A Simulation of a Suitable Habitat for Acer yangbiense and Cinnamomum chago Under Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area and Species Occurrence Data
2.2. Predictive Variables
2.3. Model Optimization and Accuracy Evaluation
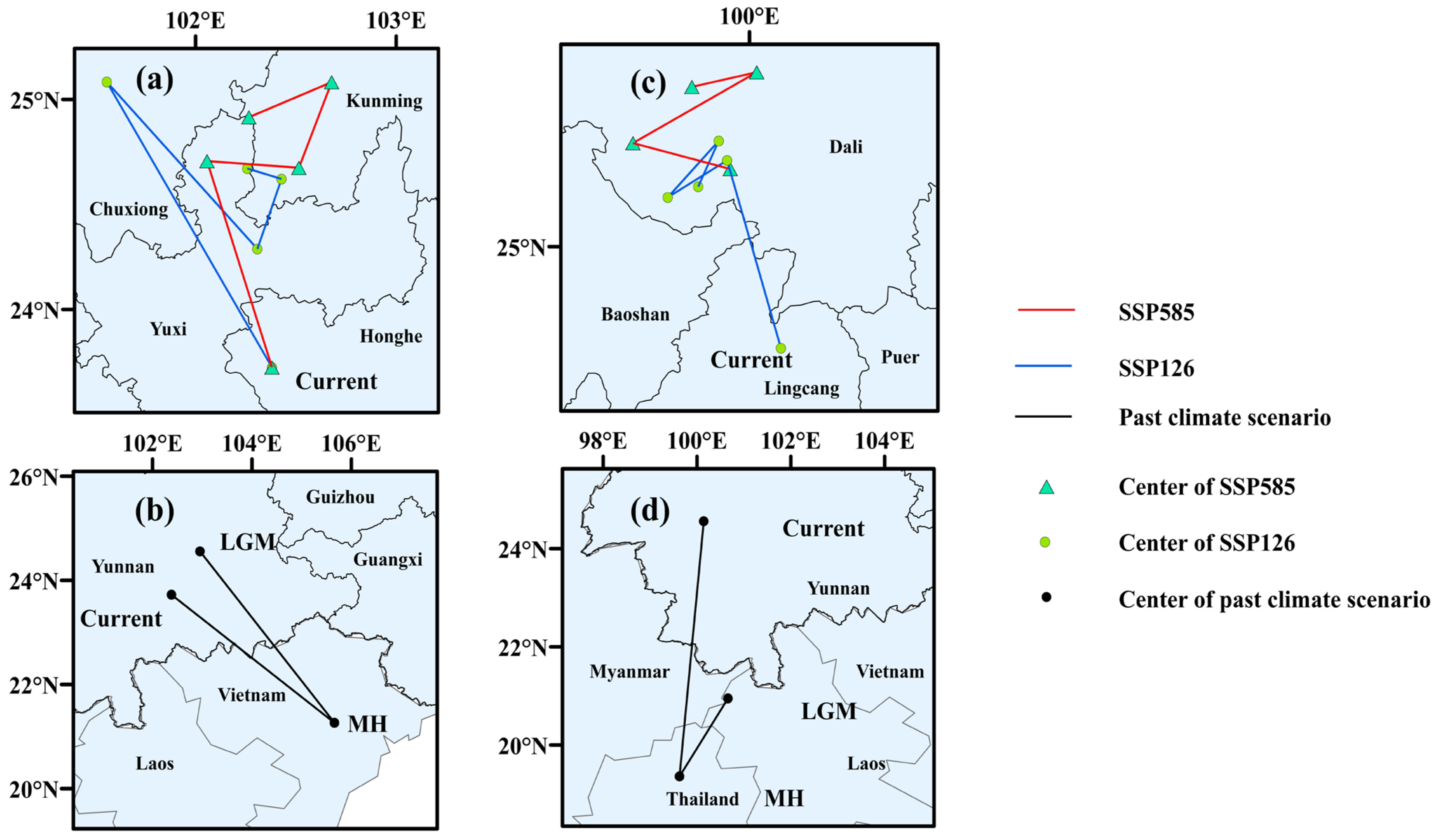
2.4. Suitable Area Division and Centroid Migration
3. Results
3.1. Optimization Outcomes and Model Accuracy Evaluation
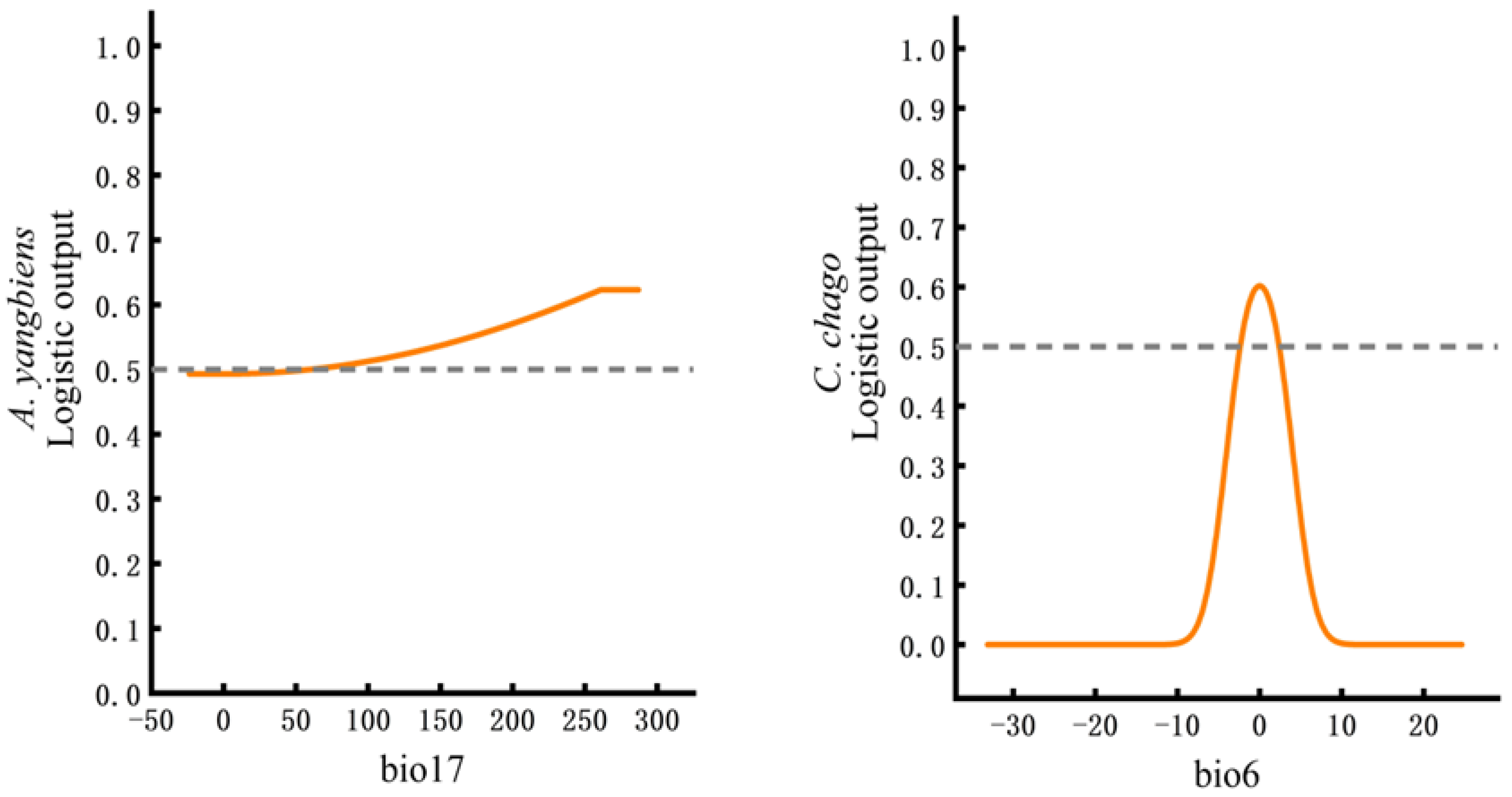
3.2. Key Factors Affecting the Potential Distributions of A. yangbiense and C. chago
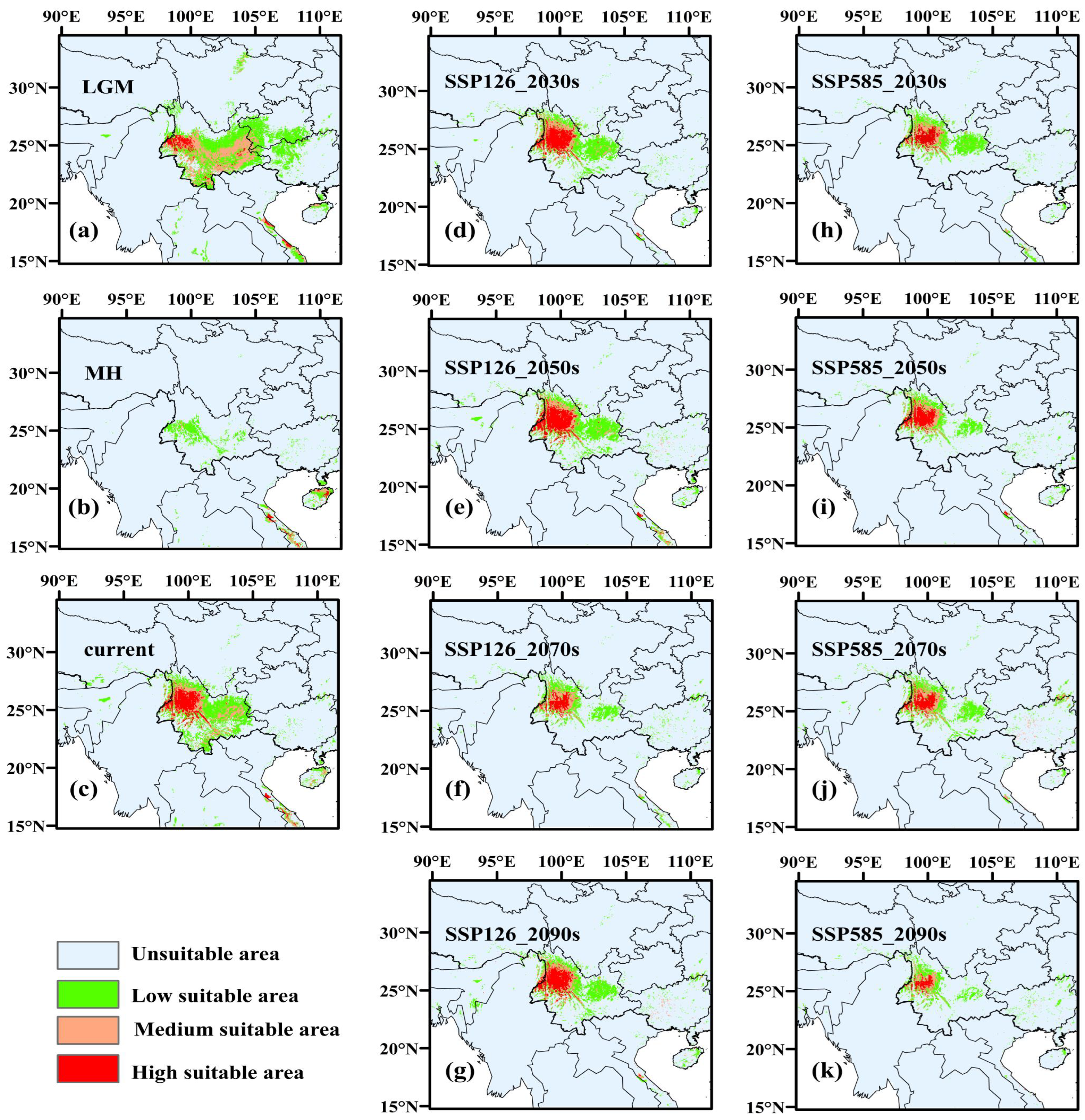
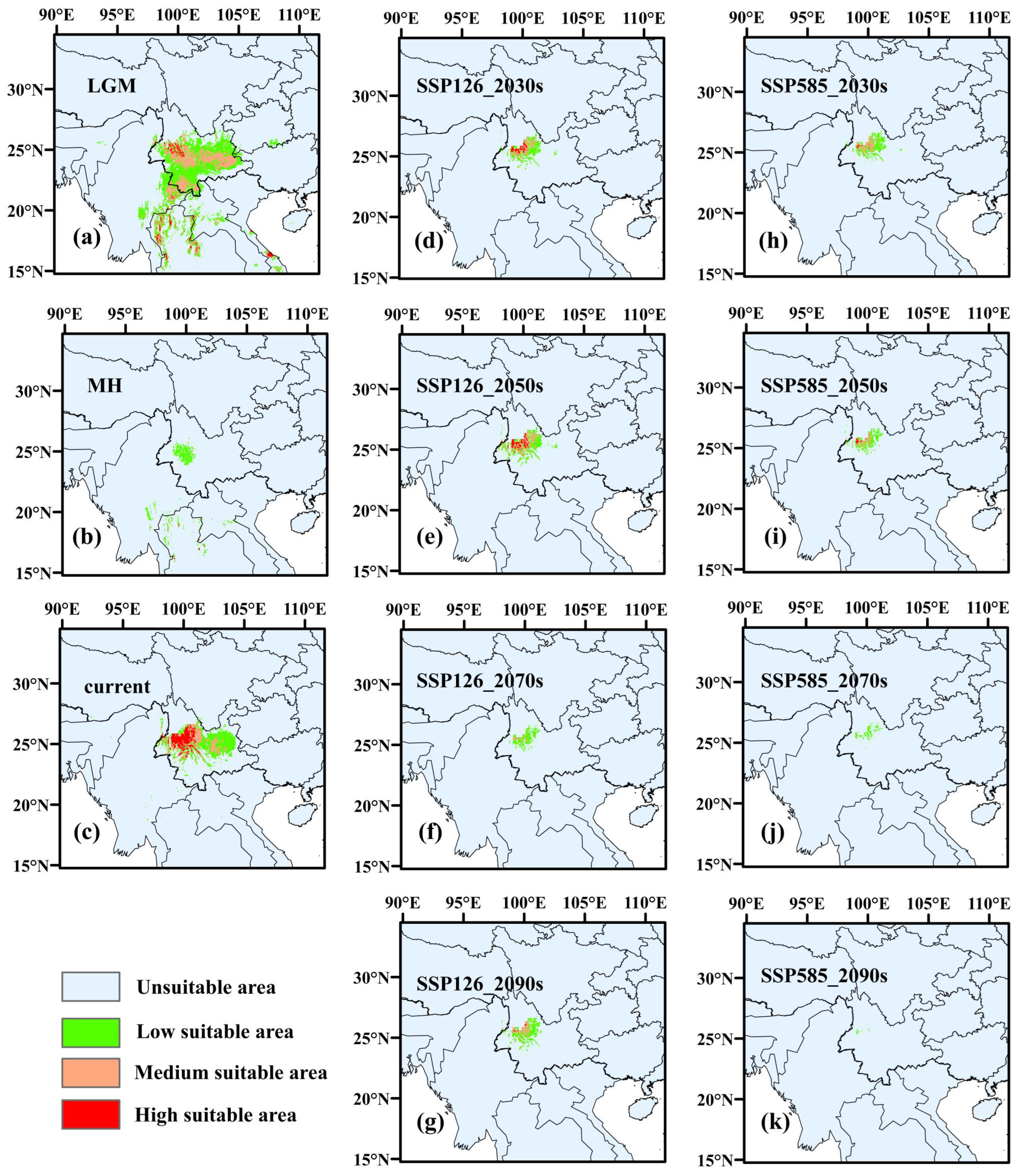
3.3. Potentially Suitable Areas for A. yangbiense and C. chago
3.3.1. Current Habitable Zones for A. yangbiense and C. chago
3.3.2. Potentially Habitable Zones for A. yangbiense and C. chago in Historical Periods
3.3.3. Potentially Habitable Zones for A. yangbiense and C. chago in Future Scenarios
3.4. Shift of Suitable Habitat Centroids
4. Discussion
4.1. Accuracy of MaxEnt After Optimization
4.2. The Impact of Key Environmental Factors on the Distributions of A. yangbiense and C. chago
4.3. Changes of Suitable Areas of A. yangbiense and C. chago from Past to Current
4.4. Changes of Suitable Areas of A. yangbiense and C. chago in Future Scenarios
4.5. Centroid Migration
4.6. Potential Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McClean, C.J.; Lovett, J.C.; Kueper, W.; Hannah, L.; Sommer, J.H.; Barthlott, W.; Termansen, M.; Smith, G.F.; Tokamine, S.; Taplin, J.R.D. African plant diversity and climate change. Ann. Mo. Bot. Gard. 2005, 92, 139–152. [Google Scholar]
- Tang, S.L.; Song, Y.B.; Zeng, B.; Dong, M. Potential distribution of the extremely endangered species Ostrya rehderiana (Betulaceae) in China under future climate change. Environ. Sci. Pollut. Res. 2022, 29, 7782–7792. [Google Scholar]
- Zhao, R.; Huang, N.; Zhang, Z.; Luo, W.; Xiang, J.; Xu, Y.; Wang, Y. Genetic Diversity Analysis and Prediction of Potential Suitable Areas for the Rare and Endangered Wild Plant Henckelia longisepala. Plants 2024, 13, 2093. [Google Scholar] [CrossRef]
