Interspecific Responses to Fire in a Mixed Forest Reveal Differences in Seasonal Growth
Abstract
1. Introduction
2. Materials and Methods
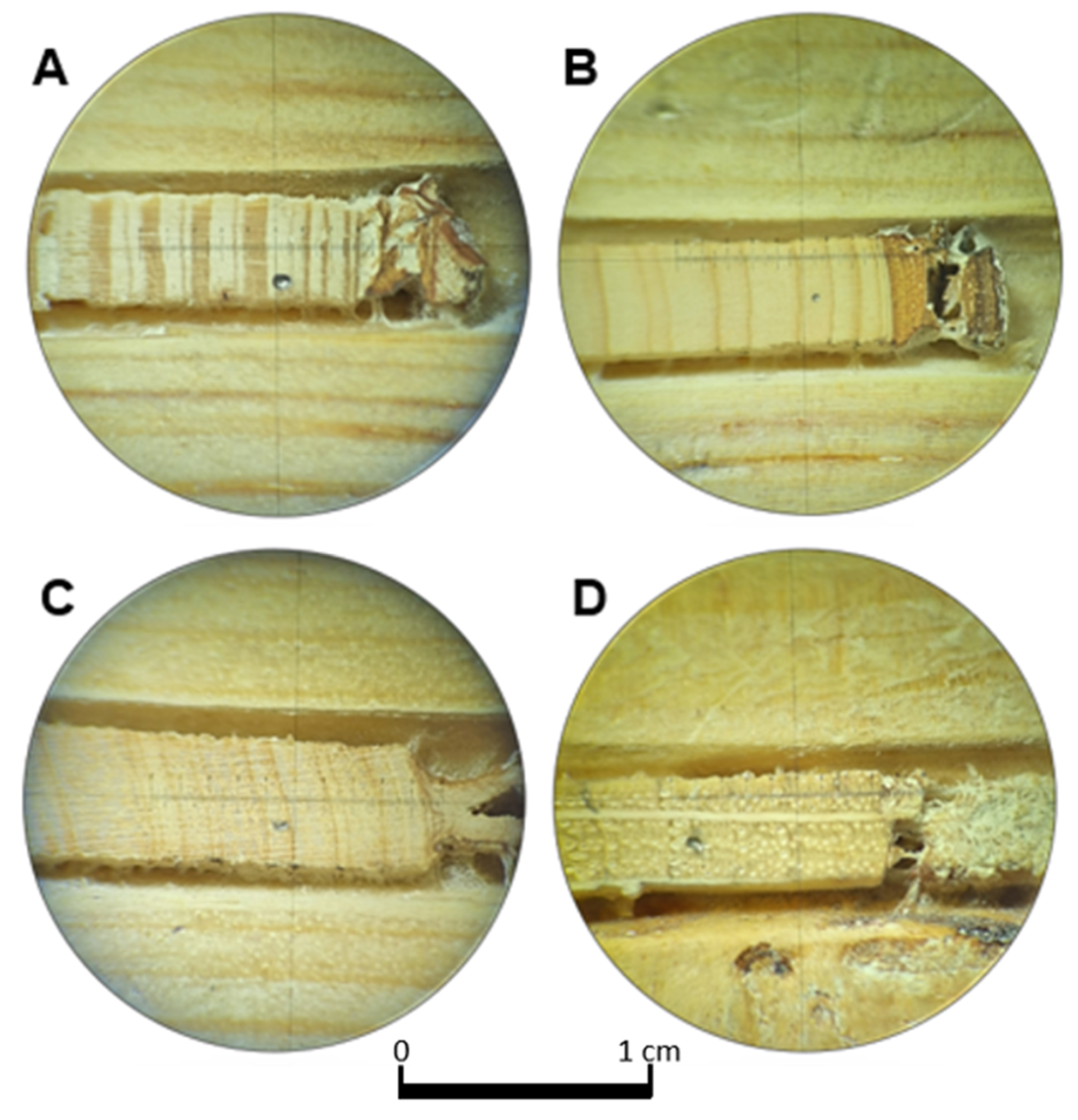
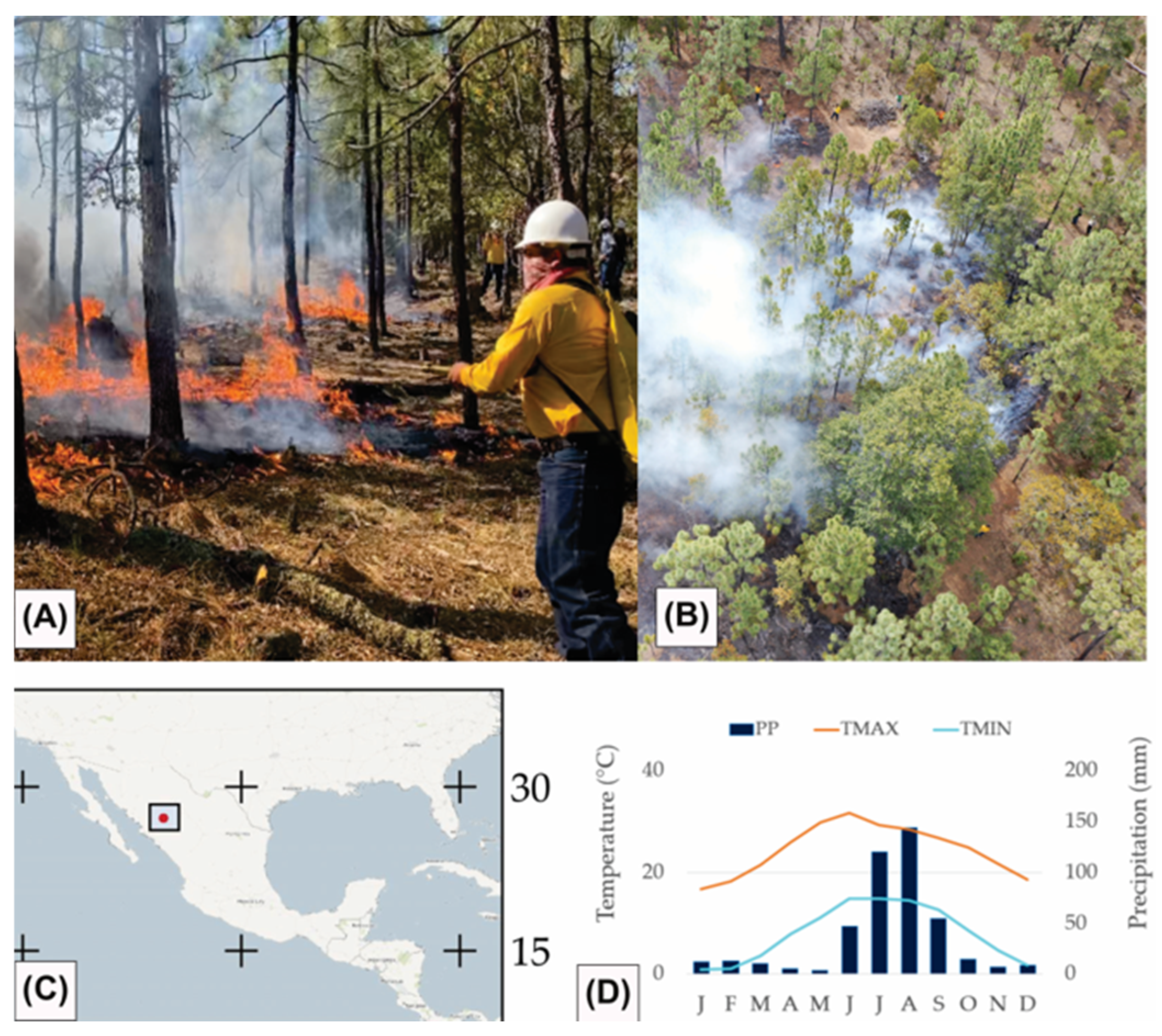
2.1. Study Area and Tree-Ring and NDVI Data
2.2. Statistical Analysis
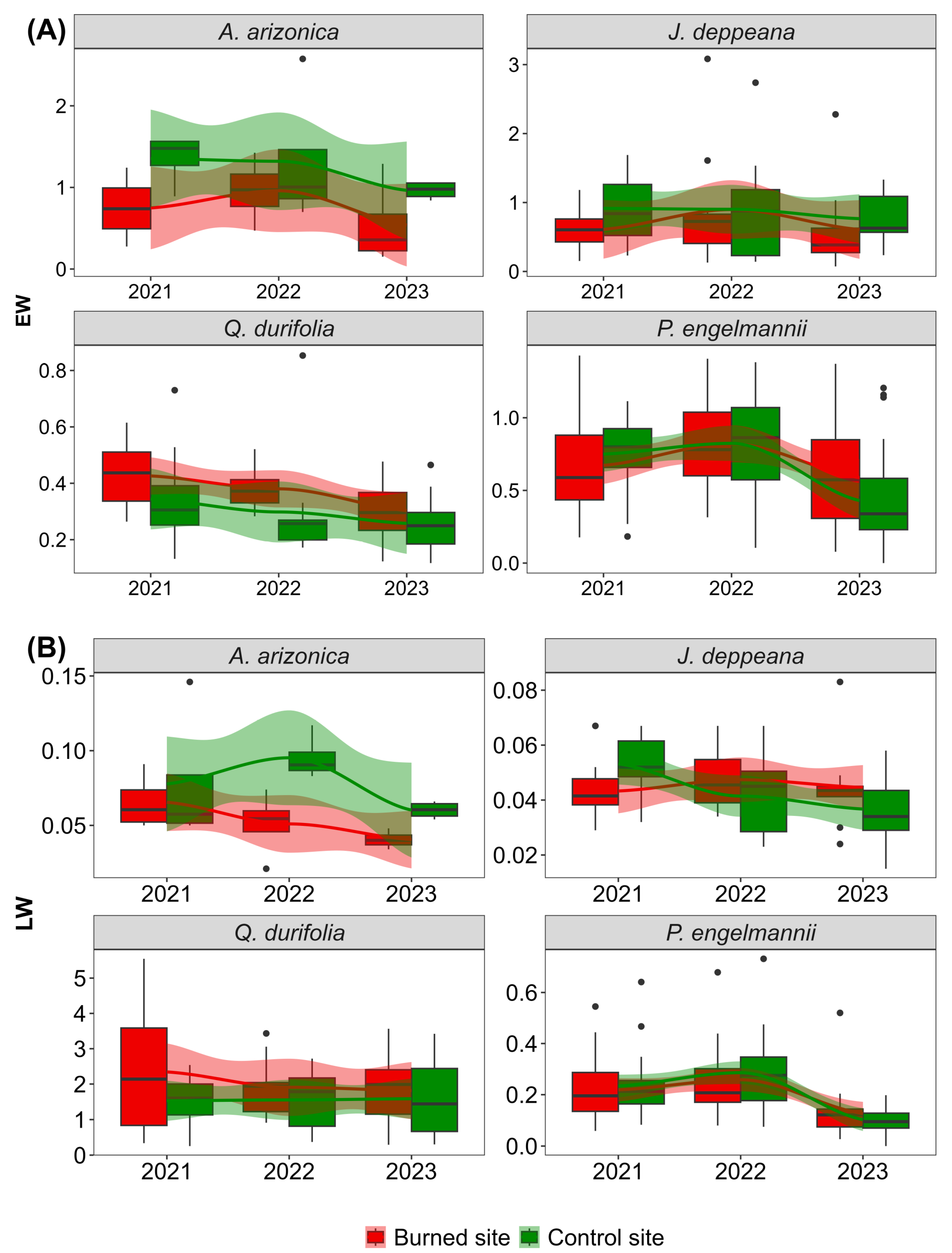
3. Results
4. Discussion
4.1. Post-Fire Seasonal Radial Growth–NDVI Relationships
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Parameters |
|---|---|
| Green material load (ton/ha) | 18.5 ton/ha estimated following the methodology of Caballero-Cruz et al. [39] |
| Ignition material load (ton/ha) | Leaf litter and dry organic matter in the soil: 2.5 ton/ha estimated following the methodology of Caballero-Cruz et al. [39] |
| Relative humidity of the fuels | According to the CNA, the relative humidity of the fuels is 26%. |
| Width | Var | Df | Sum Sq | Mean Sq | F Value | Pr(>F) |
| Site | 1 | 0.94 | 0.94 | 4.65 | 0.031 | |
| Species | 3 | 42.77 | 14.25 | 70.52 | <2 × 10−16 | |
| Year | 1 | 23.18 | 23.17 | 114.66 | <2 × 10−16 | |
| EW | Site:Species | 3 | 1.58 | 0.52 | 2.59 | 0.051 |
| Site:Year | 1 | 0.06 | 0.06 | 0.32 | 0.573 | |
| Species:Year | 3 | 12.86 | 4.28 | 21.21 | 4.70 × 10−16 | |
| Site:Species:Year | 3 | 0.85 | 0.28 | 1.39 | 0.242 | |
| Residuals | 583 | 117.85 | 0.20 | |||
| Site | 1 | 0.49 | 0.49 | 2.43 | 0.118 | |
| Species | 3 | 198.8 | 66.27 | 333.10 | <2 × 10−16 | |
| Year | 1 | 0.39 | 0.39 | 1.95 | 0.162 | |
| LW | Site:Species | 3 | 3.33 | 1.11 | 5.57 | 0.000 |
| Site:Year | 1 | 0.26 | 0.26 | 1.29 | 0.256 | |
| Species:Year | 3 | 7.15 | 2.38 | 11.97 | 1.28 × 10−16 | |
