Unveiling the Carbon Secrets: How Forestry Projects Transform Biomass and Soil Carbon on the Tibet Plateau
Abstract
1. Introduction
2. Materials and Methods
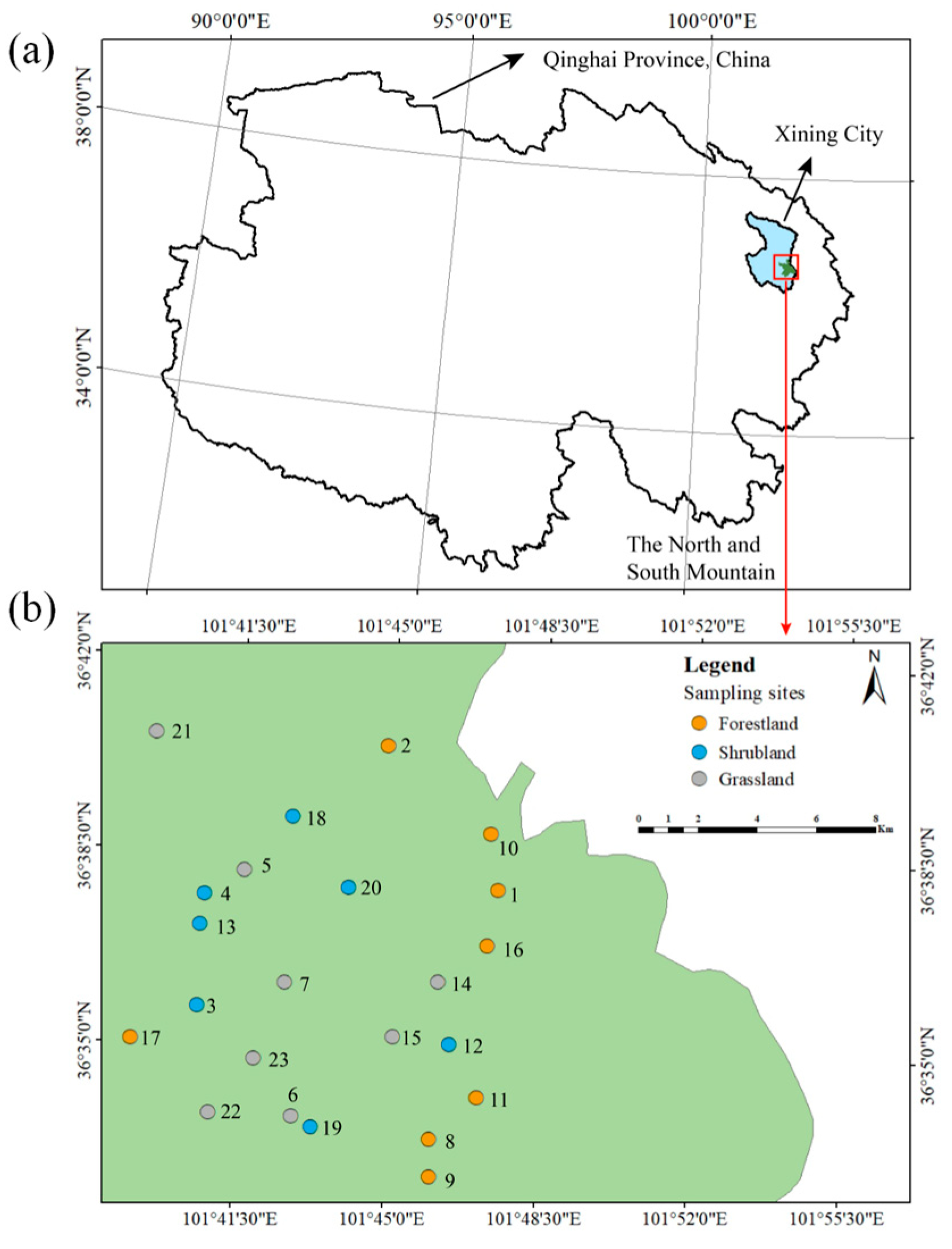
2.1. The Study Area
2.2. Vegetation Survey and Soil Sampling
2.3. Determination of Soil Physical and Chemical Properties
2.4. Calculation of Carbon Stocks in Biomass and Soil and Diversity Indices
2.5. Statistical Analysis
3. Results
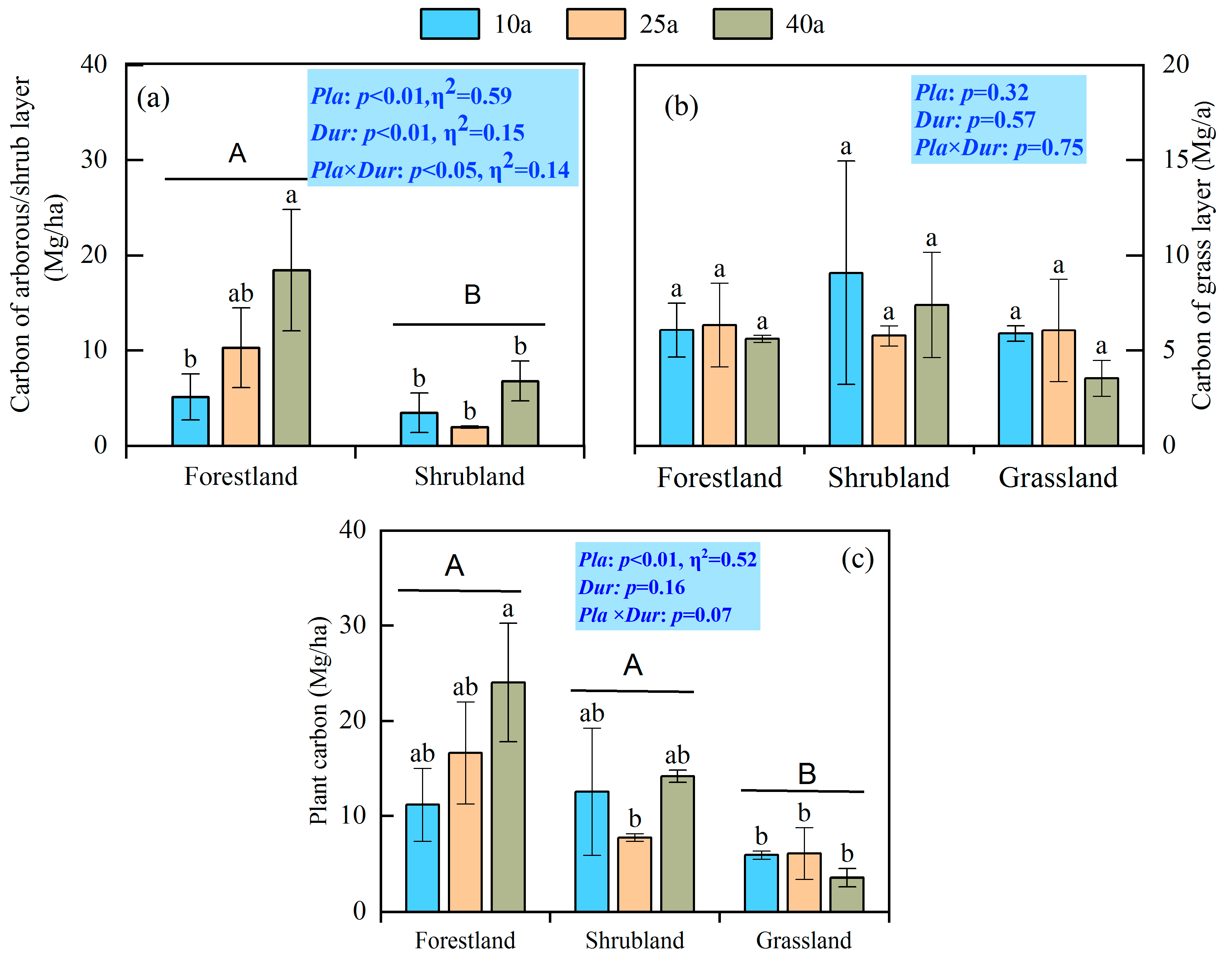
3.1. Carbon Stocks in Biomass and Soil
3.2. Carbon Stocks in Plant–Soil Continuum
3.3. Effects of Afforestation Types and Duration on Soil Properties
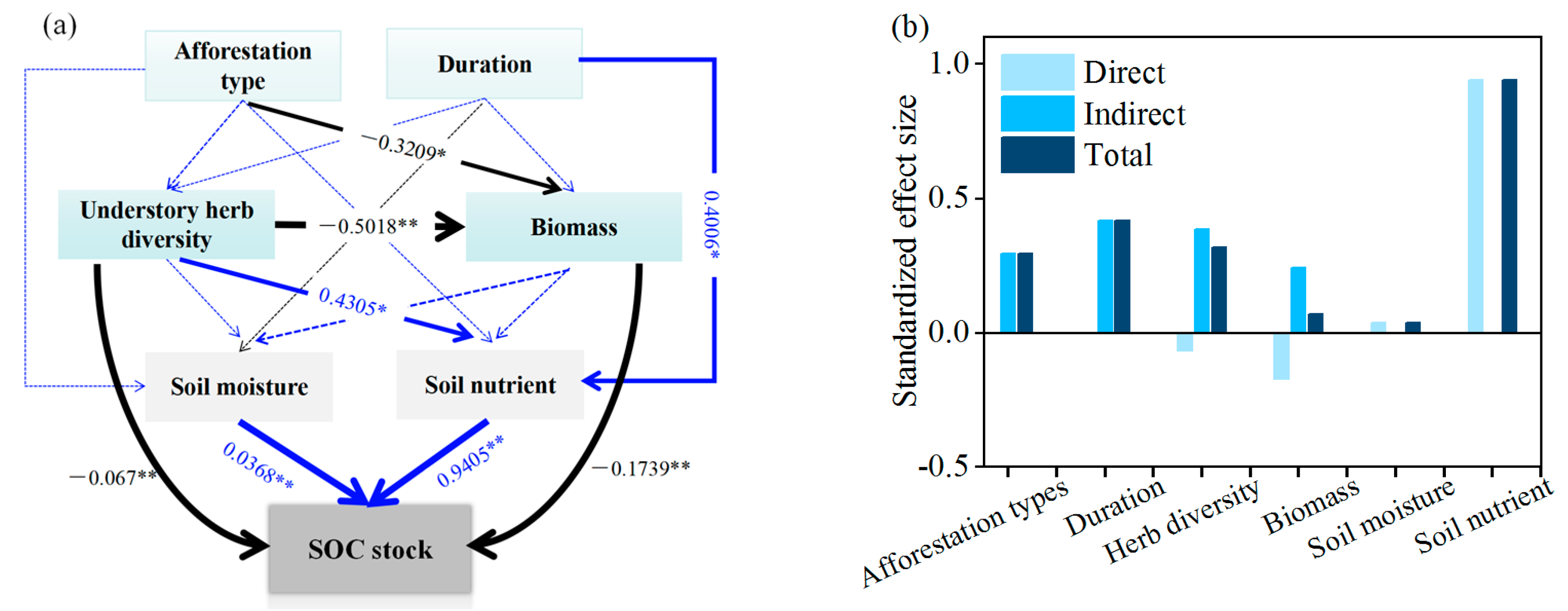
3.4. Factors Affecting the Carbon Sequestration in Plant and Soil
4. Discussion
4.1. Divergent Responses of Biomass Carbon Stocks to Afforestation
4.2. Divergent Responses of SOC Stocks to Afforestation
4.3. Regulation of Afforestation Types and Duration on Carbon Stocks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. Forest Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Hong, S.; Ding, J.; Kan, F.; Xu, H.; Chen, S.; Yao, Y.; Piao, S. Asymmetry of carbon sequestrations by plant and soil after forestation regulated by soil nitrogen. Nat. Commun. 2023, 14, 3196. [Google Scholar] [CrossRef] [PubMed]