- Tao, L.; Liu, Y.-H.; Dao, Z.-L.; Liu, D.; Yang, J.; Sun, W. Guiding Conservation Strategies for China’s Corybas Species through Species Distribution Modeling. Glob. Ecol. Conserv. 2024, 53, e03008. [Google Scholar]
- Bhat, I.A.; Fayaz, M.; Roof ul, Q.; Rafiq, S.; Guleria, K.; Qadir, J.; Wani, T.A.; Kaloo, Z.A. Predicting potential distribution and range dynamics of Aquilegia fragrans under climate change: Insights from ensemble species distribution modelling. Environ. Monit. Assess. 2023, 195, 623. [Google Scholar]
- Yu, F.; Wang, T.; Groen, T.A.; Skidmore, A.K.; Yang, X.; Ma, K.; Wu, Z. Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 2019, 659, 515–528. [Google Scholar]
- Wu, Y.-M.; Shen, X.-L.; Tong, L.; Lei, F.-W.; Mu, X.-Y.; Zhang, Z.-X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar]
- Lee-Yaw, J.; McCune, J.; Pironon, S.; Sheth, S. Species distribution models rarely predict the biology of real populations. Ecography 2021, 2022, e05877. [Google Scholar]
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar]
- Liu, L.F.; Qin, F.C.; Liu, Y.; Hu, Y.N.; Wang, W.F.; Duan, H.; Li, M.Y. Forecast of potential suitable areas for forest resources in Inner Mongolia under the Shared Socioeconomic Pathway 245 scenario. Ecol. Indic. 2024, 167, 13. [Google Scholar]
- Ji, Q.; Wang, R.; Huang, Z.; Yuan, J.; Ren, G.; Xiao, W. Effects of sample size and study range on accuracy of MaxEnt in predicting species distribution: A case study of the black-and-white snub-nosed monkey. Acta Theriol. Sin. 2019, 39, 126–133. [Google Scholar]
- Wang, Y.; Huo, W.; Wu, K.; Cao, J.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Paeonia lactiflora in China based on Maxent and Marxan models. Front. Plant Sci. 2025, 15, 1516251. [Google Scholar]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 14. [Google Scholar]
- Dong, P.B.; Wang, L.Y.; Wang, L.J.; Jia, Y.; Li, Z.H.; Bai, G.; Zhao, R.M.; Liang, W.; Wang, H.Y.; Guo, F.X.; et al. Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology 2022, 11, 1659. [Google Scholar] [CrossRef]
- Gao, X.X.; Liu, J.; Huang, Z.H. The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol. Evol. 2022, 12, 10. [Google Scholar]
