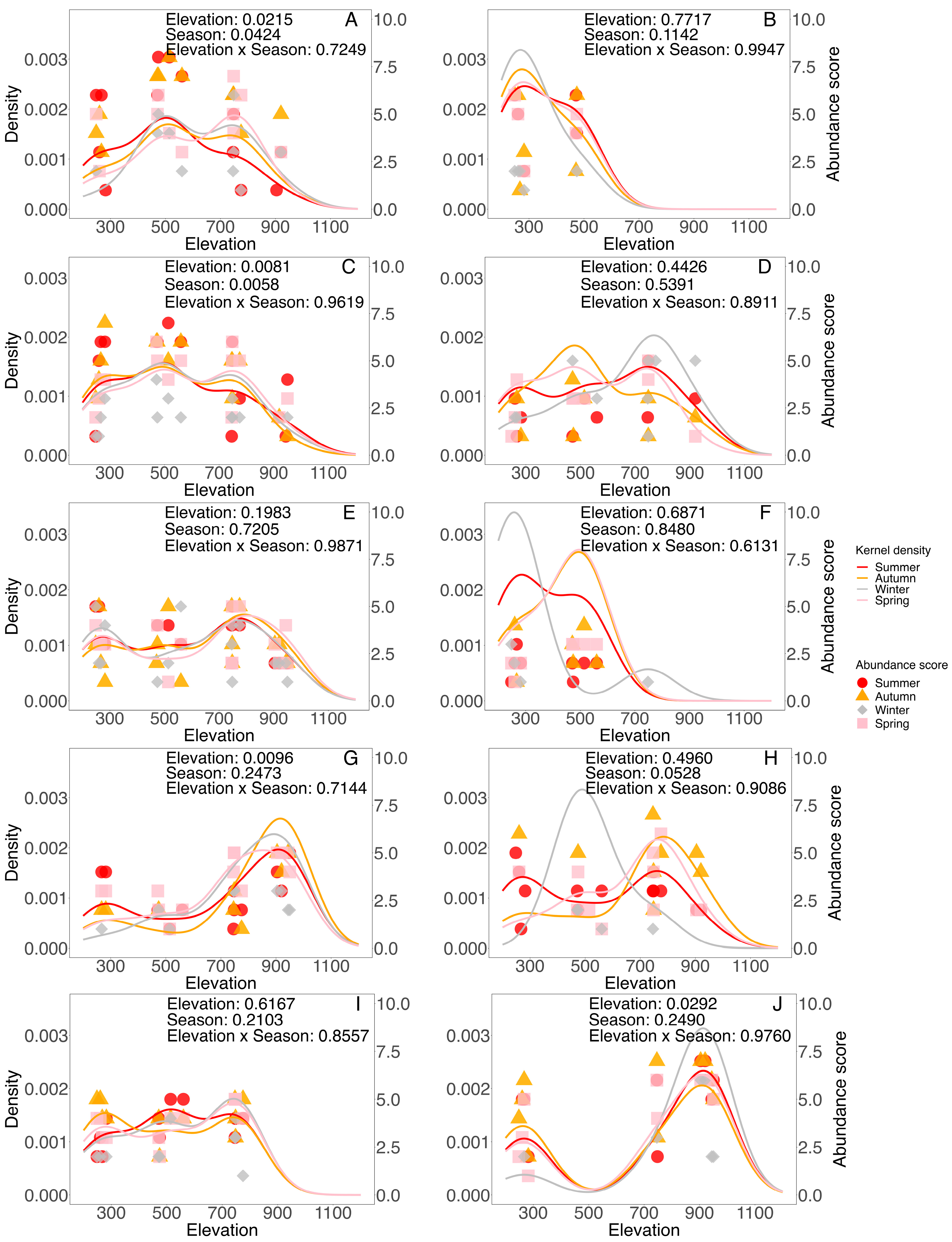
3.5. Individual Ant Species
3.6. Species Composition
3.7. Indicator Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species Number | Species Name | Arboreal | Ground |
|---|---|---|---|
| 1 | Amblyopone australis Erichson, 1842 | ✗ | ✓ |
| 2 | Anonychomyrma QM3 | ✓ | ✓ |
| 3 | Anonychomyrma QM9 | ✓ | ✓ |
| 4 | Arnoldius QM2 | ✓ | ✗ |
| 5 | Camponotus BATH1 | ✓ | ✗ |
| 6 | Camponotus BATH2 | ✓ | ✗ |
| 7 | Camponotus froggatti Forel, 1902 | ✓ | ✗ |
| 8 | Carebara BATH1 | ✓ | ✓ |
| 9 | Carebara BATH2 | ✗ | ✓ |
| 10 | Chelaner BATH3 | ✓ | ✓ |
| 11 | Chelaner BATH4 | ✓ | ✗ |
| 12 | Chelaner BATH6 | ✓ | ✗ |
| 13 | Chelaner kiliani (Forel, 1902) | ✓ | ✓ |
| 14 | Chelaner nigriceps (Heterick, 2001) | ✓ | ✗ |
| 15 | Chelaner tambourinensis (Forel, 1915) | ✓ | ✓ |
| 16 | Colobopsis BATH3 | ✓ | ✗ |
| 17 | Colobopsis BATH4 | ✓ | ✗ |
| 18 | Colobostruma biconvexa Shattuck, 2000 | ✗ | ✓ |
| 19 | Colobostruma froggatti (Forel, 1913) | ✗ | ✓ |
| 20 | Crematogaster BATH1 | ✓ | ✓ |
| 21 | Crematogaster BATH3 | ✓ | ✗ |
| 22 | Cryptopone BATH1 | ✓ | ✓ |
| 23 | Cryptopone BATH2 | ✓ | ✓ |
| 24 | Discothyrea BATH1 | ✗ | ✓ |
| 25 | Discothyrea BATH2 | ✓ | ✗ |
| 26 | Discothyrea BATH3 | ✗ | ✓ |
| 27 | Discothyrea BATH4 | ✓ | ✗ |
| 28 | Eurhopalothrix australis Brown & Kempf, 1960 | ✗ | ✓ |
| 29 | Heteroponera BATH1 | ✓ | ✓ |
| 30 | Heteroponera BATH2 | ✗ | ✓ |
| 31 | Hypoponera BATH1 | ✗ | ✓ |
| 32 | Hypoponera BATH2 | ✓ | ✓ |
| 33 | Hypoponera BATH3 | ✗ | ✓ |
| 34 | Hypoponera BATH5 | ✓ | ✗ |
| 35 | Hypoponera BATH6 | ✗ | ✓ |
| 36 | Hypoponera BATH7 | ✗ | ✓ |
| 37 | Leptogenys BATH1 | ✗ | ✓ |
| 38 | Leptogenys mjobergi Forel, 1915 | ✗ | ✓ |
| 39 | Leptogenys sjostedti Forel, 1915 | ✗ | ✓ |
| 40 | Leptomyrmex burwelli Smith, D.J. & Shattuck, 2009 | ✓ | ✗ |
| 41 | Leptomyrmex cnemidatus Wheeler, W.M., 1915 | ✓ | ✓ |
| 42 | Leptomyrmex tibialis Emery, 1895 | ✓ | ✗ |
| 43 | Lioponera BATH1 | ✗ | ✓ |
| 44 | Lioponera BATH2 | ✓ | ✗ |
| 45 | Lioponera BATH3 | ✓ | ✓ |
| 46 | Lordomyrma BATH1 | ✗ | ✓ |
| 47 | Mayriella abstinens Forel, 1902 | ✓ | ✓ |
| 48 | Mayriella overbecki Viehmeyer, 1925 | ✓ | ✗ |
| 49 | Mayriella spinosior Wheeler, W.M., 1935 | ✗ | ✓ |
| 50 | Mesoponera australis (Forel, 1900) | ✗ | ✓ |
| 51 | Monomorium BATH2 | ✓ | ✓ |
| 52 | Monomorium BATH5 | ✓ | ✓ |
| 53 | Monomorium BATH7 | ✗ | ✓ |
| 54 | Myrmecia brevinoda Forel, 1910 | ✓ | ✓ |
| 55 | Myrmecia nigrocincta Smith, F., 1858 | ✓ | ✗ |
| 56 | Myrmecina australis Wheeler, G.C. & Wheeler, J., 1973 | ✗ | ✓ |
| 57 | Myrmecorhynchus carteri Clark, 1934 | ✓ | ✓ |
| 58 | Myrmecorhynchus emeryi André, 1896 | ✓ | ✗ |