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Marcelo, R.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- He, B.; Miao, L.; Cui, X.; Wu, Z. Carbon sequestration from China’s afforestation projects. Environ. Earth Sci. 2015, 74, 5491–5499. [Google Scholar] [CrossRef]
- Nave, L.E.; Walters, B.F.; Hofmeister, K.L.; Perry, C.H.; Mishra, U.; Domke, G.M.; Swanston, C.W. The role of reforestation in carbon sequestration. New Forest. 2018, 50, 115–137. [Google Scholar] [CrossRef]
- Harden, J.W.; Hugelius, G.; Ahlstrom, A.; Blankinship, J.C.; Bond-Lamberty, B.; Lawrence, C.R.; Loisel, J.; Malhotra, A.; Jackson, R.B.; Ogle, S.; et al. Networking our science to characterize the state, vulnerabilities, and management opportunities of soil organic matter. Glob. Change Biol. 2018, 24, e705–e718. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef]
- Crowther, T.W.; Todd-Brown, K.E.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- Hong, S.; Yin, G.; Piao, S.; Dybzinski, R.; Cong, N.; Li, X.; Wang, K.; Peñuelas, J.; Zeng, H.; Chen, A. Divergent responses of soil organic carbon to afforestation. Nat. Sustain. 2020, 3, 694–700. [Google Scholar] [CrossRef]
