Diversity of Needle Terpenes Among Pinus Taxa
Abstract
1. Introduction
2. The Basis Properties of 104 Pinus Taxa
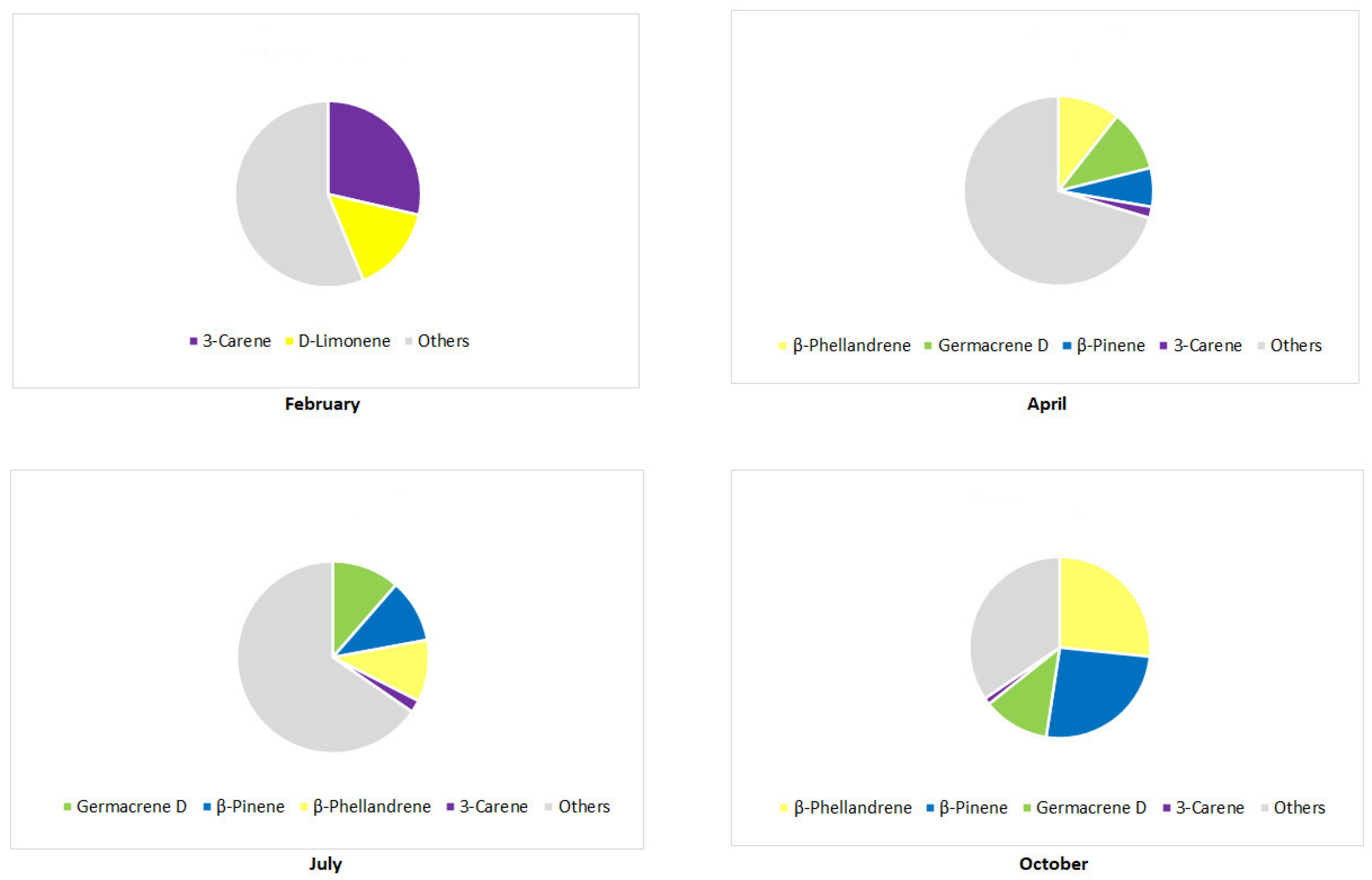
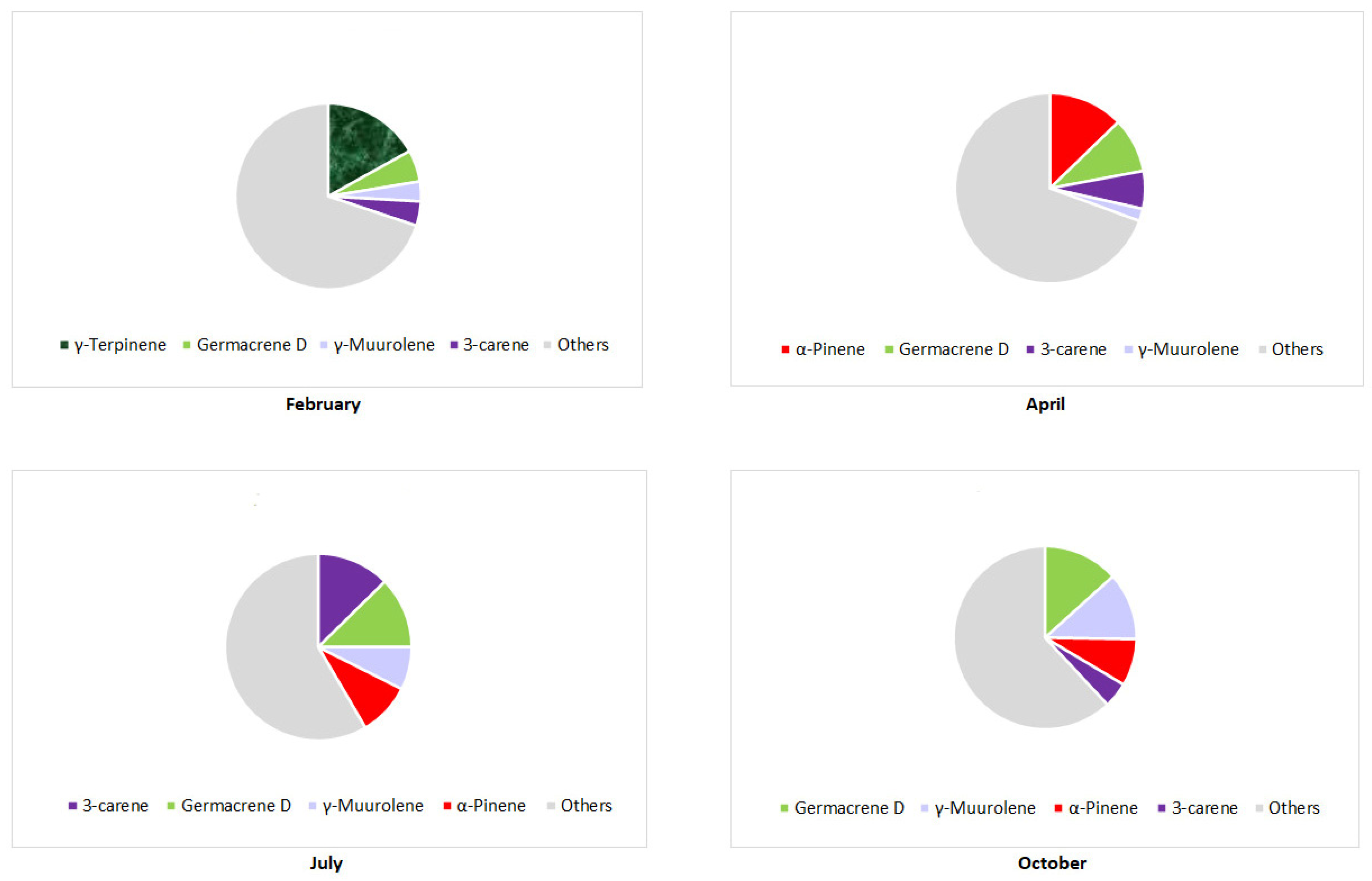
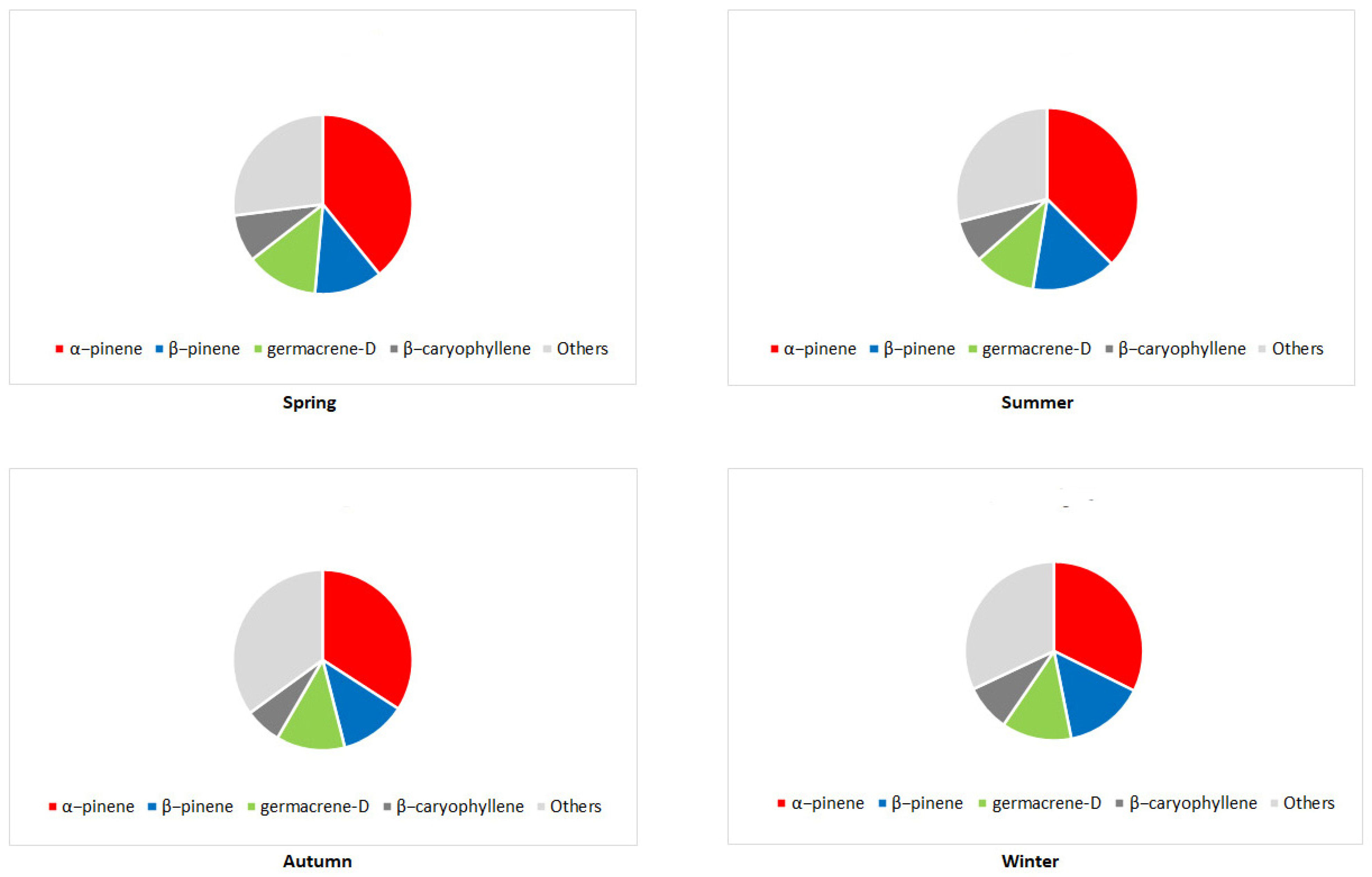
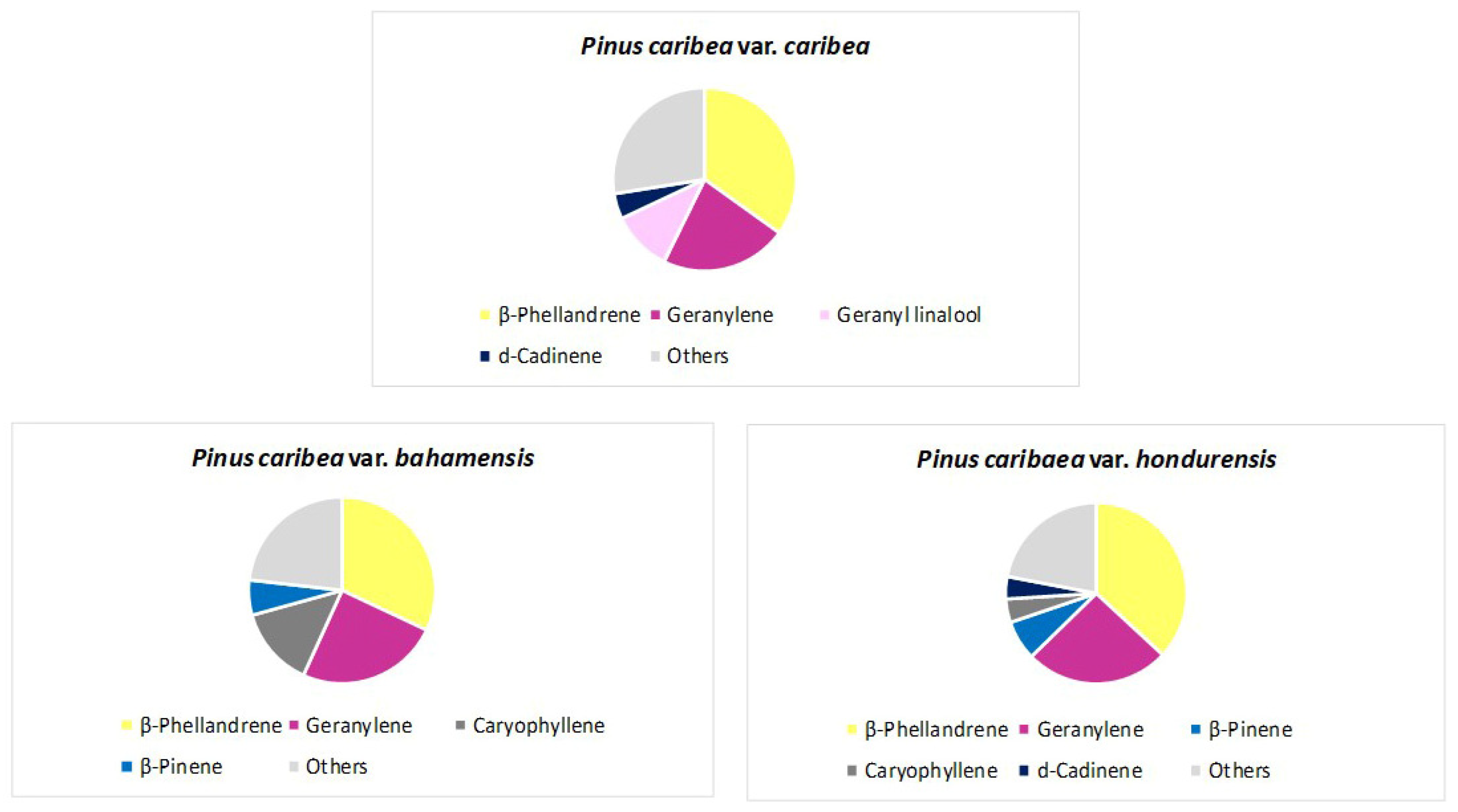
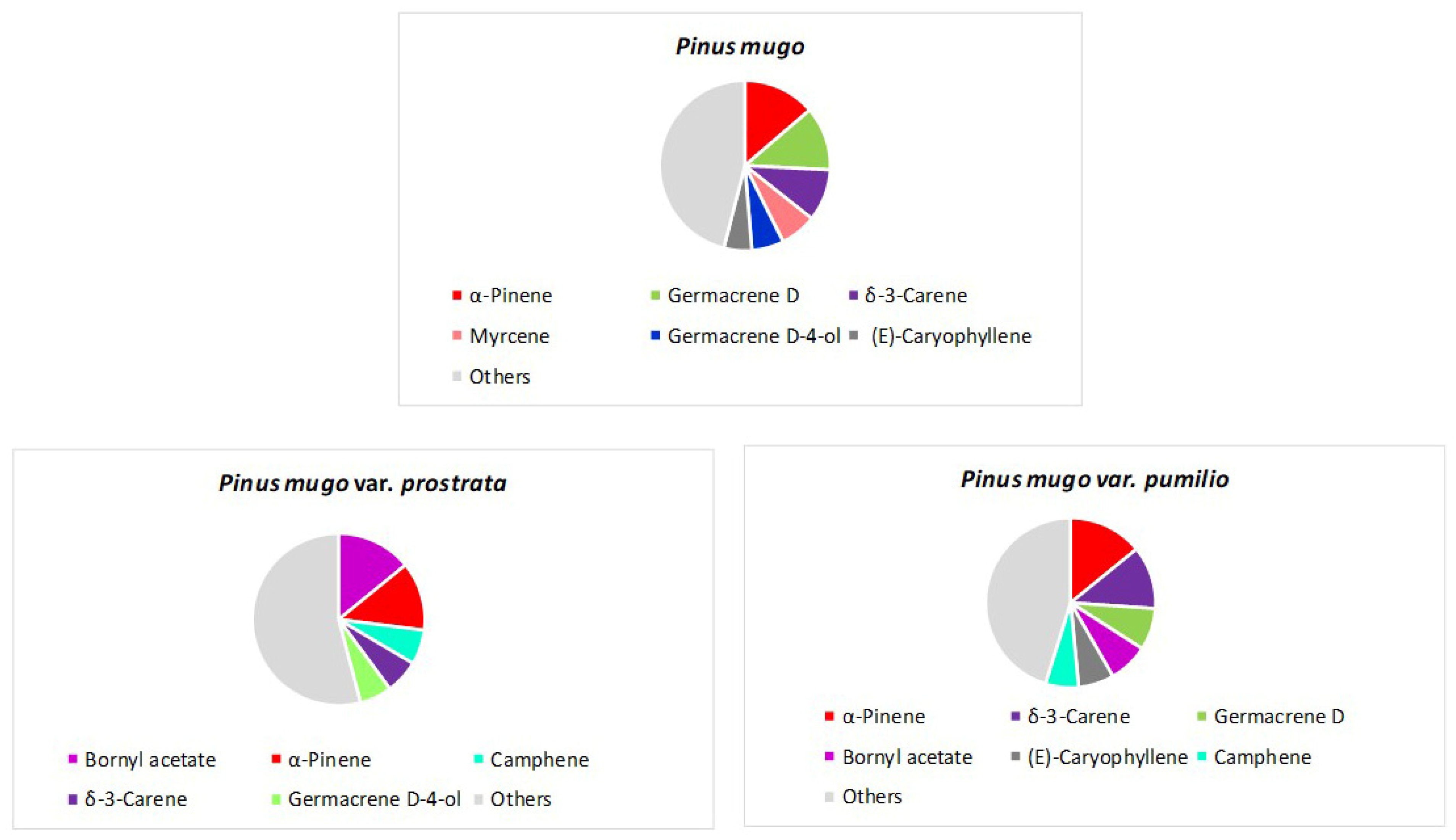
3. The Main Terpene Compounds of Pinus Taxa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Pengelly, A. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicines, 2nd ed.; Allen & Unwin Academic: Crows Nest, NSW, Australia, 2004. [Google Scholar] [CrossRef]
- Nikolić, B.; Ristić, M.; Bojović, S.; Marin, P.D. Variability of the needle essential oils of Pinus heldreichii from different populations in Montenegro and Serbia. Chem. Biodivers. 2007, 4, 905–916. [Google Scholar] [CrossRef]
- Judzentiene, A.; Stikliene, A.; Kupcinskiene, E. Changes in the essential oil composition in the needles of Scots Pine (Pinus sylvestris L.) under anthropogenic stress. Sci. World J. 2007, 7, 141–150. [Google Scholar] [CrossRef]
- Rajčević, N.; Nikolić, B.; Marin, P.D. Different responses to environmental factors in terpene composition of Pinus heldreichii and P. peuce: Ecological and chemotaxonomic considerations. Arch. Biol. Sci. 2019, 71, 629–637. Available online: https://www.serbiosoc.org.rs/arch/index.php/abs/article/view/4458 (accessed on 5 March 2025). [CrossRef]
- Nikolić, B.M.; Rajčević, N.F.; Mitić, Z.S.; Jovanović, S.S.; Čule, N.M.; Mladenović, K.; Marković, M.S.; Marin, P.D. Diversity of Picea omorika (Pančić) Purk. Populations based on morpho-anatomical needle traits and bioclimatic parameters. South-East Eur. For. 2024, 15, 131–149. [Google Scholar] [CrossRef]
- Silori, G.K.; Kushwaha, N.; Kumar, V. Essential oils from pines: Chemistry and applications. In Essential Oil Research; Malik, S., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.; Jeon, B.B.; Song, E.; Lee, H. Terpene compound composition and antioxidant activity of essential oils from needles of Pinus densiflora, Pinus koraiensis, Abies holophylla, and Juniperus chinensis by harvest period. Forests 2024, 15, 566. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.O.; Souza, E.L.; Diniz, M.F.D.M.; Trajano, V.N.; Medeiros, I.A. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Ciências Farm. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Kandi, S.; Godishala, V.; Rao, P.; Ramana, K.V. Biomedical significance of terpenes: An insight. Biomed. Biotechnol. 2015, 3, 8–10. Available online: http://pubs.sciepub.com/bb/3/1/2 (accessed on 5 March 2025).