| Site:Species:Year | 3 | 0.47 | 0.16 | 0.78 | 0.501 | |
| Residuals | 584 | 116.18 | 0.2 |
| Var | Species | Year | Treatment | n1 | n2 | statistic | df | p-Value | Conf.low | Conf.high | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 132 | 132 | 15.21 | 245.00 | 3.2 × 10−37 | 13.3639 | 17.0510 | ||||
| A. arizonica | 2022 | Control site | Burned site | 132 | 132 | 5.08 | 185.88 | 9.3 × 10−07 | 4.4605 | 5.6934 | |
| 2023 | 132 | 132 | 10.63 | 142.60 | 8.4 × 10−20 | 9.3371 | 11.9237 | ||||
| 2021 | 363 | 330 | 10.02 | 632.78 | 5.1 × 10−22 | 9.2683 | 10.7625 | ||||
| J. deppeana | 2022 | Control site | Burned site | 363 | 330 | 0.06 | 662.06 | 9.6 × 10−1 | 0.0526 | 0.0610 | |
| 2023 | 363 | 330 | 4.06 | 517.14 | 5.6 × 10−05 | 3.7597 | 4.3661 | ||||
| EW | 2021 | 793 | 858 | 5.15 | 1561.43 | 3.0 × 10−07 | 4.8990 | 5.3960 | |||
| P. engelmannii | 2022 | Control site | Burned site | 793 | 858 | −0.04 | 1628.08 | 9.7 × 10−1 | −0.0383 | −0.0422 | |
| 2023 | 793 | 858 | −9.21 | 1625.73 | 9.4 × 10−20 | −8.7692 | −9.6588 | ||||
| 2021 | 330 | 330 | −7.29 | 583.39 | 1.0 × 10−12 | −6.7308 | −7.8451 | ||||
| Q. durifolia | 2022 | Control site | Burned site | 330 | 330 | −7.34 | 410.57 | 1.1 × 10−12 | −6.7823 | −7.9063 | |
| 2023 | 330 | 330 | −5.76 | 657.81 | 1.3 × 10−08 | −5.3173 | −6.1973 | ||||
| 2021 | 132 | 132 | 3.27 | 173.55 | 1.3 × 10−03 | 2.8712 | 3.6652 | ||||
| A. arizonica | 2022 | Control site | Burned site | 132 | 132 | 21.92 | 231.61 | 2.0 × 10−58 | 19.2648 | 24.5817 | |
| 2023 | 132 | 132 | 31.67 | 261.34 | 1.9 × 10−91 | 27.8343 | 35.5110 | ||||