- Cortijos-Lόpez, M.; Sánchez-Navarrete, P.; Lasanta, T.; Cammeraat, E.L.H. Afforestation, natural secondary forest or dehesas? looking for the best post-abandonment forest management for soil organic carbon accumulation in mediterranean mountains. Forests 2024, 15, 166. [Google Scholar] [CrossRef]
- Schneider, M.V.; Oberholzer, S.; Speranza, C.I. Revegetation is key for soil organic carbon sequestation on abandoned and degraded land in northern Spain. Geoderma Reg. 2024, 38, e00835. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. Forest Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H.; Fiedler, C.E.; Ramsey, P.W.; Harrington, M.G.; Gannon, J.E. Restoration treatments in a Montana ponderosa pine forest: Effects on soil physical, chemical and biological properties. Forest Ecol. Manag. 2005, 213, 25–38. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Change Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Wang, T.; Piao, S. Estimate of terrestrial carbon balance over the Tibetan Plateau: Progresses, challenges and perspectives. Quat. Sci. 2023, 43, 313–323. [Google Scholar] [CrossRef]
- Liu, X.; Ding, J.; Zhao, W. Divergent responses of ecosystem services to afforestation and grassland restoration in the Tibetan Plateau. J. Environ. Manag. 2023, 344, 118471. [Google Scholar] [CrossRef]
- Luo, R.; Fan, J.; Wang, W.; Luo, J.; Kuzyakov, Y.; He, J.S.; Chu, H.; Ding, W. Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 650, 303–312. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Li, X.L. Implementation Study of the Master Plan for the Third Phase of the North-South Mountains Afforestation Project in Xining (2015–2020). Mod. Agric. Sci. Technol. 2021, 2, 132–135. [Google Scholar] [CrossRef]
- Chen, Z.X.; Xu, X.; Wen, Y.L.; Cheng, M.; Wang, X. The critial roles of soil ecological stoichiometric ratios: How does reforestation improve soil nitrogen and phosphorus availabiliy? Plants 2024, 13, 2320. [Google Scholar] [CrossRef]
- Fang, J.Y.; Wang, X.P.; Shen, Z.B.; Tang, Z.Y.; He, J.S.; Yu, D.; Jiang, Y.; Wang, Z.H.; Zheng, C.Y.; Zhu, J.L.; et al. Methods and protocols for plant community inventory. Biodivers. Sci. 2009, 17, 533–548. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2008; ISBN 9787109066441. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar]
- LY/T1215-1999; Forest Soil Moisture-Determination of Physical Properties. State Forestry Administration of People’s Republic of China: Beijing, China, 1999.