- Nikolić, B.M.; Ballian, D.; Mitić, Z.S. Autochthonous conifers of family Pinaceae in Europe: Broad review of morpho-anatomical and phytochemical properties of needles and genetic investigations. Forests 2024, 15, 989. [Google Scholar] [CrossRef]
- Vidaković, M.; Franjić, J. Golosjemenjače; Šumarski Fakultet Sveučilišta u Zagrebu: Zagreb, Croatia, 2004. [Google Scholar]
- Gernandt, D.S.; Geada López, G.; Ortiz García, S.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef]
- Syring, J.; Willyard, A.; Cronin, R.; Liston, A. Evolutionary relationships among Pinus (Pinaceae) subsections inferred from multiple low-copy nuclear loci. Am. J. Bot. 2005, 92, 2086–2100. [Google Scholar] [CrossRef]
- Syring, J.; Farrell, K.; Businsky, R.; Cronin, R.; Liston, A. Widespread genealogical nonmonophyly in species of Pinus subgenus Strobus. Syst. Biol. 2007, 56, 163–181. [Google Scholar] [CrossRef]
- Hernández-León, S.; Gernandt, D.S.; Pérez de la Rosa, J.A.; Jardón-Barbolla, L. Phylogenetic relationships and species delimitation in Pinus section Trifoliae inferrred from plastid DNA. PLoS ONE 2013, 8, e70501. [Google Scholar] [CrossRef]
- Jin, W.-T.; Gernandt, D.S.; Wehenkel, C.; Xia, X.-M.; Wei, X.X.; Wang, X.-Q. Phylogenomic and ecological analyses reveal the spatiotemporal evolution of global pines. Proc. Natl. Acad. Sci. USA 2021, 118, e2022302118. [Google Scholar] [CrossRef]
- Willyard, A.; Gernandt, D.S.; Cooper, B.; Douglas, C.; Finch, K.; Karemera, H.; Lindberg, E.; Langer, S.K.; Lefler, J.; Marquardt, P.; et al. Phylogenomics in the hard pines (Pinus subsection Ponderosae; Pinaceae) confirms paraphyly in Pinus ponderosa and places Pinus jeffreyi with the California big cone pines. Syst. Bot. 2021, 46, 538–561. [Google Scholar] [CrossRef]
- Montes, J.-R.; Peláez, P.; Moreno-Letelier, A.; Gernandt, D.S. Coalescent-based species delimitation in North American pinyon pines using low-copy nuclear genes and plastomes. Am. J. Bot. 2022, 109, 706–726. [Google Scholar] [CrossRef]
- Earle, C.J. (Ed.) The Gymnosperm Database. 2024. Available online: https://www.conifers.org/ (accessed on 5 March 2025).
- Vidaković, M. Četinjače—Morfologija i Varijabilnost; JAZU i Sveučilišna Naklada Liber: Zagreb, Croatia, 1982. [Google Scholar]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Moore, A.; Ankney, E.; Swor, K.; Poudel, A.; Satyal, P.; Setzer, W.N. Leaf essential oil compositions and enantiomeric distributions of monoterpenoids in Pinus species: Pinus albicaulis, Pinus flexilis, Pinus lambertiana, Pinus monticola, and Pinus sabiniana. Molecules 2025, 30, 244. [Google Scholar] [CrossRef]
- Roussis, V.; Petrakis, P.V.; Ortiz, A.; Mazomenos, B.E. Volatile constituents of needles of five Pinus species grown in Greece. Phytochemistry 1995, 39, 357–361. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.T.; Wang, J.; Meng, Q.; Zhang, H.; Yao, L.; Zhang, Y.C.; Dong, A.J.; Ma, Y.; Wang, Z.Y.; et al. Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the Southwest region of China. J. Med. Plant Res. 2010, 4, 1668–1672. [Google Scholar]
- Liu, Z.; Fan, W.; Xie, Q. Chemical composition, total phenolic content, and antioxidant activity of the essential oils extracted from the needle of ten Pinus taxa. J. Chem. 2022, 2022, 7440906. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z. Comparative analysis of essential oil components of two Pinus species from Taibai Mountain in China. Nat. Prod. Commun. 2010, 5, 1295–1298. [Google Scholar] [CrossRef]
- Dormont, L.; Roques, A.; Malosse, C. Cone and foliage volatiles emitted by Pinus cembra and some related conifer species. Phytochemistry 1998, 49, 1269–1277. [Google Scholar] [CrossRef]
- Thai, T.H.; Hien, N.T.; Ha, C.T.T.; Loc, P.K.; Dai, D.N.; Ogunwande, I.A. Chemical constituents of essential oils from three Vietnamese species of Pinus. Bol. Latinoam. Caribe Plantas Med. Aromat. 2018, 17, 53–60. Available online: www.blacpma.usach.cl (accessed on 5 March 2025).
- Petrakis, P.V.; Tsitsimpikou, C.; Tzakou, O.; Couladis, M.; Vagias, C.; Roussis, V. Needle volatiles from five Pinus species growing in Greece. Flavour. Fragr. J. 2001, 16, 249–252. [Google Scholar] [CrossRef]
- Zacher, I. A Morphological and Volatile Terpene Analysis of Pinus balfouriana to Test for the Mountain Island Effect in the Klamath Mountains. Master’s Thesis, The Faculty of Humboldt State University, Arcata, CA, USA, 2015; pp. 1–48. [Google Scholar]
- Lahlou, M. Composition and molluscidial properties of essential oils of five Moroccan Pinaceae. Pharm. Biol. 2003, 41, 207–210. [Google Scholar]
- Michelozzi, M.; Tognetti, R.; Maggino, F.; Radicati, M. Seasonal variations in monoterpene profiles and ecophysiological traits in Mediterranean pine species of group “halepensis”. iForest 2008, 1, 65–74. [Google Scholar] [CrossRef]
- Ustun, O.; Sezer Senol, F.; Kurkcuoglub, M.; Erdogan Orhan, I.; Kartal, M.; Husnu Can Baser, K. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Koutsaviti, K.; Giatropoulos, A.; Pitarokili, D.; Papachristos, D.; Michaelakis, A.; Tzakou, O. Greek Pinus essential oils: Larvicidal activity and repellency against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 583–592. [Google Scholar] [CrossRef]
- Hanana, M.; Bejia, A.; Amri, I.; Gargouri, S.; Jamoussi, B.; Hamrouni, L. Activités biologiques des huiles essentielles de pins. J. New Sci. 2014, 4, 18–32. Available online: http://www.jnsciences.org (accessed on 5 March 2025). (In French).
- Karrat, L.; Nayal, R.; Abajy, M.Y. Chemical composition of Pinus brutia ten essential oil and its in vitro anti-inflammatory activity. Int. Res. J. Pure Appl. Chem. 2020, 21, 112–121. [Google Scholar] [CrossRef]
- Ghaffari, T.; Kafil, H.S.; Asnaashari, S.; Farajnia, S.; Delazar, A.; Baek, S.C.; Hamishehkar, H.; Kim, K.H. Chemical composition and antimicrobial activity of essential oils from the aerial parts of Pinus eldarica grown in Northwestern Iran. Molecules 2019, 24, 3203. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, H.S. Volatile components of essential oils extracted from Pinus species. J. Essent. Oil Bear. Plants 2012, 15, 750–754. [Google Scholar] [CrossRef]
- Ji, W.; Ji, X. Comparative analysis of volatile terpenes and terpenoids in the leaves of Pinus species—A potentially abundant renewable resource. Molecules 2021, 26, 5244. [Google Scholar] [CrossRef]
- Pfeifhofer, H.W. Composition of the essential oil of Pinus canariensis Sweet ex Sprengel. Flav. Fragr. J. 2000, 15, 266–270. [Google Scholar] [CrossRef]
- Kamal, R.M.; Sabry, M.M.; El-Halawany, A.M.; Rabie, M.A.; El Sayed, N.S.; Hifnawy, M.S.; Younis, I.Y. GC-MS analysis and the effect of topical application of essential oils of Pinus canariensis C.Sm., Cupressus lusitanica Mill. and Cupressus arizonica Greene aerial parts in imiquimod-induced psoriasis in mice. J. Ethnopharmacol. 2024, 318 Pt B, 116947. [Google Scholar] [CrossRef] [PubMed]
- Kirima, J.M.; Okuta, M.; Omara, T. Chemical composition of essential oils from Pinus caribaea Morelet needles. Fr. Ukr. J. Chem. 2020, 8, 142–148. Available online: https://api.semanticscholar.org/CorpusID:225598804 (accessed on 5 March 2025).