| 59 | Notoncus capitatus Forel, 1915 | ✓ | ✓ |
| 60 | Notoncus spinisquamis (André, 1896) | ✓ | ✗ |
| 61 | Notostigma foreli Emery, 1920 | ✓ | ✗ |
| 62 | Nylanderia QM1 | ✓ | ✓ |
| 63 | Ochetellus BATH2 | ✗ | ✓ |
| 64 | Orectognathus antennatus Smith, F., 1853 | ✓ | ✗ |
| 65 | Orectognathus elegantulus Taylor, 1977 | ✓ | ✗ |
| 66 | Orectognathus phyllobates Brown, 1958 | ✓ | ✓ |
| 67 | Orectognathus robustus Taylor, 1977 | ✗ | ✓ |
| 68 | Orectognathus rostratus Lowery, 1967 | ✓ | ✓ |
| 69 | Orectognathus versicolor Donisthorpe, 1940 | ✓ | ✗ |
| 70 | Paraparatrechina QM4 | ✓ | ✓ |
| 71 | Paraparatrechina QM8 | ✓ | ✗ |
| 72 | Pheidole BATH1 | ✓ | ✓ |
| 73 | Pheidole BATH2 | ✓ | ✓ |
| 74 | Pheidole BATH3 | ✓ | ✓ |
| 75 | Pheidole BATH4 | ✓ | ✓ |
| 76 | Pheidole BATH5 | ✓ | ✓ |
| 77 | Pheidole BATH6 | ✓ | ✓ |
| 78 | Pheidole BATH7 | ✗ | ✓ |
| 79 | Pheidole BATH10 | ✓ | ✓ |
| 80 | Pheidole BATH11 | ✗ | ✓ |
| 81 | Pheidole dispar (Forel, 1895) | ✗ | ✓ |
| 82 | Plagiolepis QM2 | ✓ | ✓ |
| 83 | Platythyrea parallela (Smith, F., 1859) | ✓ | ✗ |
| 84 | Podomyrma BATH1 | ✓ | ✗ |
| 85 | Podomyrma BATH2 | ✓ | ✗ |
| 86 | Podomyrma BATH3 | ✓ | ✗ |
| 87 | Podomyrma BATH6 | ✓ | ✗ |
| 88 | Podomyrma BATH7 | ✓ | ✗ |
| 89 | Podomyrma BATH8 | ✓ | ✗ |
| 90 | Polyrhachis BATH1 | ✓ | ✗ |
| 91 | Polyrhachis clio Forel, 1902 | ✓ | ✗ |
| 92 | Polyrhachis maculata Forel, 1915 | ✓ | ✗ |
| 93 | Polyrhachis ornata Mayr, 1876 | ✓ | ✗ |
| 94 | Polyrhachis pilosa Donisthorpe, 1938 | ✓ | ✗ |
| 95 | Ponera leae Forel, 1913 | ✗ | ✓ |
| 96 | Prionopelta robynmae Shattuck, 2008 | ✓ | ✓ |
| 97 | Pristomyrmex wheeleri Taylor, 1965 | ✗ | ✓ |
| 98 | Probolomyrmex greavesi Taylor, 1965 | ✗ | ✓ |
| 99 | Prolasius QM1 | ✓ | ✓ |
| 100 | Prolasius QM2 | ✓ | ✓ |
| 101 | Prolasius QM4 | ✓ | ✓ |
| 102 | Prolasius QM6 | ✓ | ✓ |
| 103 | Prolasius QM7 | ✓ | ✓ |
| 104 | Prolasius QM8 | ✓ | ✗ |
| 105 | Prolasius QM9 | ✗ | ✓ |
| 106 | Pseudoneoponera BATH1 | ✗ | ✓ |
| 107 | Rhopalomastix BATH1 | ✓ | ✗ |
| 108 | Rhopalothrix orbis Taylor, 1968 | ✗ | ✓ |
| 109 | Rhytidoponera chalybaea Emery, 1901 | ✓ | ✓ |
| 110 | Rhytidoponera croesus Emery, 1901 | ✓ | ✗ |
| 111 | Rhytidoponera victoriae (André, 1896) | ✓ | ✓ |
| 112 | Solenopsis BATH1 | ✓ | ✓ |
| 113 | Stigmacros major McAreavey, 1957 | ✓ | ✗ |
| 114 | Stigmacros QM22 | ✗ | ✓ |
| 115 | Stigmatomma QM1 | ✗ | ✓ |
| 116 | Strumigenys deuteras Bolton, 2000 | ✓ | ✓ |
| 117 | Strumigenys harpyia Bolton, 2000 | ✓ | ✓ |
| 118 | Strumigenys perplexa (Smith, F., 1876) | ✓ | ✓ |
| 119 | Tapinoma QM2 | ✓ | ✗ |
| 120 | Tapinoma QM3 | ✗ | ✓ |
| 121 | Tapinoma QM4 | ✓ | ✗ |
| 122 | Tapinoma QM6 | ✓ | ✓ |