- Ma, Y.; Liu, D.; Wariss, H.M.; Zhang, R.; Tao, L.; Milne, R.I.; Sun, W. Demographic history and identification of threats revealed by population genomic analysis provide insights into conservation for an endangered maple. Mol. Ecol. 2021, 31, 767–779. [Google Scholar]
- Dong, W.; Zhang, X.; Yang, G.; Yang, L.; Wang, Y.; Shen, S. Biological characteristics and conservation genetics of the narrowly distributed rare plant Cinnamomum chago (Lauraceae). Plant Diversity 2016, 38, 247–252. [Google Scholar]
- Zhang, X. Species Germplasm Conservation of the Wild Plants with Extremely Small Populations—Cinnamomum chago. Ph.D. Thesis, Yunan University, Kunming, China, 2019. [Google Scholar]
- Tao, L.; Han, C.; Song, K.; Sun, W. A tree species with an extremely small population: Recategorizing the Critically Endangered Acer yangbiense. Oryx 2020, 54, 474–477. [Google Scholar]
- Tao, L.D.; Guo, S.W.; Xiong, Z.Z.; Zhang, R.G.; Sun, W.B. Chromosome-level genome assembly of the threatened resource plant Cinnamomum chago. Sci. Data 2024, 11, 9. [Google Scholar]
- Li, S.F.; Yang, J.H.; Li, S.L.; Hou, M.; Xi, X.L.; Chen, Q. Investigation and Protection on Germplasm Resources of Cinnamomum chago in Yangbi County. For. Investig. Plan. 2020, 45, 88–91+127. [Google Scholar]
- Hou, M.; Li, J.N.; Li, S.F.; Yang, J.H.; Xiong, X.W.; Wang, G.S. Nutritional Composition Analysis and Evaluation of Cinnamomum chago. For. Investig. Plan. 2019, 48, 80–85. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar]
- Wang, D.; Cui, B.; Duan, S.; Chen, J.; Fan, H.; Lu, B.; Zheng, J. Moving north in China: The habitat of Pedicularis kansuensis in the context of climate change. Sci. Total Environ. 2019, 697, 133979. [Google Scholar]
- Huang, E.H.; Chen, Y.X.; Fang, M.; Zheng, Y.; Yu, S.X. Environmental drivers of plant distributions at global and regional scales. Glob. Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar]
- Khalil, T.; Asad, S.A.; Khubaib, N.; Baig, A.; Atif, S.; Umar, M.; Kropp, J.P.; Pradhan, P.; Baig, S. Climate change and potential distribution of potato (Solanum tuberosum) crop cultivation in Pakistan using Maxent. AIMS Agric. Food 2021, 6, 663–676. [Google Scholar]