| 2021 | 363 | 330 | 13.02 | 672.21 | 9.8 × 10−35 | 12.0517 | 13.9944 | ||||
| J. deppeana | 2022 | Control site | Burned site | 363 | 330 | −6.61 | 668.62 | 7.9 × 10−11 | −6.1160 | −7.1019 | |
| 2023 | 363 | 330 | −7.76 | 626.30 | 3.4 × 10−14 | −7.1836 | −8.3417 | ||||
| LW | 2021 | 793 | 858 | 2.51 | 1641.64 | 1.2 × 10−02 | 2.3906 | 2.6331 | |||
| P. engelmannii | 2022 | Control site | Burned site | 793 | 858 | 4.32 | 1587.98 | 1.7 × 10−05 | 4.1127 | 4.5299 | |
| 2023 | 793 | 858 | −6.62 | 1334.42 | 5.1 × 10−11 | −6.3042 | −6.9439 | ||||
| 2021 | 330 | 330 | −8.32 | 428.91 | 1.2 × 10−15 | −7.6838 | −8.9569 | ||||
| Q. durifolia | 2022 | Control site | Burned site | 330 | 330 | −5.84 | 657.99 | 8.1 × 10−09 | −5.3956 | −6.2887 | |
| 2023 | 330 | 330 | −3.03 | 653.31 | 2.5 × 10−03 | −2.8026 | −3.2664 | ||||
| Var | Site | Group1 | Group2 | Estimate | se | df | Conf.Low | Conf.High | Statistic | p | p.Adj | p.Adj.Signif |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EW | Burned site | A. arizonica | J. deppeana | 0.043 | 0.135 | 292 | −0.224 | 0.309 | 0.316 | 0.753 | 1 | ns |
| A. arizonica | Q. durifolia | 0.379 | 0.135 | 292 | 0.113 | 0.646 | 2.800 | 0.005 | 0.033 | * | ||
| A. arizonica | P. engelmannii | 0.055 | 0.123 | 292 | −0.187 | 0.297 | 0.451 | 0.653 | 1 | ns | ||
| J. deppeana | Q. durifolia | 0.336 | 0.102 | 292 | 0.135 | 0.538 | 3.286 | 0.001 | 0.007 | ** | ||
| J. deppeana | P. engelmannii | 0.013 | 0.085 | 292 | −0.155 | 0.180 | 0.149 | 0.882 | 1 | ns | ||
| Q. durifolia | P. engelmannii | −0.324 | 0.085 | 292 | −0.491 | −0.156 | −3.801 | 0.000 | 0.001 | ** | ||
| Control site | A. arizonica | J. deppeana | 0.353 | 0.134 | 292 | 0.090 | 0.616 | 2.638 | 0.009 | 0.053 | ns | |