- Xie, J.; Yang, Z.; Feng, Y.; Chen, H.; Hu, L.; Jia, J.; Wu, D. Optimization of extraction process of pine needle essential oil by response surface methodology and its chemical composition analysis. BioResources 2022, 17, 5890–5904. Available online: https://ojs.bioresources.com/index.php/BRJ/article/view/22051 (accessed on 5 March 2025).
- Apetrei, C.L.; Șpac, A.F.; Brebu, M.; Tuchilus, C.; Miron, A. Composition, and antioxidant and antimicrobial activities of the essential oils of a full-grown Pinus cembra L. tree from the Calimani Mountains (Romania). J. Serb. Chem. Soc. 2013, 78, 27–37. [Google Scholar] [CrossRef]
- Nikolić, B.; Todosijević, M.; Ratknić, M.; Đorđević, I.; Stanković, J.; Cvetković, M.; Marin, P.D.; Tešević, V. Terpenes and n-alkanes in needles of Pinus cembra. Nat. Prod. Commun. 2018, 13, 1035–1037. [Google Scholar] [CrossRef]
- Chizzola, R.; Billiani, F.; Singer, S.; Novak, J. Diversity of essential oils and the respective hydrolates obtained from three Pinus cembra populations in the Austrian Alps. Appl. Sci. 2021, 11, 5686. [Google Scholar] [CrossRef]
- Lis, A.; Kalinowska, A.; Krajewska, A.; Mellor, K. Chemical composition of the essential oils from different morphological parts of Pinus cembra L. Chem. Biodivers. 2017, 14, e1600345. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.H.; Hien, N.T.; Diep, L.N.; Paoli, M.; Casanova, J.; Tomi, F. Chemical composition of needle, cone, and branch oils from Vietnamese Pinus cernua. Nat. Prod. Commun. 2019, 14, 1934578X19850992. [Google Scholar] [CrossRef]
- Ankney, E.; Swor, K.; Satyal, P.; Setzer, W.N. Essential oil compositions of Pinus species (P. contorta ssp. contorta, P. ponderosa var. ponderosa, and P. flexilis); Enantiomeric distribution of terpenoids in Pinus species. Molecules 2022, 27, 5658. [Google Scholar] [CrossRef]
- Swor, K.; Satyal, P.; Poudel, A.; Setzer, W.N. Gymnosperms of Idaho: Chemical compositions and enantiomeric distributions of essential oils of Abies lasiocarpa, Picea engelmannii, Pinus contorta, Pseudotsuga menziesii, and Thuja plicata. Molecules 2023, 28, 2477. [Google Scholar] [CrossRef]
- Sakhno, T.M.; Plugatar, Y.V.; Shevchuk, O.M.; Feskov, S.A.; Bulavin, I.V. Component composition of essential oil in the North American Pinus L. species introduced to the Southern Coast of Crimea. Proc. Appl. Bot. Genet. Breed. 2021, 182, 44–53. [Google Scholar] [CrossRef]
- Idžojtić, M. Sastav eteričnih ulja iz iglica Pinus sylvestris L., P. nigra Arnold, P. densiflora Sieb. et Zucc. i P. thunbergiana Franco. Šumar. List 2000, 5–6, 263–270. (In Croatian) [Google Scholar]
- Hong, E.J.; Na, K.J.; Choi, I.G.; Choi, K.C.; Jeung, E.B. Antibacterial and antifungal effects of essential oils from coniferous trees. Biol. Pharm. Bull. 2004, 27, 863–866. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cho, B.J.; Ko, M.S.; Jung, J.M.; Kim, H.R.; Song, H.S.; Lee, J.Y.; Sim, S.S.; Kim, C.J. Anti-oxidant and anti-aging activities of essential oils of Pinus densiflora needles and twigs. Yakhak Hoeji 2010, 54, 215–225. [Google Scholar]
- Park, J.S.; Lee, G.H. Volatile compounds and antimicrobial and antioxidant activities of the essential oils of the needles of Pinus densiflora and Pinus thunbergii. J. Sci. Food Agric. 2011, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, B.; Yun, K.W. Comparison of chemical composition and antimicrobial activity of essential oils from three Pinus species. Ind. Crop. Prod. 2013, 44, 323–329. [Google Scholar] [CrossRef]
- Deo, L.P.; Aguiar, C.G.; Oliveira, C.D.; Garcia, K.J.; Cardoso, M.d.G. Extraction and characterization of essential oils from fresh and dry leaves of Pinus elliottii. Eclética Química 2024, 49, e1531. [Google Scholar] [CrossRef]
- Tran, H.T.; Nguyen, T.H.; Trinh, X.T.; Nguyen, Q.H.; Paoli, M.; Bighelli, A.; Casanova, J. Chemical composition of the needle and cone essential oils of (Pinaceae) from Vietnam. Acad. J. Biol. 2023, 45, 127–135. [Google Scholar] [CrossRef]
- Kilic, O.; Kocak, A. Essential oil composition of six Pinus L. taxa (Pinaceae) from Canada and their chemotaxonomy. J. Agric. Sci. Technol. B 2014, 4, 67–73. Available online: https://api.semanticscholar.org/CorpusID:82029474 (accessed on 5 March 2025).
- Dob, T.; Berramdane, T.; Chelgoum, C. Chemical composition of essential oil of Pinus halepensis Miller growing in Algeria. C. R. Chim. 2005, 8, 1939–1945. [Google Scholar] [CrossRef]
- Mohareb, A.S.O.; Kherallah, I.E.A.; Badawy, M.E.I.; Salem, M.Z.M.; Faraj, H.A.Y. Chemical composition and antibacterial activity of essential oils isolated from leaves of different woody trees grown in Al-Jabel Al-Akhdar Region, Libya. Alex. Sci. Exch. J. 2016, 37, 358–371. [Google Scholar]
- Nam, A.M.; Tomi, F.; Gibernau, M.; Casanova, J.; Bighelli, A. Composition and chemical variability of the needle oil from Pinus halepensis growing in Corsica. Chem. Biodivers. 2016, 13, 380–386. [Google Scholar] [CrossRef]
- Djerrad, Z.; Djouahri, A.; Kadik, L. Variability of Pinus halepensis Mill. essential oils and their antioxidant activities depending on the stage of growth during vegetative cycle. Chem. Biodivers. 2017, 14, 201600340. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Abrini, J.; Dakka, N.; Bakri, Y. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac. J. Trop. Med. 2019, 12, 117–122. [Google Scholar] [CrossRef]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef] [PubMed]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Stojanović-Radić, Z.Z.; Mihajilov-Krstev, T.; Jovanović, N.M.; Nikolić, B.M.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Essential oils of Pinus halepensis and P. heldreichii: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crop. Prod. 2019, 140, 111702. [Google Scholar] [CrossRef]
- Khouja, M.; Elaissi, A.; Ghazghazi, H.; Boussaid, M.; Khouja, M.L.; Khaldi, A.; Messaoud, C. Variation of essential oil composition, antioxidant and anticholinesterase activities between Pinus halepensis Mill. plant organs. J. Essent. Oil Bear. Plants 2020, 23, 1450–1462. [Google Scholar] [CrossRef]
- Ameur, E.; Sarra, M.E.; Takoua, K.; Mariem, K.; Nabil, A.; Lynen, F.; Larbi, K.M. Chemical composition of five Tunisian Pinus species’ essential oils and effect of their blends on Otitis infection. Ind. Crop. Prod. 2022, 180, 114688. [Google Scholar] [CrossRef]
- Hamzaoui, E.; Zallez, O.B.Y.; Buñay, J.; Leremboure, M.; Argui, H.; Baron, S.; Said, H.; Lobaccaro, J.A.; Akriche, S. Comparative study of essential oils from Tunisian Pinus Halepensis Mill. by hydrodistillation and microwave-assisted processes: Chemical composition and antioxidant and cytotoxic potential against prostate and cervical cancer cells. ACS Omega 2024, 9, 34128–34139. [Google Scholar] [CrossRef]
- Hmamouchi, M.; Hamamouchi, J.; Zouhdi, M.; Bessiere, J.M. Chemical and antimicrobial properties of essential oils of five Moroccan Pinaceae. J. Essent. Oil Res. 2001, 13, 298–302. [Google Scholar] [CrossRef]
- Elkady, W.M.; Gonaid, M.H.; Yousif, M.F.; El-Sayed, M.; Omar, H.A.N. Impact of altitudinal variation on the phytochemical profile, anthelmintic and antimicrobial activity of two Pinus species. Molecules 2021, 26, 3170. [Google Scholar] [CrossRef]
- Gad, H.; Al-Sayed, E.; Ayoub, I. Phytochemical discrimination of Pinus species based on GC-MS and ATR-IR analyses and their impact on Helicobacter pylori. Phytochem. Anal. 2021, 32, 820–835. [Google Scholar] [CrossRef]
- Hjouji, K.; Atmni, I.; Mehdaoui, I.; Ainane, A.; Berrada, S.; Rais, Z.; Taleb, M.; Ainane, T. Essential oil of Aleppo pine needles: Antioxidant and antibacterial activities. Pharmacologyonline 2021, 2, 556–565. [Google Scholar]
- Ezzine, O.; Mannai, Y.; Ben Jamâa, M.L.; Hamrouni, L. Evaluation of the larvicidal activity of essential oil from needles of Pinus halepensis against defoliating caterpillars of Quercus spp. in North Africa. J. Chem. Eng. Theor. Appl. Chem. 2022, 79, 539–545. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Gargouri, S.; Fezzani, T.; Jamoussi, B. Chemical composition, physico-chemical properties, antifungal and herbicidal activities of Pinus halepensis Miller essential oils. Biol. Agric. Hortic. 2013, 29, 91–106. [Google Scholar] [CrossRef]
- Djerrad, Z.; Kadik, L.; Djouahri, A. Chemical variability and antioxidant activities among Pinus halepensis Mill. essential oils provenances, depending on geographic variation and environmental conditions. Ind. Crops Prod. 2015, 74, 440–449. [Google Scholar] [CrossRef]
- Macchioni, F.; Cioni, P.L.; Flamini, G.; Morelli, I.; Maccioni, S.; Ansaldi, M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central Italy. Flavour. Fragr. J. 2003, 18, 139–143. [Google Scholar] [CrossRef]
- Fekih, N.; Allali, H.; Merghache, S.; Chaib, F.; Merghache, D.; Dib, M.A.; Djabou, N.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition and antibacterial activity of Pinus halepensis Miller growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Mirković, S.; Tadić, V.; Milenković, M.T.; Ušjak, D.; Racić, G.; Bojović, D.; Žugić, A. Antimicrobial activities of essential oils of different Pinus species from Bosnia and Herzegovina. Pharmaceutics 2024, 16, 1331. [Google Scholar] [CrossRef]
- Abi-Ayad, M.; Abi-Ayad, F.Z.; Lazzouni, H.A.; Rebiahi, S.A. Antibacterial activity of Pinus halepensis essential oil from Algeria (Tlemcen). J. Nat. Prod. Plant Resour. 2011, 1, 33–36. Available online: https://api.semanticscholar.org/CorpusID:212480582 (accessed on 5 March 2025).