| 123 | Technomyrmex jocosus Forel, 1910 | ✓ | ✗ |
| 124 | Zasphinctus BATH1 | ✗ | ✓ |
References
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Magurran, A.E. Species abundance distributions over time. Ecol. Lett. 2007, 10, 347–354. [Google Scholar] [CrossRef]
- Willig, M.R.; Presley, S.J. Latitudinal gradients of biodiversity. In Reference Module in Life Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–17. [Google Scholar]
- Mannion, P.D.; Upchurch, P.; Benson, R.B.J.; Goswami, A. The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 2014, 29, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Lundholm, J.T. Plant species diversity and environmental heterogeneity: Spatial scale and competing hypotheses. J. Veg. Sci. 2009, 20, 377–391. [Google Scholar] [CrossRef]
- Körner, C. The use of “altitude” in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Sanders, N.J.; Rahbek, C. The patterns and causes of elevational diversity gradients. Ecography 2012, 35, 1–3. [Google Scholar] [CrossRef]
- Barry, R.G. Mountain Weather and Climate; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Zhang, L.; Hay, W.W.; Wang, C.; Gu, X. The evolution of latitudinal temperature gradients from the latest cretaceous through the present. Earth-Sci. Rev. 2019, 189, 147–158. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Kessler, M.; Kluge, J.; Hemp, A.; Ohlemüller, R. A global comparative analysis of elevational species richness patterns of ferns. Glob. Ecol. Biogeogr. 2011, 20, 868–880. [Google Scholar] [CrossRef]
- Sanders, N.J. Elevational gradients in ant species richness: Area, geometry, and Rapoport’s rule. Ecography 2002, 25, 25–32. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J. Elevational gradients in species richness. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Dehling, D.M.; Töpfer, T.; Schaefer, H.M.; Jordano, P.; Böhning-Gaese, K.; Schleuning, M. Functional relationships beyond species richness patterns: Trait matching in plant-bird mutualisms across scales. Glob. Ecol. Biogeogr. 2014, 23, 1085–1093. [Google Scholar] [CrossRef]
- Stark, J.; Lehman, R.; Crawford, L.; Enquist, B.J.; Blonder, B. Does environmental heterogeneity drive functional trait variation? A test in montane and alpine meadows. Oikos 2017, 126, 1650–1659. [Google Scholar] [CrossRef]
- Rahbek, C. The Role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Brehm, G.; Colwell, R.K.; Kluge, J. The role of environment and mid-domain effect on moth species richness along a tropical elevational gradient. Glob. Ecol. Biogeogr. 2007, 16, 205–219. [Google Scholar] [CrossRef]