- Gan, T.; Qian, Q.; Liu, Z.; Xu, D. Simulation of Suitable Distribution Areas of Magnolia officinalis in China Based on the MaxEnt Model and Analysis of Key Environmental Variables. Agriculture 2024, 14, 2303. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Wang, S.; Xu, Y.; Du, C.; Rohani, E.R.; Tong, X.; Wang, T.; Han, R. Predicting the potential distribution of Arisaema heterophyllum in China under current and future climate change based on ArcGIS and MaxEnt model. Plant Biosyst. 2024, 158, 1326–1334. [Google Scholar]
- Wang, Y.S.; Wang, Z.H.; Xing, H.F.; Li, J.W.; Sun, S. Prediction of potential suitable distribution of Davidia involucrata Baill in China based on MaxEnt. Chin. J. Ecol. 2019, 38, 1230–1237. [Google Scholar]
- Zhu, D.P.; Yang, L.; Li, Y.H.; Huang, P.; Yao, B.; Kong, Z.; Xiang, Y.Z. Predicting the Potential Distribution of the Endangered Pyrethrum tatsienense in China Using an Optimized Maxent Model Under Climate Change Scenarios. Ecol. Evol. 2024, 14, 12. [Google Scholar]
- Wu, H.-Y.; Liu, Y.-H.; He, Q.-X.; Ye, J.-W.; Tian, B. Differential distribution shifts in two subregions of East Asian subtropical evergreen broadleaved forests—A case of Magnoliaceae. Front. Plant Sci. 2024, 14, 1326207. [Google Scholar]
- Gu, C.; Tu, Y.; Liu, L.; Wei, B.; Zhang, Y.; Yu, H.; Wang, X.; Yangjin, Z.; Zhang, B.; Cui, B. Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 2021, 11, 12092–12113. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar]
- Chen, C.; Zhou, L.L.; Han, Y.; Wen, J.W.; Liu, L.Q.; Liu, Q.P.; Peng, C.; He, Y. Comprehensive analysis of the effects of climate change on the species distribution and active components of Leonurus japonicus Houtt. Ind. Crop. Prod. 2024, 218, 11. [Google Scholar]
- Chen, J.; He, D. Potential geographical distribution of Cordyceps cicadae and its two hosts in China under climate change. Front. Microbiol. 2025, 15, 1519560. [Google Scholar] [PubMed]