- Aloui, F.; Baraket, M.; Jedidi, S.; Hosni, K.; Bouchnak, R.; Salhi, O.; Jdaidi, N.; Selmi, H.; Ghazghazi, H.; Khadhri, A.; et al. Chemical composition, anti-radical and antibacterial activities of essential oils from needles of Pinus halepensis Mill., P. pinaster Aiton., and P. pinea L. J. Essent. Oil Bear. Plants 2021, 24, 453–460. [Google Scholar] [CrossRef]
- Kurose, K.; Okamura, D.; Yatagai, M. Composition of the essential oils from the leaves of nine Pinus species and the cones of three of Pinus species. Flavour. Fragr. J. 2007, 22, 10–20. [Google Scholar] [CrossRef]
- Simić, N.; Palić, R.; Andelković, S.; Vajs, V.; Milosavljević, S. Essential oil of Pinus heldreichii, needles. J. Essent. Oil Res. 1996, 8, 1–5. [Google Scholar]
- Bojović, S.; Nikolić, B.; Ristić, M.; Orlović, S.; Veselinović, M.; Rakonjac, L.; Dražić, D. Variability in chemical composition and abundance of the rare Tertiary relict Pinus heldreichii in Serbia. Chem. Biodivers. 2011, 8, 1754–1765. [Google Scholar] [CrossRef]
- Nikolić, B.; Ristić, M.; Bojović, S.; Krivošej, Z.; Matevski, V.; Marin, P.D. Population variability of essential oils of Pinus heldreichii from the Scardo-Pindic mountains Ošljak and Galičica. Chem. Biodivers. 2015, 12, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.; Ristić, M.; Janaćković, P.; Novaković, J.; Šarac, Z.; Rajčević, N.; Marin, P.D. Essential oil composition of one-year-old Bosnian pine needles. In Proceedings of the International Conference Reforestation Challenges, Belgrade, Serbia, 3–6 June 2015; pp. 282–287, ISBN 978-86-918861-0-3. [Google Scholar]
- Mitić, Z.S.; Jovanović, S.Č.; Zlatković, B.K.; Nikolić, B.M.; Stojanović, G.S.; Marin, P.D. Needle terpenes as chemotaxonomic markers in Pinus: Subsections Pinus and Pinaster. Chem. Biodivers. 2017, 14, e1600453. [Google Scholar] [CrossRef]
- Naydenov, K.; Tremblay, F.; Bergeron, Y.; Alexandrov, A.; Fenton, N. Dissimilar patterns of Pinus heldreichii Christ. populations in Bulgaria revealed by chloroplast microsatellites and terpenes analysis. Biochem. Syst. Ecol. 2005, 33, 133–148. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Nikolić, B.M.; Ristić, M.S.; Tešević, V.V.; Bojović, S.R.; Marin, P.D. Terpenes as useful markers in differentiation of natural populations of relict pines Pinus heldreichii, P. nigra, and P. peuce. Chem. Biodivers. 2017, 14, e1700093. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, E.; Pentimalli, D.; et al. Chemical composition, antioxidant and antimicrobial activities of essential oils of different Pinus species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Maric, S.; Jukic, M.; Katalinic, V.; Milos, M. Comparison of chemical composition and free radical scavenging ability of glycosidically bound and free volatiles from Bosnian Pine (Pinus heldreichii Christ. var. leucodermis). Molecules 2007, 12, 283–289. [Google Scholar] [CrossRef]
- Adams, R.P.; Tashev, A.N.; Tashev, N. The leaf volatile terpenoids of Pinus heldreichii Christ from Bulgaria and comparisons with Greece and Montenegro-Serbia oils, and P. leucodermis oil, Italy. Phytologia 2020, 102, 124–130. Available online: https://www.phytologia.org/uploads/2/3/4/2/23422706/102_3_124-130adamstashevpinusheldreichii7-8-20.pdf (accessed on 5 March 2025).
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Z.; Li, Z. Chemical composition and antioxidant activity of essential oil of six Pinus taxa native to China. Molecules 2015, 20, 9380–9392. [Google Scholar] [CrossRef]
- Adams, R.P.; Wright, J.W. Alkanes and terpenes in wood and leaves of Pinus jeffreyi and P. sabiniana. J. Essent. Oil Res. 2012, 24, 435–440. [Google Scholar] [CrossRef]
- Thai, T.H.; Cuong, N.; Hien, N.T.; Paoli, M.; Casanova, J.; Tomi, F. Chemical composition of needle and twig essential oils from Pinus krempfii Lecomte, an endemic species to Vietnam. J. Essent. Oil Res. 2020, 33, 63–68. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Gallucci, M.N.; Luna, A.; Gonzalez, S.B.; Guerra, P.E.; Zunino, M.P. Composition, antifungal and antifumonisin activity of Pinus wallichiana, Pinus monticola and Pinus strobus essential oils from Patagonia Argentina. J. Essent. Oil Bear. Plants 2016, 19, 1769–1775. [Google Scholar] [CrossRef]
- Tsitsimpikou, C.; Petrakis, P.V.; Ortiz, A.; Harvala, C.; Roussis, V. Volatile needle terpenoids of six Pinus species. J. Essent. Oil Res. 2001, 13, 174–178. [Google Scholar] [CrossRef]
- Celiński, K.; Bonikowski, R.; Wojnicka-Półtorak, A.; Chudzińska, E.; Maliński, T. Volatiles as chemosystematic markers for distinguishing closely related species within the Pinus mugo complex. Chem. Biodivers. 2015, 12, 1208–1213. [Google Scholar] [CrossRef]
- Hajdari, A.; Mustafa, B.; Ahmeti, G.; Pulaj, B.; Lukas, B.; Ibraliu, A.; Stefkov, G.; Quave, C.L.; Novak, J. Essential oil composition variability among natural populations of Pinus mugo Turra in Kosovo. SpringerPlus 2015, 4, 828. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, S.Č.; Zlatković, B.K.; Milanovici, S.J.; Nikolić, B.M.; Petrović, G.M.; Stojanović, G.S.; Marin, P.D. Variation of needle volatiles in native populations of Pinus mugo—Evidence from multivariate statistical analysis. Plant Biosyst. 2021, 155, 700–710. [Google Scholar] [CrossRef]
- Lis, A.; Lukas, M.; Mellor, K. Comparison of chemical composition of the essential oils from different botanical organs of Pinus mugo growing in Poland. Chem. Biodivers. 2019, 16, e1900397. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Vittori, S.; Papa, F.; Maggi, F.; Di Cecco, M.; Ciaschetti, G.; Bruno, M.; Rosselli, S.; Bianco, A. Secondary metabolites from Pinus mugo TURRA subsp. mugo growing in the Majella National Park (Central Apennines, Italy). Chem. Biodivers. 2013, 10, 2091–2100. [Google Scholar] [CrossRef]
- Bojović, S.; Jurc, M.; Ristić, M.S.; Popović, Z.; Matić, R.; Vidaković, V.; Stefanović, M.; Jurc, D. Essential-oil variability in natural populations of Pinus mugo Turra from the Julian Alps. Chem. Biodivers. 2016, 13, 181–187. [Google Scholar] [CrossRef]
- Adams, R.P.; Tashev, A.N. Composition of the leaf volatile terpenoids of Pinus mugo Turra from Bulgaria compared with oils from other regions. Phytologia 2019, 101, 74–80. Available online: https://www.phytologia.org/uploads/2/3/4/2/23422706/101_1_74-80adams_and_tashevpinus_mugo_bulgaria.pdf (accessed on 5 March 2025).