- Wang, J.; Che, K.; Jiang, Z. A Study on Carbon Balance of Picea crassifolia in Qilian Mountains. J. Northwest For. Univ. 2000, 15, 9–14. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Chen, X.; Zhang, R.; Chanlumen, B.; Bayaer, A.; Bilige, S.; Wang, Y. Establishment of biomass models for artificial shrubbery in the Kubuqi desert area. J. Northwest A&F Univ (Nat. Sci. Ed.) 2022, 50, 74–82. [Google Scholar] [CrossRef]
- Wang, X.K.; Liu, W.W.; Lu, F. 166 Questions & Answers Carbon Sequestration in Terrestrial Ecosystems; Science Press: Beijing, China, 2015; ISBN 9787030439208. [Google Scholar]
- Song, Y.C. Vegetation Ecology; East China Normal University Press: Shanghai, China, 2001; ISBN 9787561726402. [Google Scholar]
- Meng, X.T.; Zhang, X.C.; Li, Y.N.; Jiao, Y.P.; Fan, L.C.; Jiang, Y.J.; Qu, C.Y.; Filimonenko, E.; Jiang, Y.H.; Tian, X.H.; et al. Nitrogen fertilizer builds soil organic carbon under straw return mainly via microbial necromass formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Gorazda, K.; Mikula, K.; Mironiuk, M.; Kominko, H.; Sawska, K.; Evrard, D.; Trzaska, K.; Moustakas, K.; Chojnacka, K. Towards carbon neutrality: Enhancing CO2 sequestration by plants to reduce carbon footprint. Sci. Total Environ. 2025, 966, 178763. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, L.; Wen, D.; Zhou, Z.; Li, M.; Wang, T.; He, N. The carbon sequestration potential of vegetation over the Tibetan Plateau. Renew. Sust. Energ. Rev. 2025, 207, 114937. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, X.; Bai, Y.; Tang, Z.; Wang, W.; Zhao, Y.; Wan, H.; Xie, Z.; Shi, X.; Wu, B.; et al. Carbon pools in China’s terrestrial ecosystems: New estimates based on an intensive field survey. Proc. Natl. Acad. Sci. USA 2018, 115, 4021–4026. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Menezes, R.S.C.; Sales, A.T.; Primo, D.C.; Albuquerque, E.R.G.M.d.; Jesus, K.N.d.; Pareyn, F.G.C.; Santana, M.d.S.; Santos, U.J.d.; Martins, J.C.R.; Althoff, T.D.; et al. Soil and vegetation carbon stocks after land-use changes in a seasonally dry tropical forest. Geoderma 2021, 390, 114943. [Google Scholar] [CrossRef]
- Yue, J.; Guan, J.; Deng, L.; Zhang, J.; Li, G.; Du, S. Dynamics and allocation patterns of carbon and nitrogen storage in Picea asperata plantations in subalpine areas of Gansu Province. Acta Ecol. Sin. 2018, 38, 7790–7800. [Google Scholar]
- Deng, L.; Wang, K.B.; Chen, M.L.; Shangguan, Z.P.; Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Moura, P.M.; Althoff, T.D.; Oliveira, R.A.; Souto, J.S.; Souto, P.C.; Menezes, R.S.C.; Sampaio, E.V.S.B. Carbon and nutrient fluxes through litterfall at four succession stages of Caatinga dry forest in Northeastern Brazil. Nutr. Cycl. Agroecosyst. 2016, 105, 25–38. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, M.K.; Lee, C.B. Combined effects of environmental conditions and stand age–induced tree size variation on temperate forest carbon pools among stand types in South Korea. Forest Ecol. Manag. 2025, 581, 122553. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, H.I.; Lee, C.B.; Lee, K.H.; Kim, R.H.; Ali, A. Abiotic and stand age-induced changes in tree diversity and size inequality regulate aboveground biomass and soil organic carbon stock in temperate forests of South Korea. Catena 2024, 237, 107827. [Google Scholar] [CrossRef]