- Zhang, X.; Zhou, X.-L.; Liu, Y.-H.; Mo, J.-Q.; Zhang, L.-Q.; Wang, Y.-H.; Shen, S.-K. Investigating the status of Cinnamomum chago (Lauraceae), a plant species with an extremely small population endemic to Yunnan, China. Oryx 2020, 54, 470–473. [Google Scholar]
- He, H.; Zheng, X.; Wang, Y.; Wang, W.; Li, M.; Wang, S.; Wang, J.; Wang, C.; Zhan, H. Effects of Climate Change and Environmental Factors on Bamboo (Ferrocalamus strictus), a PSESP Unique to China. Forests 2022, 13, 2108. [Google Scholar] [CrossRef]
- Janitza, S.; Strobl, C.; Boulesteix, A.-L. An AUC-based permutation variable importance measure for random forests. BMC Bioinform. 2013, 14, 119. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Suárez, M.; Ramírez-Albores, J.E.; Martinez-Campos, A.R. Predicting the impacts of climate change on potential suitability habitats of three Juniperus trees in Mexico. Plant Ecol. 2024, 225, 37–51. [Google Scholar] [CrossRef]
- Warren, D.; Seifert, S. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, Y.; Liu, W.; Yang, X.; Wang, L. Predicting the Global Potential Suitable Areas of Sweet Osmanthus (Osmanthus fragrans) Under Current and Future Climate Scenarios. Ecol. Evol. 2024, 14, e70435. [Google Scholar] [CrossRef]
- Qiu, L.; Jacquemyn, H.; Burgess, K.S.; Zhang, L.G.; Zhou, Y.D.; Yang, B.Y.; Tan, S.L. Contrasting range changes of terrestrial orchids under future climate change in China. Sci. Total Environ. 2023, 895, 13. [Google Scholar] [CrossRef]
- Tao, H.; Kingston, K.; Xu, Z.H.; Bai, S.H.; Guo, L.; Liu, G.L.; Hui, C.M.; Liu, W.Y. Predicting Potential Suitable Areas of Dendrocalamus brandisii under Global Climate Change. Forests 2024, 15, 1301. [Google Scholar] [CrossRef]
- Kim, C.; Kim, W.; Song, W.K.Y.; Cho, J.P.; Choi, J. Prediction of Native Seed Habitat Distribution According to SSP Scenario and Seed Transfer Zones: A Focus on Acer pictum subsp. mono and Quercus acuta. Forests 2023, 14, 87. [Google Scholar] [CrossRef]
- Qi, S.; Luo, W.; Chen, K.-L.; Li, X.; Luo, H.-L.; Yang, Z.-Q.; Yin, D.-M. The Prediction of the Potentially Suitable Distribution Area of Cinnamomum mairei H. Lév in China Based on the MaxEnt Model. Sustainability 2022, 14, 7682. [Google Scholar] [CrossRef]