- Garzoli, S.; Masci, V.L.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and vapor phase of four conifer-derived essential oils: Comparison of chemical compositions and antimicrobial and antioxidant properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Vaglia, V.; Iriti, M.; Vitalini, S. Vapor and liquid phase profiles of essential oils from Abies, Picea and Pinus species and their phytotoxic interactions with weed growth in pre- and post-emergence conditions. Plants 2023, 12, 1172. [Google Scholar] [CrossRef] [PubMed]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Mihajilov-Krstev, T.; Stojanović-Radić, Z.Z.; Cvetković, V.J.; Mitrović, T.L.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crop. Prod. 2018, 111, 55–62. [Google Scholar] [CrossRef]
- Bonikowski, R.; Celiński, K.; Wojnicka-Półtorak, A.; Maliński, T. Composition of essential oils isolated from the needles of Pinus uncinata and P. uliginosa grown in Poland. Nat. Prod. Commun. 2015, 10, 371–373. [Google Scholar] [CrossRef]
- Rafii, Z.A.; Dodd, R.S.; Zavarin, E. Genetic diversity in foliar terpenoids among natural populations of European black pine. Biochem. Syst. Ecol. 1996, 24, 325–339. [Google Scholar] [CrossRef]
- Bojović, S.; Jurc, M.; Drazic, D.; Pavlovic, P.; Mitrovic, M.; Djurdjevic, L.; Dodd, R.S.; Afzal-Rafii, Z.; Barbero, M. Origin identification of Pinus nigra populations in southwestern Europe using terpene composition variations. Trees 2005, 19, 357–368. [Google Scholar] [CrossRef]
- Naydenov, K.D.; Tremblay, F.M.; Fenton, N.J.; Alexandrov, A. Structure of Pinus nigra Arn. populations in Bulgaria revealed by chloroplast microsatellites and terpene analysis: Provenance tests. Biochem. Syst. Ecol. 2006, 34, 562–574. [Google Scholar] [CrossRef]
- Sezik, E.; Üstün, O.; Demirci, B.; Başer, K.H.C. Composition of the essential oils of Pinus nigra Arnold from Turkey. Turk. J. Chem. 2010, 34, 313–325. [Google Scholar] [CrossRef]
- Šarac, Z.; Bojović, S.; Nikolić, B.; Tešević, V.; Đorđević, I.; Marin, P.D. Chemotaxonomic significance of the terpene composition in natural populations of Pinus nigra J.F.ARNOLD from Serbia. Chem. Biodivers. 2013, 10, 1507–1520. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Nikolić, B.M.; Stojković, J.P.; Jevtović, S.Č.; Stojanović, G.S.; Zlatković, B.K.; Marin, P.D. Morpho-anatomical characteristics and volatile profiles of Pinus nigra J.F.Arnold from the Balkan Peninsula and Southern Carpathians. Forests 2024, 15, 739. [Google Scholar] [CrossRef]
- Politeo, O.; Botica, I.; Bilusic, T.; Jukic, M.; Carev, I.; Burcul, F.; Milos, M. Chemical composition and evaluation of acetylcholinesterase inhibition and antioxidant activity of essential oil from Dalmatian endemic species Pinus nigra Arnold ssp. dalmatica (Vis.) Franco. J. Med. Plant Res. 2011, 5, 6590–6596. [Google Scholar] [CrossRef]
- Rezzi, S.; Bighelli, A.; Mouillot, D.; Casanova, J. Composition and chemical variability of the needle essential oil of Pinus nigra ssp. laricio from Corsica. Flavour. Fragr. J. 2001, 16, 379–383. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Gorunović, M.S. Chemotaxonomy of pines native to Balkans. Part 2. Illiryan black pine Pinus nigra Arnold ssp. nigra, Pinaceae according to location, plant part and age of specimens. Pharmazie 1995, 50, 281–283. [Google Scholar] [CrossRef]
- Supuka, J.; Berta, F.; Chladná, A. The influence of the urban environment on the composition of terpenes in the needles of Black Pine (Pinus nigra Arnold). Trees 1997, 11, 176–182. [Google Scholar] [CrossRef]
- Amri, I.; Hanana, M.; Jamoussi, B.; Hamrouni, L. Essential oils of Pinus nigra J.F. Arnold subsp. laricio Maire: Chemical composition and study of their herbicidal potential. Arab. J. Chem. 2017, 10, S3877–S3882. [Google Scholar] [CrossRef]
- Fkiri, S.; Mezni, F.; Rigane, G.; Ben Salem, R.; Ghazghazi, H.; Khouja, M.L.; Nasr, Z.; Khaldi, A. Chemotaxonomic study of four subspecies of Pinus nigra Arn. grown in common garden based on essential oil composition. J. Food Qual. 2021, 2021, 5533531. [Google Scholar] [CrossRef]
- Adjaoud, A.; Laouer, H.; Braca, A.; Cioni, P.; Moussi, K.; Berboucha-Rahmani, M.; Abbaci, H.; Falconieri, D. Chemical composition, antioxidant and insecticidal activities of a new essential oil chemotype of Pinus nigra ssp. mauritanica (Pinaceae), Northern Algeria. Plant Biosyst. 2022, 156, 358–369. [Google Scholar] [CrossRef]
- Zanoni, T.A.; Adams, R.P.; Miller, E.J. Essential Oils of Plants from Hispaniola West Indies 2. The Volatile Leaf Oil of Pinus occidentalis Pinaceae. Moscoso Contrib. Científicas Jardín Botánico Naci. Dr. Rafael M. Moscoso 1990, 6, 219–222. Available online: https://www.biodiversitylibrary.org/part/148625 (accessed on 5 March 2025).
- Amri, I.; Lamia, H.; Gargouri, S.; Hanana, M.; Mahfoudhi, M.; Fezzani, T.; Ezzeddine, F.; Jamoussi, B. Chemical composition and biological activities of essential oils of Pinus patula. Nat. Prod. Commun. 2011, 6, 1531–1536. [Google Scholar] [CrossRef]
- Simić, N.; Palić, R.; Anđelković, S.; Kitić, D.; Gašić, M.J. Essential oil of Pinus peuce needles. Facta Univ. Ser. Phys. Chem. Technol. 1995, 1, 188–192. [Google Scholar]
- Koukos, P.K.; Papadopoulou, K.I.; Patiaka, D.T.; Papagiannopoulos, A.D. Chemical composition of essential oils from needles and twigs of Balkan pine (Pinus peuce Grisebach) grown in Northern Greece. J. Agric. Food Chem. 2000, 48, 1266–1268. [Google Scholar] [CrossRef]
- Nikolić, B.; Ristić, M.; Bojović, S.; Marin, P.D. Variability of the needle essential oils of Pinus peuce from different populations in Montenegro and Serbia. Chem. Biodivers. 2008, 5, 1377–1388. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Kulevanova, S. Essential oils composition of Pinus peuce Griseb. (Pinaceae) growing on Pelister Mtn., Republic of Macedonia. Maced. Pharm. Bull. 2010, 56, 13–22. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Trajkovska-Dokic, E.; Kaftandzieva, A.; Kulevanova, S. Antimicrobial activity of needle essential oil of Pinus peuce Griseb. (Pinaceae) from Macedonian flora. Maced. Pharm. Bull. 2011, 57, 25–36. [Google Scholar] [CrossRef]
- Nikolić, B.; Ristić, M.; Bojović, S.; Matevski, V.; Krivošej, Z.; Marin, P.D. Essential-oil composition of the needles collected from natural populations of Macedonian pine (Pinus peuce Griseb.) from the Scardo-Pindic mountain system. Chem. Biodivers. 2014, 11, 934–948. [Google Scholar] [CrossRef]
- Hajdari, A.; Mustafa, B.; Nebija, D.; Selimi, H.; Veselaj, Z.; Breznica, P.; Quave, C.L.; Novak, J. Essential oil composition of Pinus peuce Griseb. needles and twigs from two National Parks of Kosovo. Sci. World J. 2016, 2016, 5393079. [Google Scholar] [CrossRef]
- Sana, K.; Marwad, K.; Ismail, A.; Yassine, M.; Dhaouadi, F.; Samia, G.; Mohsen, H.; Bassem, J.; Lamia, H. Phytochemical studies on essential oils of Pinus pinaster Aiton and evaluation of their biological activities. Arab. J. Med. Aromat. Plants 2022, 8, 75–98. [Google Scholar] [CrossRef]
- Dob, T.; Berramdane, T.; Chelghoum, C. Analysis of essential oil from the needles of Pinus pinaster growing in Algeria. Chem. Nat. Compd. 2005, 41, 545–548. [Google Scholar] [CrossRef]
- Mimoune, N.A.; Mimoune, D.A.; Yataghene, A. Chemical composition and antimicrobial activity of the essential oils of Pinus pinaster. J. Coast. Life Med. 2013, 1, 55–59. [Google Scholar] [CrossRef]
- Tümen, I.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef]
- Ottavioli, J.; Bighelli, A.; Casanova, J. Diterpene-rich needle oil of Pinus pinaster Ait. from Corsica. Flavour. Fragr. J. 2008, 23, 121–125. [Google Scholar] [CrossRef]
- Arrabal, C.; García-Vallejo, M.C.; Cadahia, E.; Cortijo, M.; De Simón, F.; Brígida, M. Characterization of two chemotypes of Pinus pinaster by their terpene and acid patterns in needles. Plant Syst. Evol. 2012, 298, 511–522. [Google Scholar] [CrossRef]
- Fkiri, S.; Ghazghazi, H.; Rigane, G.; Ben Salem, R.; Mezni, F.; Khaldi, A.; Khouja, M.L.; Nasr, Z. Chemical compositions and biological activities essential oil from the needles of North African Pinus pinaster var. Rev. Roum. Chim. 2019, 64, 511–517. [Google Scholar] [CrossRef]
- Nasri, N.; Tlili, N.; Triki, S.; Elfalleh, W.; Chéraif, I.; Khaldi, A. Volatile constituents of Pinus pinea L. needles. J. Essent. Oil Res. 2011, 23, 15–19. [Google Scholar] [CrossRef]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest Sci. 2012, 85, 199–207. Available online: https://api.semanticscholar.org/CorpusID:14384333 (accessed on 5 March 2025). [CrossRef]
- Demirci, F.; Bayramiç, P.; Göger, G.; Demirci, B.; Başer, K.H.C. Characterization and antimicrobial evaluation of the essential oil of Pinus pinea L. from Turkey. Nat. Vol. Essent. Oil 2015, 2, 39–44. [Google Scholar]
- Balaban Uçar, M.; Gönültaş, O. Geographical variation of essential oil of Pinus pinea needles in Turkey. Kastamonu Univ. J. For. Fac. 2022, 22, 78–84. [Google Scholar] [CrossRef]
- Halub, B.; Shakya, A.K.; Elagbar, Z.A.; Naik, R.R. GC-MS Analysis and biological activity of essential oil of fruits, needles and bark of Pinus pinea grown wildly in Jordan. Acta Pol. Pharm. 2019, 76, 825–831. [Google Scholar] [CrossRef]
- Adams, R.P.; Edmunds, G.F., Jr. A re-examination of the volatile leaf oils of Pinus ponderosa Dougl. ex. P. Lawson using ion trap mass spectroscopy. Flavour. Fragr. J. 1989, 4, 19–23. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Mardarowicz, M.; Wiwart, M.; Pobłocka, L.; Dynowska, M. Antifungal activity of the essential oils from some species of the genus Pinus. Z. Naturforsch. C J. Biosci. 2002, 57, 478–482. [Google Scholar] [CrossRef]
- Adams, R.P.; Ferguson, G.M.; Thornburg, D. First comprehensive report on the composition of the leaf volatile terpenoids of Pinus arizonica Engelm. and P. ponderosa var. brachyptera (Engelm.) Lemmon. Phytologia 2015, 97, 45–50. Available online: https://www.phytologia.org/uploads/2/3/4/2/23422706/97145-50adams_et_al_leaf_oils_of_p_arizonica_and_p_pond_brachyptera.pdf (accessed on 5 March 2025).