- Sanders, N.J.; Moss, J.; Wagner, D. Patterns of ant species richness along elevational gradients in an arid ecosystem. Glob. Ecol. Biogeogr. 2003, 12, 93–102. [Google Scholar] [CrossRef]
- Szewczyk, T.; McCain, C.M. A systematic review of global drivers of ant elevational diversity. PLoS ONE 2016, 11, e0155404. [Google Scholar] [CrossRef]
- Bishop, T.R.; Robertson, M.P.; van Rensburg, B.J.; Parr, C.L. Elevation–diversity patterns through space and time: Ant communities of the Maloti-Drakensberg Mountains of southern Africa. J. Biogeogr. 2014, 41, 2256–2268. [Google Scholar] [CrossRef]
- Marathe, A.; Priyadarsanan, D.R.; Krishnaswamy, J.; Shanker, K. Spatial and climatic variables independently drive elevational gradients in ant species richness in the eastern Himalaya. PLoS ONE 2020, 15, e0227628. [Google Scholar] [CrossRef]
- Sanders, N.J.; Lessard, J.; Fitzpatrick, M.C.; Dunn, R.R. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 2007, 16, 640–649. [Google Scholar] [CrossRef]
- Schebeck, M.; Lehmann, P.; Laparie, M.; Bentz, B.J.; Ragland, G.J.; Battisti, A.; Hahn, D.A. Seasonality of forest insects: Why diapause matters. Trends Ecol. Evol. 2024, 39, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Maicher, V.; Sáfián, S.; Murkwe, M.; Delabye, S.; Przybyłowicz, Ł.; Potocký, P.; Kobe, I.N.; Janeček, Š.; Mertens, J.E.J.; Fokam, E.B.; et al. Seasonal shifts of biodiversity patterns and species’ elevation ranges of butterflies and moths along a complete rainforest elevational gradient on Mount Cameroon. J. Biogeogr. 2020, 47, 342–354. [Google Scholar] [CrossRef]
- Kishimoto-Yamada, K.; Itioka, T. How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? Entomol. Sci. 2015, 18, 407–419. [Google Scholar] [CrossRef]
- Araújo, C.d.O.; Hortal, J.; de Macedo, M.V.; Monteiro, R.F. Elevational and seasonal distribution of scarabaeinae dung beetles (Scarabaeidae: Coleoptera) at Itatiaia National Park (Brazil). Int. J. Trop. Insect Sci. 2022, 42, 1579–1592. [Google Scholar] [CrossRef]
- Kaspari, M.; Donnell, S.; Kercher, J.R. Energy, density, and constraints to species richness: Ant assemblages along a productivity gradient. Am. Nat. 2000, 155, 280–293. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Phillips, B.L.; Laurance, W.F.; Sodhi, N.S.; Diesmos, A.; Williams, S.E. Increasing arboreality with altitude: A novel biogeographic dimension. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131581. [Google Scholar] [CrossRef]
- Leahy, L.; Scheffers, B.R.; Andersen, A.N.; Hirsch, B.T.; Williams, S.E. Vertical niche and elevation range size in tropical ants: Implications for climate resilience. Divers. Distrib. 2021, 27, 485–496. [Google Scholar] [CrossRef]