- Rao, Z.; Qin, Q.; Wei, S.; Guo, H.; Li, Y. Holocene temperature history and its significance to studies on historical human-land relationship in China. Acta Geogr. Sin. 2022, 77, 1169–1180. [Google Scholar]
- Tian, Z.P.; Jiang, D.B. Mid-holocene and last glacial maximum changes in monsoon area and precipitation over China. Chin. Sci. Bull. 2015, 60, 400–410. [Google Scholar]
- Chen, F.; Duan, Y.; Hao, S.; Chen, J.; Feng, X.; Hou, J.; Cao, X.; Zhang, X.; Zhou, T. Holocene thermal maximum mode versus the continuous warming mode: Problems of data-model comparisons and future research prospects. Sci. China Earth Sci. 2023, 53, 1699–1717. [Google Scholar] [CrossRef]
- He, Y. Changes over the past 5000 years in the warming and cooling of southern China. Chin. Hist. Geogr. 1999, 197–207. [Google Scholar]
- Chen, D.; Kang, H.; Liu, C. An Overview on the Potential Quaternary Glacial Refugia of Plants in China Mainland. Bull. Bot. Res. 2011, 31, 623–632. [Google Scholar]
- Shen, J. The Holocene vegetation history of Lake Erhai, Yunnan province southwestern China: The role of climate and human forcings. Holocene 2006, 16, 265–276. [Google Scholar] [CrossRef]
- Dearing, J.A.; Jones, R.T.; Shen, J.; Yang, X.; Boyle, J.F.; Foster, G.C.; Crook, D.S.; Elvin, M.J.D. Using multiple archives to understand past and present climate–human–environment interactions: The lake Erhai catchment, Yunnan Province, China. J. Paleolimnol. 2008, 40, 3–31. [Google Scholar] [CrossRef]
- Uchale, N.N.; Singh, B.B. Characteristics and projected changes in maximum daily precipitation across the globe. Q. J. R. Meteorol. Soc. 2024, 151, e4912. [Google Scholar]
- Chmura, D.J.; Banach, J.; Kempf, M.; Kowalczyk, J.; Mohytych, V.; Szeligowski, H.; Buraczyk, W.; Kowalkowski, W. Growth and productivity of European beech populations show plastic response to climatic transfer at the north-eastern border of the species range. For. Ecol. Manag. 2024, 565, 9. [Google Scholar] [CrossRef]