| A. arizonica | Q. durifolia | 0.913 | 0.135 | 292 | 0.647 | 1.180 | 6.744 | 8.25 × 10−11 | 4.95 × 10−10 | **** | ||
| A. arizonica | P. engelmannii | 0.545 | 0.123 | 292 | 0.303 | 0.788 | 4.424 | 1.37 × 10−05 | 8.22 × 10−05 | **** | ||
| J. deppeana | Q. durifolia | 0.561 | 0.100 | 292 | 0.364 | 0.757 | 5.606 | 4.79 × 10−08 | 2.87 × 10−07 | **** | ||
| J. deppeana | P. engelmannii | 0.193 | 0.083 | 292 | 0.030 | 0.356 | 2.328 | 0.021 | 0.124 | ns | ||
| Q. durifolia | P. engelmannii | −0.368 | 0.086 | 292 | −0.536 | −0.199 | −4.296 | 2.37 × 10−05 | 0.000 | *** | ||
| LW | Burned site | A. arizonica | J. deppeana | 0.007 | 0.163 | 292 | −0.314 | 0.328 | 0.044 | 0.965 | 1 | ns |
| A. arizonica | Q. durifolia | −1.970 | 0.163 | 292 | −2.291 | −1.649 | −12.087 | 1.52 × 10−27 | 9.09 × 10−27 | **** | ||
| A. arizonica | P. engelmannii | −0.149 | 0.148 | 292 | −0.440 | 0.142 | −1.006 | 0.315 | 1 | ns | ||
| J. deppeana | Q. durifolia | −1.977 | 0.123 | 292 | −2.219 | −1.735 | −16.049 | 5.44 × 10−42 | 3.27 × 10−41 | **** | ||
| J. deppeana | P. engelmannii | −0.156 | 0.103 | 292 | −0.358 | 0.046 | −1.523 | 0.129 | 0.773 | ns | ||
| Q. durifolia | P. engelmannii | 1.821 | 0.103 | 292 | 1.619 | 2.023 | 17.765 | 2.23 × 10−48 | 1.34 × 10−47 | **** | ||
| Control site | A. arizonica | J. deppeana | 0.034 | 0.161 | 292 | −0.283 | 0.351 | 0.211 | 0.833 | 1 | ns | |
| A. arizonica | Q. durifolia | −1.479 | 0.163 | 292 | −1.799 | −1.158 | −9.074 | 1.75 × 10−17 | 1.05 × 10−16 | **** | ||
| A. arizonica | P. engelmannii | −0.130 | 0.148 | 292 | −0.422 | 0.162 | −0.874 | 0.383 | 1 | ns | ||