- Leahy, L.; Scheffers, B.R.; Andersen, A.N.; Williams, S.E. Rates of species turnover across elevation vary with vertical stratum in rainforest ant assemblages. Ecography 2024, 2024, e06972. [Google Scholar] [CrossRef]
- Nakamura, A.; Kitching, R.L.; Cao, M.; Creedy, T.J.; Fayle, T.M.; Freiberg, M.; Hewitt, C.N.; Itioka, T.; Koh, L.P.; Ma, K.; et al. Forests and their canopies: Achievements and horizons in canopy science. Trends Ecol. Evol. 2017, 32, 438–451. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Scheffers, B.R. Vertical stratification influences global patterns of biodiversity. Ecography 2019, 42, 249. [Google Scholar] [CrossRef]
- Arruda, F.V.; Camarota, F.; Ramalho, W.P.; Izzo, T.J.; Santos Almeida, R.P. Seasonal variation of ground and arboreal ants in forest fragments in the highly-threatened Cerrado-Amazon transition. J. Insect Conserv. 2021, 25, 897–904. [Google Scholar] [CrossRef]
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Novotny, V.; Ødegaard, F.; Roslin, T.; Tishechkin, A.K.; Schmidl, J.; Winchester, N.N.; et al. Arthropod distribution in a tropical rainforest: Tackling a four dimensional puzzle. PLoS ONE 2015, 10, e0144110. [Google Scholar] [CrossRef]
- Burwell, C.J.; Nakamura, A. Distribution of ant species along an altitudinal transect in continuous rainforest in subtropical Queensland, Australia. Mem. Queensl. Mus. 2011, 55, 403–411. [Google Scholar]
- Kitching, R.L.; Putland, D.; Ashton, L.A.; Laidlaw, M.J.; Boulter, S.L.; Christensen, H.; Lambkin, C.L. Detecting biodiversity changes along climatic gradients: The IBISCA-Queensland project. Mem. Queensl. Mus. 2011, 55, 235–250. [Google Scholar]
- Laidlaw, M.J.; McDonald, W.J.F.; Hunter, R.J.; Kitching, R.L. Subtropical rainforest turnover along an altitudinal gradient. Mem. Queensl. Mus. 2011, 55, 271–290. [Google Scholar]
- Strong, C.L.; Boulter, S.L.; Laidlaw, M.J.; Maunsell, S.C.; Putland, D.; Kitching, R.L. The physical environment of an altitudinal gradient in the rainforest of Lamington National Park, Southeast Queensland. Mem. Queensl. Mus. 2011, 55, 251–270. [Google Scholar]
- Northcote, K.H. A Factual Key for the Recognition of Australian Soils; Rellium Technical Publications: Glenside, Australia, 1979. [Google Scholar]
- FAO. World Reference Base for Soil Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Bolton, B. Synopsis and classification of Formicidae. Mem. Am. Entomol. Inst. 2003, 71, 1–370. [Google Scholar]
- Shattuck, S.; Barnett, N. Australian Ants Online: The Guide to Australian Ant Fauna. 2001. Available online: http://www.ento.csiro.au/science/ants/ (accessed on 29 October 2007).