- Adhikari, D.; Tiwary, R.; Singh, P.P.; Upadhaya, K.; Singh, B.; Haridasan, K.E.; Bhatt, B.B.; Chettri, A.; Barik, S.K. Ecological niche modeling as a cumulative environmental impact assessment tool for biodiversity assessment and conservation planning: A case study of critically endangered plant Lagerstroemia minuticarpa in the Indian Eastern Himalaya. J. Environ. Manag. 2019, 243, 299–307. [Google Scholar]
- Stanton, J.C.; Pearson, R.G.; Horning, N.; Ersts, P.; Akcakaya, H.R. Combining static and dynamic variables in species distribution models under climate change. Methods Ecol. Evol. 2012, 3, 349–357. [Google Scholar]
- Wilson, R.J.; Gutiérrez, D.; Gutiérrez, J.; Monserrat, V.J. An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob. Change Biol. 2007, 13, 1873–1887. [Google Scholar]
- Xian-Ge, H.; Yuqing, J.; Xiao-Ru, W.; Jian-Feng, M.; Yue, L. Predicting Impacts of Future Climate Change on the Distribution of the Widespread Conifer Platycladus orientalis. PLoS ONE 2015, 10, e0132326. [Google Scholar]
- Newman, B.J.; Ladd, P.; Brundrett, M.; Dixon, K.W. Effects of habitat fragmentation on plant reproductive success and population viability at the landscape and habitat scale. Biol. Conserv. 2013, 159, 16–23. [Google Scholar]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar]
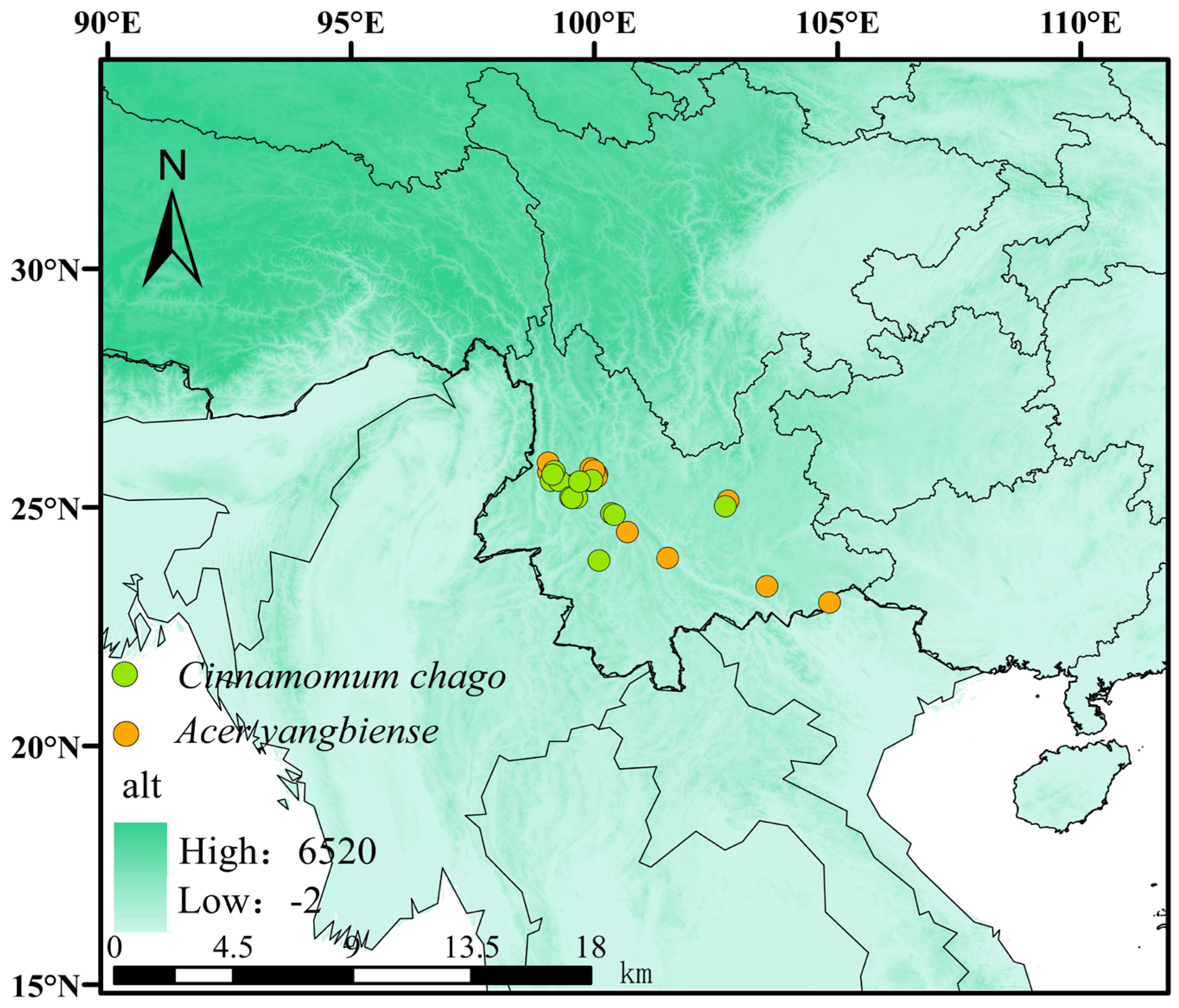

| Species | Retained Environmental Factors |
|---|---|
| A. yangbiense | Bio3, bio4, bio13, bio15, bio17, alt, aspect, slope, uvb1, T_sand, T_silt |
| C. chago | Bio3, bio4, bio6, bio13, bio15, bio17, alt, uvb2, uvb6, T_sand, T_silt, T_ph |
| Species | RM | FC | Omission Rate | Delta AICc | Mean AUc | Standard Deviation | AUCDiff |
|---|---|---|---|---|---|---|---|
| A. yangbiense | 3.5 | QP | 0.25 | 0 | 0.982 | 0.029 | 0.0079 |
| C. chago | 2 | QPT | 0.33 | 0 | 0.993 | 0.013 | 0.0029 |
| The Percentage Contributions of the Environmental Factors of A. yangbiense | ||
|---|---|---|
| Environment | Description | Contribution |
| Variables | Rate/% | |
| bio3 | Isothermality (bio2/bio7) (×100) | 12.4 |
| bio4 | Temperature seasonality (standard deviation ×100) | 22.2 |
| bio13 | Precipitation of the wettest month | 3.9 |