- Peng, X.; Yang, X.; Gu, H.; Yang, L.; Gao, H. Essential oil extraction from fresh needles of Pinus pumila (Pall.) Regel using a solvent-free microwave-assisted methodology and an evaluation of acetylcholinesterase inhibition activity in vitro compared to that of its main components. Ind. Crop. Prod. 2021, 167, 113549. [Google Scholar] [CrossRef]
- Ekundayo, O. Technical Note: Essential Oils III: Analysis of Table Mountain Pine (Pinus Pungens Lamb) needle oil by Gas Chromatography/Mass Spectrometry (GC/MS). J. Chromatogr. Sci. 1980, 18, 368–369. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Ali, H.M.; Basalah, M.O. Essential oils from wood, bark, and needles of Pinus roxburghii Sarg. from Alexandria, Egypt: Antibacterial and antioxidant activities. BioResources 2014, 9, 7454–7466. [Google Scholar]
- Satyal, P.; Paudel, P.; Raut, J.; Deo, A.; Dosoky, N.S.; Setzer, W.N. Volatile constituents of Pinus roxburghii from Nepal. Pharmacogn. Res. 2013, 5, 43. [Google Scholar] [CrossRef]
- Bhagat, M.; Bandrall, A.; Bashir, M.; Bindu, K. GC–MS analysis of essential oil of Pinus roxburghii Sarg. (Chir pine) needles and evaluation of antibacterial and anti-proliferative properties. Indian J. Nat. Prod. Resour. 2018, 9, 34–38. Available online: https://api.semanticscholar.org/CorpusID:91936003 (accessed on 5 March 2025).
- Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical application of Siberian pine essential oil formulations enhance diabetic wound healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Venskutonis, L.; Vyskupaityte, K.; Plausinatis, R. Composition of essential oils of Pinus sylvestris L. from different locations of Lithuania. J. Essent. Oil Res. 2000, 12, 559–565. [Google Scholar] [CrossRef]
- Manninen, A.M.; Tarhanen, S.; Vuorinen, M.; Kainulaine, P. Comparing the variation of needle and wood terpenoids in Scots pine provenances. J. Chem. Ecol. 2002, 28, 211–228. [Google Scholar] [CrossRef]
- Naydenov, K.D.; Tremblay, F.M.; Alexandrov, A.; Fenton, N.J. Structure of Pinus sylvestris L. populations in Bulgaria revealed by chloroplast microsatellites and terpenes analysis: Provenance tests. Biochem. Syst. Ecol. 2005, 33, 1226–1245. [Google Scholar] [CrossRef]
- Ustun, O.; Sezik, E.; Kurkcuoglu, M.; Baser, K.H.C. Study of the essential oil composition of Pinus sylvestris from Turkey. Chem. Nat. Compd. 2006, 42, 26–31. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic effects of commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris essential oils on weeds, crops, and invasive species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef]