- Ismail, M.; Roy, S.K.; Dey, T.; Basak, J.K.; Chowdhury, M.A.A.; Ullah, F.; Ahmed, S. Carbon sequestration potential of Sonneratia apetala plantation forests in the Chakaria Sundarbans: Effects of stand age and structure. Reg. Stud. Mar. Sci. 2025, 84, 104104. [Google Scholar] [CrossRef]
- Cao, J.; Gong, Y.; Adamowski, J.F.; Deo, R.C.; Zhu, G.; Dong, X.; Zhang, X.; Liu, H.; Xin, C. Effects of stand age on carbon storage in dragon spruce forest ecosystems in the upper reaches of the Bailongjiang River basin, China. Sci. Rep. 2019, 9, 3005. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Liu, D.; Lv, G.; Ren, S.; Ding, J.; Chen, B.; Qu, J.; Wang, Y.; Piao, S.; et al. The current and future of terrestrial carbon balance over the Tibetan Plateau. Sci. China Earth Sci. 2023, 66, 1493–1503. [Google Scholar] [CrossRef]
- Lewińska, K.E.; Hostert, P.; Buchner, J.; Bleyhl, B.; Radeloff, V.C. Short-term vegetation loss versus decadal degradation of grasslands in the Caucasus based on Cumulative Endmember Fractions. Remote Sens. Environ. 2020, 248, 111969. [Google Scholar] [CrossRef]
- Andrade, B.O.; Koch, C.; Boldrini, I.I.; Vélez-Martin, E.; Hasenack, H.; Hermann, J.M.; Kollmann, J.; Pillar, V.D.; Overbeck, G.E. Grassland degradation and restoration: A conceptual framework of stages and thresholds illustrated by southern Brazilian grasslands. Nat. Conserv. 2015, 13, 95–104. [Google Scholar] [CrossRef]
- Dlamini, L.X.; Lévêque, J.; Thevenot, M.; Feig, G.T.; Mathieu, O.; Kotzé, E. Afforestation and wildfire-induced grassland degradation: Impact on soil carbon dynamics in temperate grasslands, Drakensberg, South Africa. Catena 2025, 252, 108886. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Li, L.; Fritz, A. Mapping degraded grassland on the Eastern Tibetan Plateau with multi-temporal Landsat 8 data—Where do the severely degraded areas occur? Int. J. Appl. Earth Obs. 2015, 42, 115–127. [Google Scholar] [CrossRef]
- Li, T.; Ren, B.; Wang, D.; Liu, G. Spatial variation in the storages and age-related dynamics of forest carbon sequestration in different climate zones-evidence from black locust plantations on the Loess Plateau of China. PLoS ONE 2015, 10, e0121862. [Google Scholar] [CrossRef] [PubMed]
- Todd-Brown, K.E.O.; Randerson, J.T.; Post, W.M.; Hoffman, F.M.; Tarnocai, C.; Schuur, E.A.G.; Allison, S.D. Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 2013, 10, 1717–1736. [Google Scholar] [CrossRef]
- Davidson, E.A.; Trumbore, S.E.; Amundson, R. Soil warming and organic carbon content. Nature 2000, 408, 789–790. [Google Scholar] [CrossRef]