| bio15 | Precipitation seasonality (Coefficient of variation) | 2.7 |
| bio17 | Precipitation of the driest quarter | 24.3 |
| alt | Altitude | 17.2 |
| aspect | Aspect | 0.6 |
| slope | Slope | 0.3 |
| uvb1 | Annual mean UV-B | 3.8 |
| T_sand | Topsoil sand fraction | 3.4 |
| T_silt | Topsoil silt fraction | 9.2 |
| The Percentage Contributions of the Environmental Factors of C. chago | ||
|---|---|---|
| Environment | Description | Contribution |
| Variables | Rate/% | |
| bio3 | Isothermality (bio2/bio7) (×100) | 13.7 |
| bio4 | Temperature seasonality (standard deviation ×100) | 5.6 |
| Bio6 | Min temperature of the coldest month | 34.8 |
| bio13 | Precipitation of the wettest month | 5.2 |
| bio15 | Precipitation seasonality (Coefficient of variation) | 2.1 |
| bio17 | Precipitation of the driest quarter | 7.2 |
| alt | Altitude | 15.4 |
| uvb2 | UV-B seasonality | 14 |
| uvb6 | Sum of monthly mean UV-B during lowest quarter | 0.3 |
| T_sand | Topsoil sand fraction | 0.5 |
| T_silt | Topsoil silt fraction | 1 |
| T_ph | Topsoil pH (H2O) | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, K.; Wu, H.; Li, C.; Luo, G.; Zhao, T.; Chen, C.; Liu, Y.; Duan, M.; Wang, C. A Simulation of a Suitable Habitat for Acer yangbiense and Cinnamomum chago Under Climate Change. Forests 2025, 16, 621. https://doi.org/10.3390/f16040621
Gao K, Wu H, Li C, Luo G, Zhao T, Chen C, Liu Y, Duan M, Wang C. A Simulation of a Suitable Habitat for Acer yangbiense and Cinnamomum chago Under Climate Change. Forests. 2025; 16(4):621. https://doi.org/10.3390/f16040621
Chicago/Turabian StyleGao, Kemei, Haiyang Wu, Chunping Li, Guomi Luo, Taiyang Zhao, Chunpu Chen, Yuting Liu, Mengsi Duan, and Changming Wang. 2025. "A Simulation of a Suitable Habitat for Acer yangbiense and Cinnamomum chago Under Climate Change" Forests 16, no. 4: 621. https://doi.org/10.3390/f16040621
APA StyleGao, K., Wu, H., Li, C., Luo, G., Zhao, T., Chen, C., Liu, Y., Duan, M., & Wang, C. (2025). A Simulation of a Suitable Habitat for Acer yangbiense and Cinnamomum chago Under Climate Change. Forests, 16(4), 621. https://doi.org/10.3390/f16040621