| J. deppeana | Q. durifolia | −1.513 | 0.120 | 292 | −1.750 | −1.276 | −12.568 | 2.99 × 10−29 | 1.79 × 10−28 | **** | ||
| J. deppeana | P. engelmannii | −0.164 | 0.100 | 292 | −0.360 | 0.032 | −1.642 | 0.102 | 0.610 | ns | ||
| Q. durifolia | P. engelmannii | 1.349 | 0.103 | 292 | 1.146 | 1.552 | 13.089 | 4.08 × 10−31 | 2.45 × 10−30 | **** |
References
- Pyne, S.J. The Pyrocene: How We Created an Age of Fire, and What Happens Next; The University of California Press: Berkeley, CA, USA, 2022. [Google Scholar]
- Pompa-García, M.; Camarero, J.J.; Valeriano, C.; Vivar-Vivar, E.D. Variable growth responses of four tree species to climate and drought in a Madrean pine-oak forest. For. Ecosyst. 2025, 12, 100292. [Google Scholar] [CrossRef]
- Alfaro-Sánchez, R.; Camarero, J.J.; Sánchez-Salguero, R.; Trouet, V.; de Las Heras, J. How do droughts and wildfires alter seasonal radial growth in Mediterranean Aleppo pine forests? Tree-Ring Res. 2018, 74, 1–14. [Google Scholar] [CrossRef]
- Pompa-García, M.; Rodríguez-Flores, F.d.J.; Sigala, J.A.; Rodríguez-Trejo, D.A. Does fire influence the greenness index of trees? Twelve months to decode the answer in a Rarámuri Mixed Forest. Fire 2024, 7, 282. [Google Scholar] [CrossRef]
- Mašek, J.; Tumajer, J.; Lange, J.; Kaczka, R.; Fišer, P.; Treml, V. Variability in tree-ring width and NDVI responses to climate at a landscape level. Ecosystems 2023, 26, 1144–1157. [Google Scholar] [CrossRef]
- Gallardo, V.B.; Hadad, M.A.; Roig, F.A.; Gatica, G.; Chen, F. Spatio-temporal linkage variations between NDVI and tree rings on the leeward side of the northern Patagonian Andes. For. Ecol. Manage. 2024, 553, 121593. [Google Scholar] [CrossRef]