- Csikós, E.; Csekő, K.; Kemény, Á.; Draskóczi, L.; Kereskai, L.; Kocsis, B.; Böszörményi, A.; Helyes, Z.; Horváth, G. Pinus sylvestris L. and Syzygium aromaticum (L.) Merr. & L. M. Perry essential oils inhibit endotoxin-induced airway hyperreactivity despite aggravated inflammatory mechanisms in mice. Molecules 2022, 27, 3868. [Google Scholar] [CrossRef]
- Namshir, J.; Shatar, A.; Khandaa, O.; Tserennadmid, R.; Shiretorova, V.G.; Nguyen, M.C. Antimicrobial, antioxidant and cytotoxic activity on human breast cancer cells of essential oil from Pinus sylvestris var. mongolica needle. Mong. J. Chem. 2020, 21, 19–26. [Google Scholar] [CrossRef]
- Adams, R.P. Comparison of the volatile leaf and wood oils of the subspecies of Pinus torreyana: Two isolated, narrow endemics in California. Phytologia 2013, 95, 188–191. Available online: https://biostor.org/reference/175919 (accessed on 5 March 2025).
- Dar, M.Y.; Shah, W.A.; Mubashir, S.; Rather, M.A. Chromatographic analysis, anti-proliferative and radical scavenging activity of Pinus wallichiana essential oil growing in high altitude areas of Kashmir, India. Phytomedicine 2012, 19, 1228–1233. [Google Scholar] [CrossRef]
- Pagula, F.P.; Baeckström, P. Studies on essential oil-bearing plants from Mozambique: Part II. Volatile leaf oil of needles of Pinus elliottii Engelm. and Pinus taeda L. J. Essent. Oil Res. 2006, 18, 32–34. [Google Scholar] [CrossRef]
- Hamrouni, L.; Hanana, M.; Amri, I.; Romane, A.E.; Gargouri, S.; Jamoussi, B. Allelopathic effects of essential oils of Pinus halepensis Miller: Chemical composition and study of their antifungal and herbicidal activities. Arch. Phytopathol. Pflanzenschutz 2014, 48, 145–158. [Google Scholar] [CrossRef]
- Neverova, O.A.; Tsandekova, O.L.; Domrachev, D.V. Study of the composition of ether oils from pine needles of Pinus sylvestris L. growing in various edaphic conditions of Kuzbass surface coal mines dumps. Global J. Pharmacol. 2014, 8, 415–419. [Google Scholar]
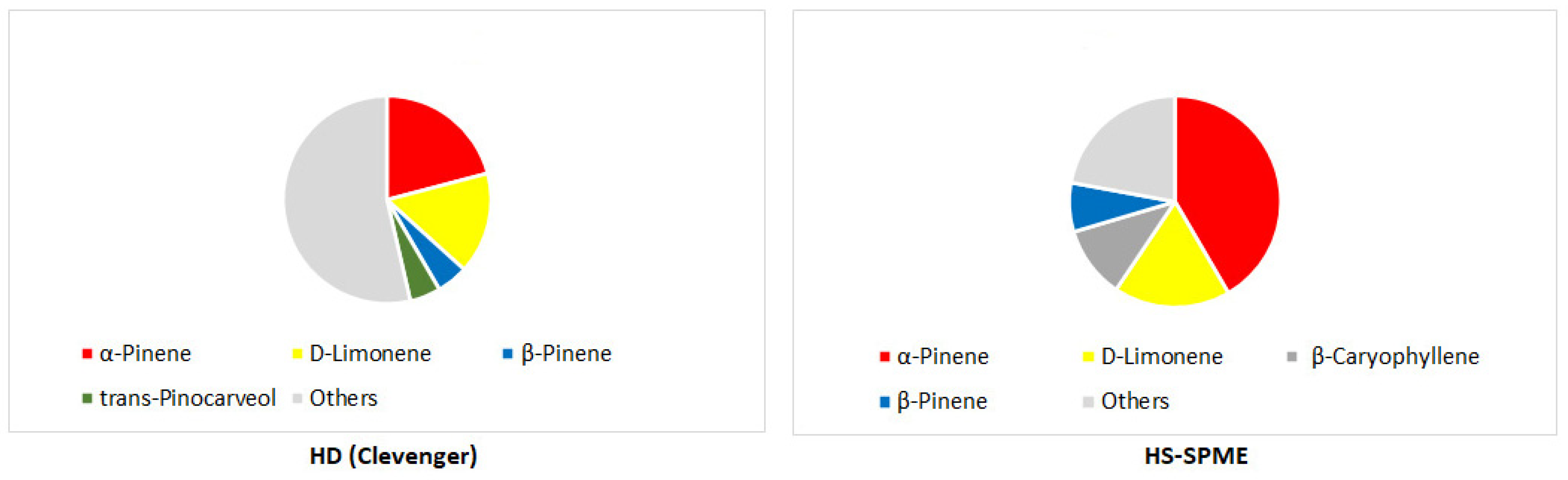




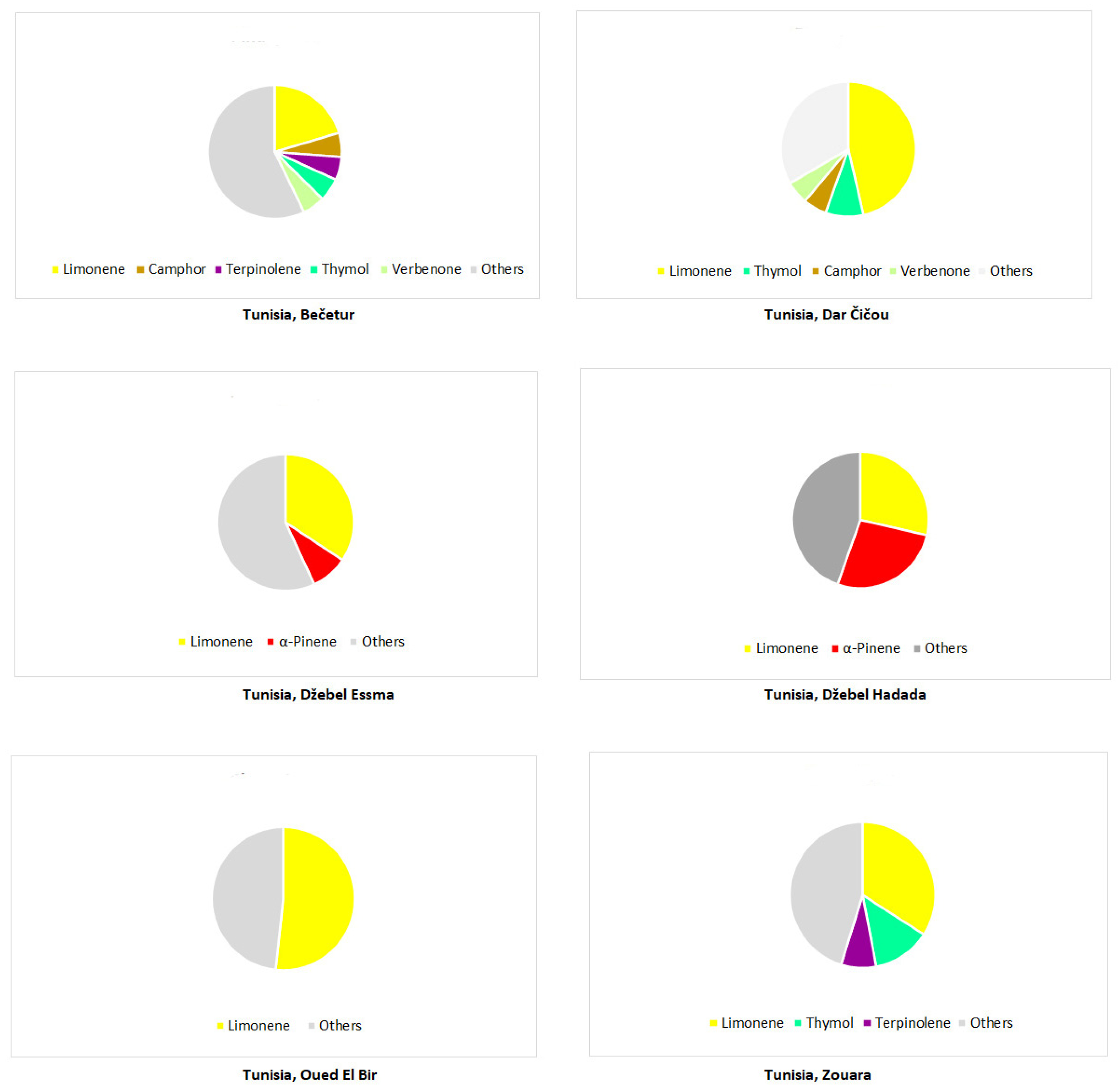
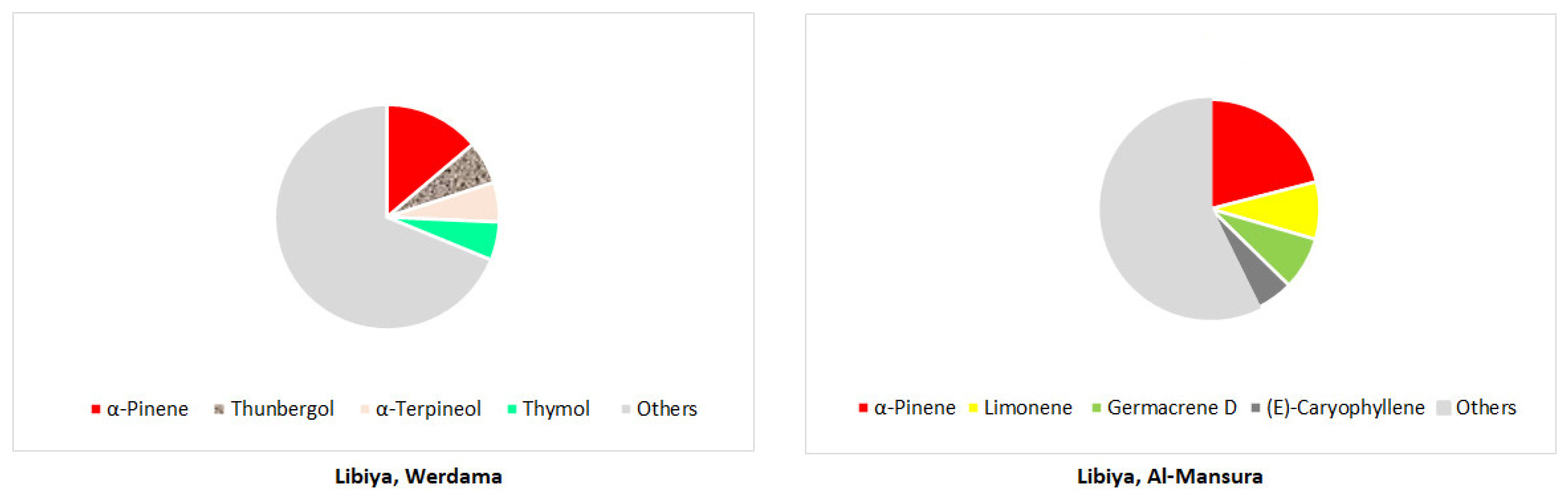


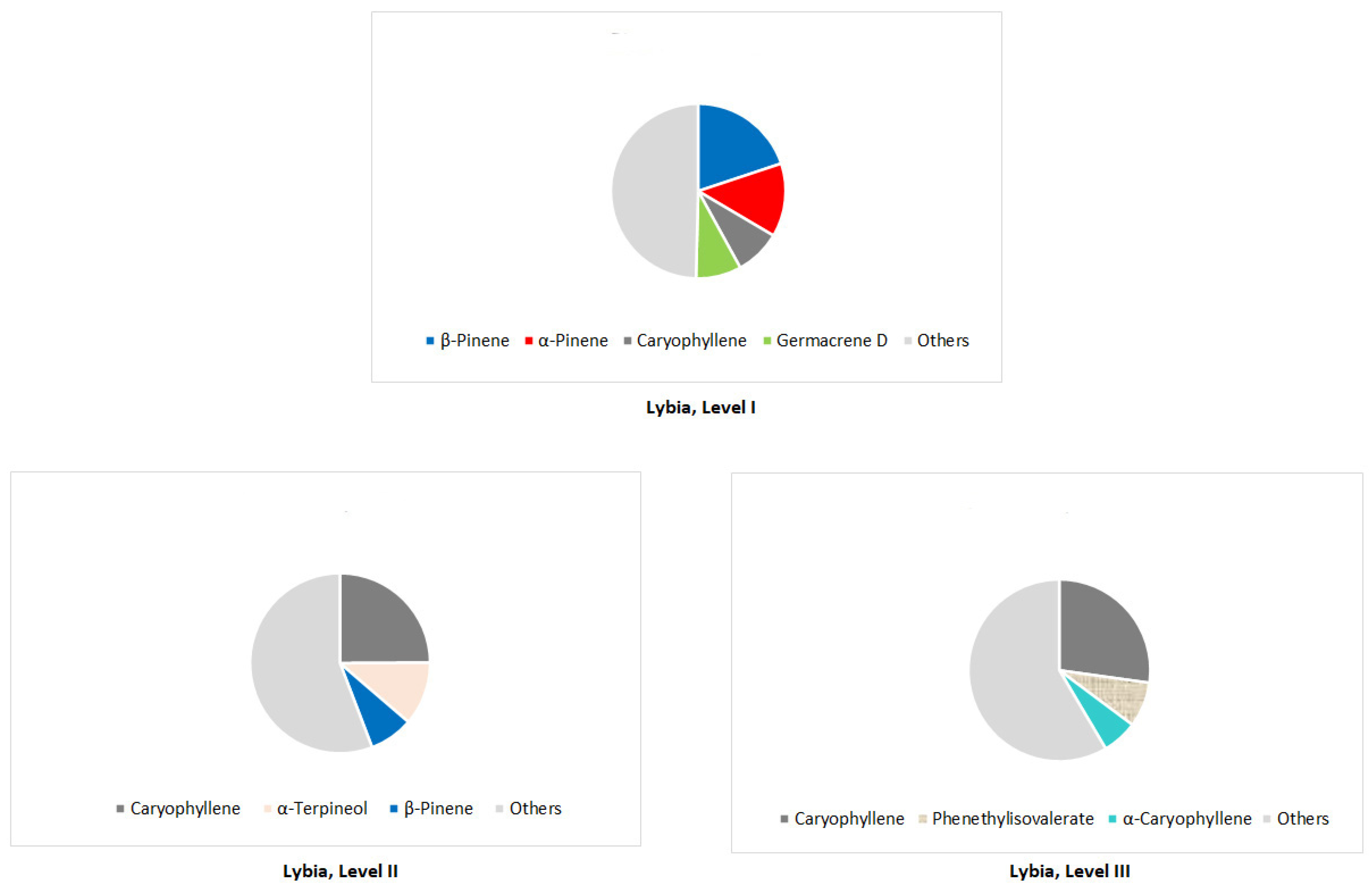

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, B.M.; Ballian, D.; S. Mitić, Z. Diversity of Needle Terpenes Among Pinus Taxa. Forests 2025, 16, 623. https://doi.org/10.3390/f16040623
Nikolić BM, Ballian D, S. Mitić Z. Diversity of Needle Terpenes Among Pinus Taxa. Forests. 2025; 16(4):623. https://doi.org/10.3390/f16040623
Chicago/Turabian StyleNikolić, Biljana M., Dalibor Ballian, and Zorica S. Mitić. 2025. "Diversity of Needle Terpenes Among Pinus Taxa" Forests 16, no. 4: 623. https://doi.org/10.3390/f16040623
APA StyleNikolić, B. M., Ballian, D., & S. Mitić, Z. (2025). Diversity of Needle Terpenes Among Pinus Taxa. Forests, 16(4), 623. https://doi.org/10.3390/f16040623