- Hoffmann, B.D.; Kay, A. Pisonia grandis monocultures limit the spread of an invasive ant—A case of carbohydrate quality? Biol. Invasions 2009, 11, 1403–1410. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 25 March 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. Using Car and Effects Functions in Other Functions. 2020. Available online: http://download.nust.na/pub3/cran/web/packages/car/vignettes/embedding.pdf (accessed on 25 March 2025).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.2. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 25 March 2025).
- Baselga, A.; Orme, D.; Villéger, S.; De Bortoli, J.; Leprieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5.6. 2022. Available online: https://cran.r-project.org/web/packages/betapart/index.html (accessed on 25 March 2025).
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 March 2025).
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Van Rensburg, B.J.; Botes, A. The verification and application of bioindicators: A case study of dung beetles in a savanna ecosystem. J. Appl. Ecol. 2002, 39, 661–672. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Burwell, C.J.; Nakamura, A. Can changes in ant diversity along elevational gradients in tropical and subtropical Australian rainforests be used to detect a signal of past lowland biotic attrition? Austral Ecol. 2016, 41, 209–218. [Google Scholar] [CrossRef]
- Liu, C.; Dudley, K.L.; Xu, Z.; Economo, E.P. Mountain metacommunities: Climate and spatial connectivity shape ant diversity in a complex landscape. Ecography 2018, 41, 101–112. [Google Scholar] [CrossRef]
- Fontanilla, A.M.; Nakamura, A.; Xu, Z.; Cao, M.; Kitching, R.L.; Tang, Y.; Burwell, C.J. Taxonomic and functional ant diversity along tropical, subtropical, and subalpine elevational transects in Southwest China. Insects 2019, 10, 128. [Google Scholar] [CrossRef]