- Brehaut, L.; Danby, R.K. Inconsistent relationships between annual tree ring-widths and satellite-measured NDVI in a mountainous subarctic environment. Ecol. Indic. 2018, 91, 698–711. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Li, S.; Yang, C.; Chen, C.; Yun, W. Changes in vegetation resistance and resilience under different drought disturbances based on NDVI and SPEI time series data in Jilin Province, China. Remote Sens. 2023, 15, 3280. [Google Scholar] [CrossRef]
- Vivar-Vivar, E.D.; Pompa-García, M.; Martínez-Rivas, J.A.; Mora-Tembre, L.A. UAV-based characterization of tree attributes and multispectral indices in an uneven-aged mixed conifer-broadleaf forest. Remote Sens. 2022, 14, 2775. [Google Scholar] [CrossRef]
- Seifert, T.; Meincken, M.; Odhiambo, B.O. The effect of surface fire on tree ring growth of Pinus radiata trees. Ann. For. Sci. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- Azpeleta-Tarancón, A.; Fulé, P.Z.; García-Arévalo, A. Mexican mixed-species forest shows resilience to high-intensity fire. Can. J. For. Res. 2023, 54, 500–511. [Google Scholar] [CrossRef]
- Stokes, M.A.; Smiley, T.L. Tree-Ring Dating; The University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Braeker, O.U.; Schoch, W.; Innes, J.L. Identification, measurement and interpretation of tree rings in woody species from Mediterranean climates. Biol. Rev. 2007, 78, 119–148. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 3.0.1; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org. (accessed on 10 October 2024).