- Tang, K.; Li, F.Y.; Jaesong, S.; Liu, Y.; Sun, T.; Liu, J.; Gao, X.; Wang, Y. Soil water retention capacity surpasses climate humidity in determining soil organic carbon content but not plant production in the steppe zone of Northern China. Ecol. Indic. 2022, 141, 109129. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Das, S.; Inagaki, T.M.; Azimzadeh, B.; Richards, B.; Martínez, C.E.; Kögel-Knabner, I.; Lehmann, J. Soil organic carbon accrual due to more efficient microbial utilization of plant inputs at greater long-term soil moisture. Geochim. Cosmochim. Acta 2022, 327, 170–185. [Google Scholar] [CrossRef]
- Butcher, K.R.; Nasto, M.K.; Norton, J.M.; Stark, J.M. Physical mechanisms for soil moisture effects on microbial carbon-use efficiency in a sandy loam soil in the western United States. Soil Biol. Biochem. 2020, 150, 107969. [Google Scholar] [CrossRef]
- Kazempour Larsary, M.; Pourbabaei, H.; Salehi, A.; Yousefpour, R.; Ali, A. Few functionally acquisitive big-sized trees restrict but soil nutrients promote soil organic carbon storage in temperate deciduous forests. Forest Ecol. Manag. 2023, 541, 121059. [Google Scholar] [CrossRef]
- Ahirwal, J.; Gogoi, A.; Sahoo, U.K. Stability of soil organic carbon pools affected by land use and land cover changes in forests of eastern Himalayan region, India. Catena 2022, 215, 106308. [Google Scholar] [CrossRef]
- Dai, L.; Ge, J.; Wang, L.; Zhang, Q.; Liang, T.; Bolan, N.; Lischeid, G.; Rinklebe, J. Influence of soil properties, topography, and land cover on soil organic carbon and total nitrogen concentration: A case study in Qinghai-Tibet plateau based on random forest regression and structural equation modeling. Sci. Total Environ. 2022, 821, 153440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; He, T.; Liu, L.; Wu, J. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Liao, C.; Men, X.; Wang, C.; Chen, R.; Cheng, X. Nitrogen availability and mineral particles contributed fungal necromass to the newly formed stable carbon pool in the alpine areas of Southwest China. Soil Biol. Biochem. 2022, 173, 108788. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Zhang, W.; Wang, Q.; Guo, P.; Wang, Q. Nitrogen deposition caused higher increases in plant-derived organic carbon than microbial-derived organic carbon in forest soils. Sci. Total Environ. 2024, 925, 171752. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Xu, X.; Chen, Z.; Xiang, Y.; Wen, Y.; Wang, X. Unveiling the Carbon Secrets: How Forestry Projects Transform Biomass and Soil Carbon on the Tibet Plateau. Forests 2025, 16, 631. https://doi.org/10.3390/f16040631
Cheng M, Xu X, Chen Z, Xiang Y, Wen Y, Wang X. Unveiling the Carbon Secrets: How Forestry Projects Transform Biomass and Soil Carbon on the Tibet Plateau. Forests. 2025; 16(4):631. https://doi.org/10.3390/f16040631
Chicago/Turabian StyleCheng, Man, Xia Xu, Zhixuan Chen, Yun Xiang, Yongli Wen, and Xiao Wang. 2025. "Unveiling the Carbon Secrets: How Forestry Projects Transform Biomass and Soil Carbon on the Tibet Plateau" Forests 16, no. 4: 631. https://doi.org/10.3390/f16040631
APA StyleCheng, M., Xu, X., Chen, Z., Xiang, Y., Wen, Y., & Wang, X. (2025). Unveiling the Carbon Secrets: How Forestry Projects Transform Biomass and Soil Carbon on the Tibet Plateau. Forests, 16(4), 631. https://doi.org/10.3390/f16040631