- Bertuzzo, E.; Carrara, F.; Mari, L.; Altermatt, F.; Rodriguez-Iturbe, I.; Rinaldo, A. Geomorphic controls on elevational gradients of species richness. Proc. Natl. Acad. Sci. USA 2016, 113, 1737–1742. [Google Scholar] [CrossRef]
- Longino, J.T.; Branstetter, M.G.; Ward, P.S. Ant diversity patterns across tropical elevation gradients: Effects of sampling method and subcommunity. Ecosphere 2019, 10, e02798. [Google Scholar] [CrossRef]
- McGlynn, T.P. The ecology of nest movement in social insects. Annu. Rev. Entomol. 2012, 57, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Reeve, C.; Robichaud, J.A.; Fernandes, T.; Bates, A.E.; Bramburger, A.J.; Brownscombe, J.W.; Davy, C.M.; Henry, H.A.L.; McMeans, B.C.; Moise, E.R.D.; et al. Applied winter biology: Threats, conservation and management of biological resources during winter in cold climate regions. Conserv. Physiol. 2023, 11, coad027. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Morimoto, Y.; Widodo, E.S.; Mohamed, M.; Fellowes, J.R. Vertical habitat use and foraging activities of arboreal and ground ants (Hymenoptera: Formicidae) in a Bornean tropical rainforest. Sociobiology 2010, 56, 435–448. [Google Scholar]
- Floren, A.; Wetzel, W.; Staab, M. The contribution of canopy species to overall ant diversity (Hymenoptera: Formicidae) in temperate and tropical ecosystems. Myrmecol. News 2014, 19, 65–74. [Google Scholar]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Change Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef]
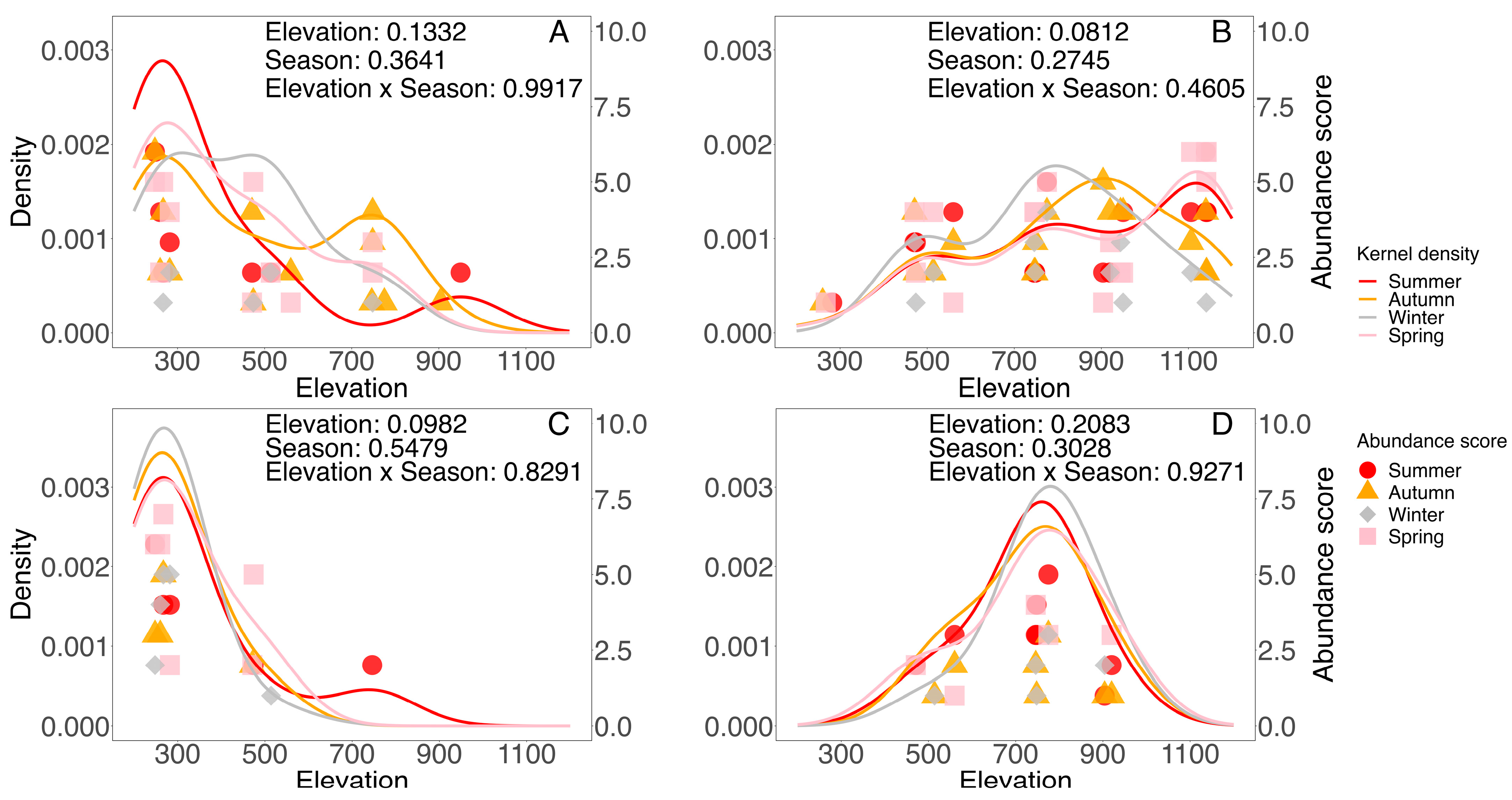
| Habitat | Model | Chi-Square | df | p |
|---|---|---|---|---|
| Ground | Species richness (Poisson) | |||
| Elevation band | 138.97 | 4 | <0.0001 | |
| Season | 23.58 | 3 | <0.0001 | |
| Elevation band × Season | 10.19 | 12 | 0.5993 | |
| Abundance score (Poisson) | ||||
| Elevation band | 175.83 | 4 | <0.0001 | |
| Season | 101.10 | 3 | <0.0001 | |
| Elevation band × Season | 45.42 | 12 | <0.0001 | |
| Arboreal | Species abundance (Poisson) | |||