- Fu, X.; Meinzer, F.C. Metrics and proxies for stringency of regulation of plant water status (iso/anisohydry): A global data set reveals coordination and trade-offs among water transport traits. Tree Physiol. 2019, 39, 122–134. [Google Scholar] [CrossRef]
- Reinke, R.K.; de Oliveira, A.C.; Pilon, N.; Kolb, R.M. Time since fire and soil depth shapes grass regeneration niche in Cerrado. Plant Ecol. 2025, 226, 171–183. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Búrquez, A.; Martínez-Yrízar, A.; Teece, M.; Yépez, E.A.; Dovciak, M. Resource partitioning by evergreen and deciduous species in a tropical dry forest. Oecologia 2017, 183, 607–618. [Google Scholar] [CrossRef]
- Pineda-García, F.; Paz, H.; Meinzer, F.C.; Angeles, G. Exploiting water versus tolerating drought: Water-use strategies of trees in a secondary successional tropical dry forest. Tree Physiol. 2016, 36, 208–217. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef]
- Alderott, F.; Verdiani, E. God save the queen! How and why the dominant evergreen species of the Mediterranean Basin is declining? AoB Plants 2023, 15, plad051. [Google Scholar] [CrossRef]
- Quevedo, L.; Arnan, X.; Rodrigo, A. Post-fire forestry management improves fruit weight and seed set in forest coppices dominated by Arbutus unedo L. For. Ecol. Manage. 2015, 345, 65–72. [Google Scholar] [CrossRef]
- González-Pelayo, O.; Prats, S.A.; Vieira, A.M.D.; Vieira, D.C.S.; Maia, P.; Keizer, J.J. Impacts of barley (Hordeum vulgare L.) straw mulch on post-fire soil erosion and ground vegetation recovery in a strawberry tree (Arbutus unedo L.) stand. Ecol. Eng. 2023, 195, 107074. [Google Scholar] [CrossRef]
- De Micco, V.; Zalloni, E.; Balzano, A.; Battipaglia, G. Fire influence on Pinus halepensis: Wood responses close and far from scar. IAWA J. 2013, 34, 446–458. [Google Scholar] [CrossRef]
- Santiso, X.; Retuerto, R. Low among-provenance differences in structural and functional plasticity in response to nutrients in saplings of the circum-Mediterranean tree Arbutus unedo L. Tree Physiol. 2015, 35, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Trejo, D.A.; Pausas, J.G.; Miranda-Moreno, A.G. Plant responses to fire in a Mexican arid shrubland. Fire Ecol. 2019, 15, 1–9. [Google Scholar] [CrossRef]
- Cuevas-Guzmán, R.; Canales-Piña, S.; Sánchez-Rodríguez, E.V.; Morales-Arias, J.G.; Guzmán-Hernández, L.; Núñez-López, N.M. Structural attributes and habitat of Juniperus jaliscana in Talpa de Allende, Jalisco, Mexico. Bot. Sci. 2023, 101, 670–684. [Google Scholar] [CrossRef]
- Strand, E.K.; Bunting, S.C. Effects of pre-fire vegetation on the post-fire plant community response to wildfire along a successional gradient in western juniper woodlands. Fire 2023, 6, 141. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Smith, D.D.; Woodruff, D.R.; Marias, D.E.; McCulloh, K.A.; Howard, A.R.; Magedman, A.L. Stomatal kinetics and photosynthetic gas exchange along a continuum of isohydric to anisohydric regulation of plant water status. Plant Cell Environ. 2017, 40, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Keeley, J.E.; Schwilk, D.W. Flammability as an ecological and evolutionary driver. J. Ecol. 2017, 105, 289–297. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Johnson, E.A.; Tyree, M.T. Moving beyond the cambium necrosis hypothesis of post-fire tree mortality: Cavitation and deformation of xylem in forest fires. New Phytol. 2012, 194, 254–263. [Google Scholar] [CrossRef]
- Wessels, C.B.; Malan, F.S.; Seifert, T.; Louw, J.H.; Rypstra, T. The prediction of the flexural lumber properties from standing South African-grown Pinus patula trees. Eur. J. For. Res. 2015, 134, 1–18. [Google Scholar] [CrossRef]
- Ivanova, Y.; Kovalev, A.; Soukhovolsky, V. Modeling the radial stem growth of the pine (Pinus sylvestris L.) forests using the satellite-derived NDVI and LST (MODIS/AQUA) data. Atmosphere 2020, 12, 12. [Google Scholar] [CrossRef]