| Elevation band | 70.37 | 4 | <0.0001 | |
| Season | 87.23 | 3 | <0.0001 | |
| Elevation band × Season | 4.50 | 12 | 0.9727 | |
| Abundance score (negative Binomial) | ||||
| Elevation band | 69.25 | 4 | <0.0001 | |
| Season | 165.98 | 3 | <0.0001 | |
| Elevation band × Season | 16.15 | 12 | 0.1844 |
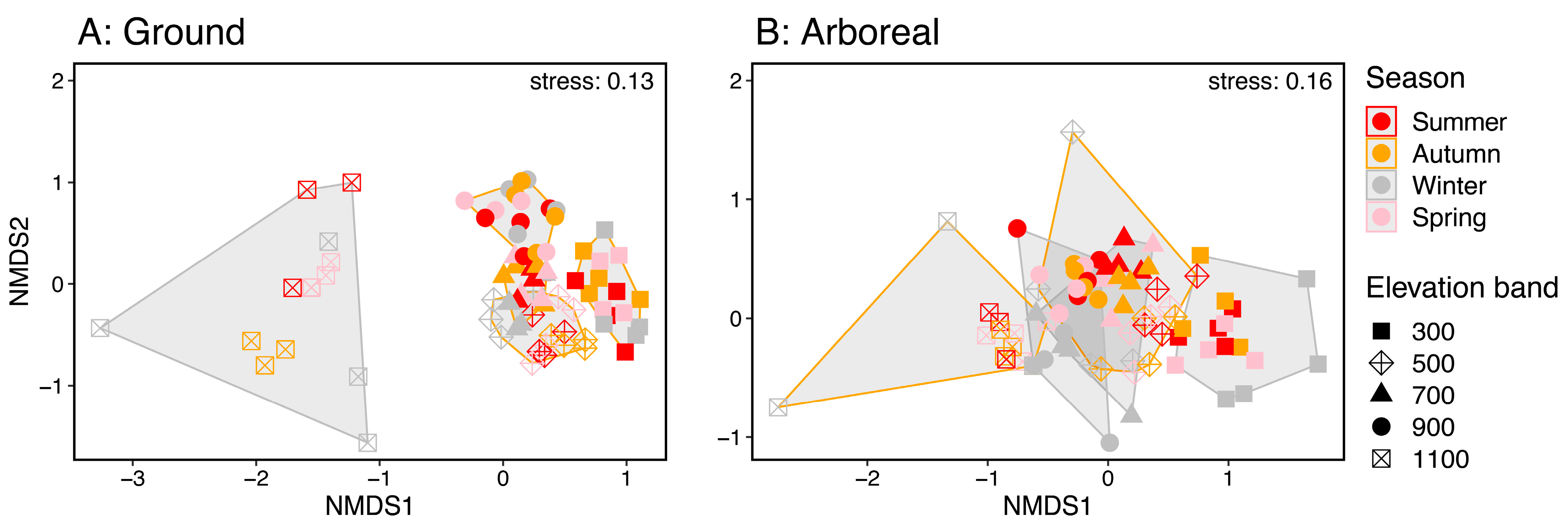
| Habitat | Factors | Pseudo-F | df | p |
|---|---|---|---|---|
| Ground | Elevation band | 22.46 | 4 | 0.0001 |
| Season | 2.60 | 3 | 0.0011 | |
| Elevation band–Season | 1.57 | 12 | 0.0059 | |
| Arboreal | Elevation band | 13.65 | 4 | 0.0001 |
| Season | 3.87 | 3 | 0.0001 | |
| Elevation band–Season | 1.64 | 12 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongnoo, P.; Burwell, C.J.; Blanchard, B.D.; Punthuwat, L.; Alcantara, M.J.M.; Ashton, L.A.; Kitching, R.L.; Cao, M.; Nakamura, A. Elevational Distribution of Ants Across Seasons in a Subtropical Rainforest of Eastern Australia. Forests 2025, 16, 664. https://doi.org/10.3390/f16040664
Kongnoo P, Burwell CJ, Blanchard BD, Punthuwat L, Alcantara MJM, Ashton LA, Kitching RL, Cao M, Nakamura A. Elevational Distribution of Ants Across Seasons in a Subtropical Rainforest of Eastern Australia. Forests. 2025; 16(4):664. https://doi.org/10.3390/f16040664
Chicago/Turabian StyleKongnoo, Pitoon, Chris J. Burwell, Benjamin D. Blanchard, Laksamee Punthuwat, Mark Jun M. Alcantara, Louise A. Ashton, Roger L. Kitching, Min Cao, and Akihiro Nakamura. 2025. "Elevational Distribution of Ants Across Seasons in a Subtropical Rainforest of Eastern Australia" Forests 16, no. 4: 664. https://doi.org/10.3390/f16040664
APA StyleKongnoo, P., Burwell, C. J., Blanchard, B. D., Punthuwat, L., Alcantara, M. J. M., Ashton, L. A., Kitching, R. L., Cao, M., & Nakamura, A. (2025). Elevational Distribution of Ants Across Seasons in a Subtropical Rainforest of Eastern Australia. Forests, 16(4), 664. https://doi.org/10.3390/f16040664