- Lusk, C.H.; Wright, I.; Reich, P.B. Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. New Phytol. 2003, 160, 32–336. [Google Scholar] [CrossRef]
- Ladd, P.G.; Zhao, X.; Enright, N.J. Fire regime and climate determine spatial variation in level of serotiny and population structure in a fire-killed conifer. Plant Ecol. 2022, 223, 849–862. [Google Scholar] [CrossRef]
- Asbjornsen, H.; McIntire, C.D.; Vadeboncoeur, M.A.; Jennings, K.A.; Coble, A.P.; Berry, Z.C. Sensitivity and threshold dynamics of Pinus strobus and Quercus spp. in response to experimental and naturally occurring severe droughts. Tree Physiol. 2021, 41, 1819–1835. [Google Scholar] [CrossRef] [PubMed]
- Voelker, S.L.; DeRose, R.J.; Bekker, M.F.; Sriladda, C.; Leksungnoen, N.; Kjelgren, R.K. Anisohydric water use behavior links growing season evaporative demand to ring-width increment in conifers from summer-dry environments. Trees 2018, 32, 735–749. [Google Scholar] [CrossRef]
- Konstantinidis, P.; Tsiourlis, G.; Xofis, P. Effect of fire season, aspect and pre-fire plant size on the growth of Arbutus unedo L. (strawberry tree) resprouts. For. Ecol. Manage. 2006, 225, 359–367. [Google Scholar] [CrossRef]
- Rother, M.T.; Huffman, J.M.; Harley, G.L.; Platt, W.J.; Jones, N.; Robertson, K.M.; Orzell, S.L. Cambial phenology informs tree-ring analysis of fire seasonality in Coastal Plain pine savannas. Fire Ecol. 2018, 14, 164–185. [Google Scholar] [CrossRef]
- Caballero-Cruz, P.; Santiago-Juárez, W.; Martínez-Santiago, D.; Cruz-Santiago, O.L.; Pérez-Silva, E.R.; Aguirre-Calderón, O.A. Combustibles forestales y susceptibilidad a incendios de un bosque templado en la Mixteca Alta, Oaxaca, México. For. Veracruzana 2018, 20, 9–14. [Google Scholar]


| Site | Species | No. of Trees | DBH (cm) Mean ± SD | TH (m) Mean ± SD |
|---|---|---|---|---|
| Control (unburned) | A. arizonica | 5 | 14.6 ± 4.9 | 5.8 ± 1.1 |
| J. deppeana | 10 | 13.4 ± 3.5 | 4.7 ± 1.1 | |
| P. engelmannii | 25 | 15.7 ± 8.5 | 8.1 ± 3.8 | |
| Q. durifolia | 10 | 41.4 ± 4.1 | 11.7 ± 2.1 | |
| Burned | A. arizonica | 5 | 15.9 ± 2.5 | 5.5 ± 1.6 |
| J. deppeana | 10 | 10.3 ± 1.8 | 4.8 ± 0.7 | |
| P. engelmannii | 25 | 15.1 ± 6.0 | 8.6 ± 2.9 | |
| Q. durifolia | 10 | 39.6 ± 6.2 | 14.1 ± 1.5 |
| Site | Species | No. of Trees | EW and LW Width Mean ± SD (mm) | |
|---|---|---|---|---|
| EW | LW | |||
| Control (unburned) | A. arizonica | 5 | 1.275 ± 0.62 | 0.072 ± 0.03 |
| J. deppeana | 10 | 0.795 ± 0.47 | 0.043 ± 0.01 | |
| P. engelmannii | 25 | 0.677 ± 0.34 | 0.210 ± 0.13 | |
| Q. durifolia | 10 | 0.300 ± 0.16 | 1.557 ± 0.84 | |
| Burned | A. arizonica | 5 | 0.655 ± 0.44 | 0.051 ± 0.02 |
| J. deppeana | 10 | 0.757 ± 0.65 | 0.046 ± 0.02 | |
| P. engelmannii | 25 | 0.690 ± 0.33 | 0.207 ± 0.13 | |
| Q. durifolia | 10 | 0.370 ± 0.11 | 2.022 ± 1.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Gutiérrez, J.E.; Martínez-Rivas, J.A.; Acosta-Hernández, A.C.; Rodríguez-Flores, F.d.J.; Pompa-García, M. Interspecific Responses to Fire in a Mixed Forest Reveal Differences in Seasonal Growth. Forests 2025, 16, 633. https://doi.org/10.3390/f16040633
Gutiérrez-Gutiérrez JE, Martínez-Rivas JA, Acosta-Hernández AC, Rodríguez-Flores FdJ, Pompa-García M. Interspecific Responses to Fire in a Mixed Forest Reveal Differences in Seasonal Growth. Forests. 2025; 16(4):633. https://doi.org/10.3390/f16040633
Chicago/Turabian StyleGutiérrez-Gutiérrez, Jesús Efrén, José Alexis Martínez-Rivas, Andrea Cecilia Acosta-Hernández, Felipa de Jesús Rodríguez-Flores, and Marín Pompa-García. 2025. "Interspecific Responses to Fire in a Mixed Forest Reveal Differences in Seasonal Growth" Forests 16, no. 4: 633. https://doi.org/10.3390/f16040633
APA StyleGutiérrez-Gutiérrez, J. E., Martínez-Rivas, J. A., Acosta-Hernández, A. C., Rodríguez-Flores, F. d. J., & Pompa-García, M. (2025). Interspecific Responses to Fire in a Mixed Forest Reveal Differences in Seasonal Growth. Forests, 16(4), 633. https://doi.org/10.3390/f16040633











