Annual Tree Biomass Increment Is Positively Related to Nonstructural Carbohydrate Pool Size and Depletion: Evidence for Carbon Limitation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Field Sampling
2.1.1. Tree Species Selection and Organ Separation
2.1.2. Sampling Date
2.1.3. Sampling of Foliage and Branch
2.1.4. Sampling of Stem Tissues
2.1.5. Sampling of Belowground Tissues
2.1.6. Sample Processing
2.1.7. NSC Concentrations
2.2. Data Analysis
2.2.1. Tree Organ Biomass
2.2.2. Diameter or Age Effects on Organ NSC Estimation
2.2.3. Proxy of NSC Storage
2.2.4. Annual Biomass Increment
2.2.5. Difference Among Organs, Species, and Plant Groups
2.2.6. Significance of NSC Storage to Growth
3. Results
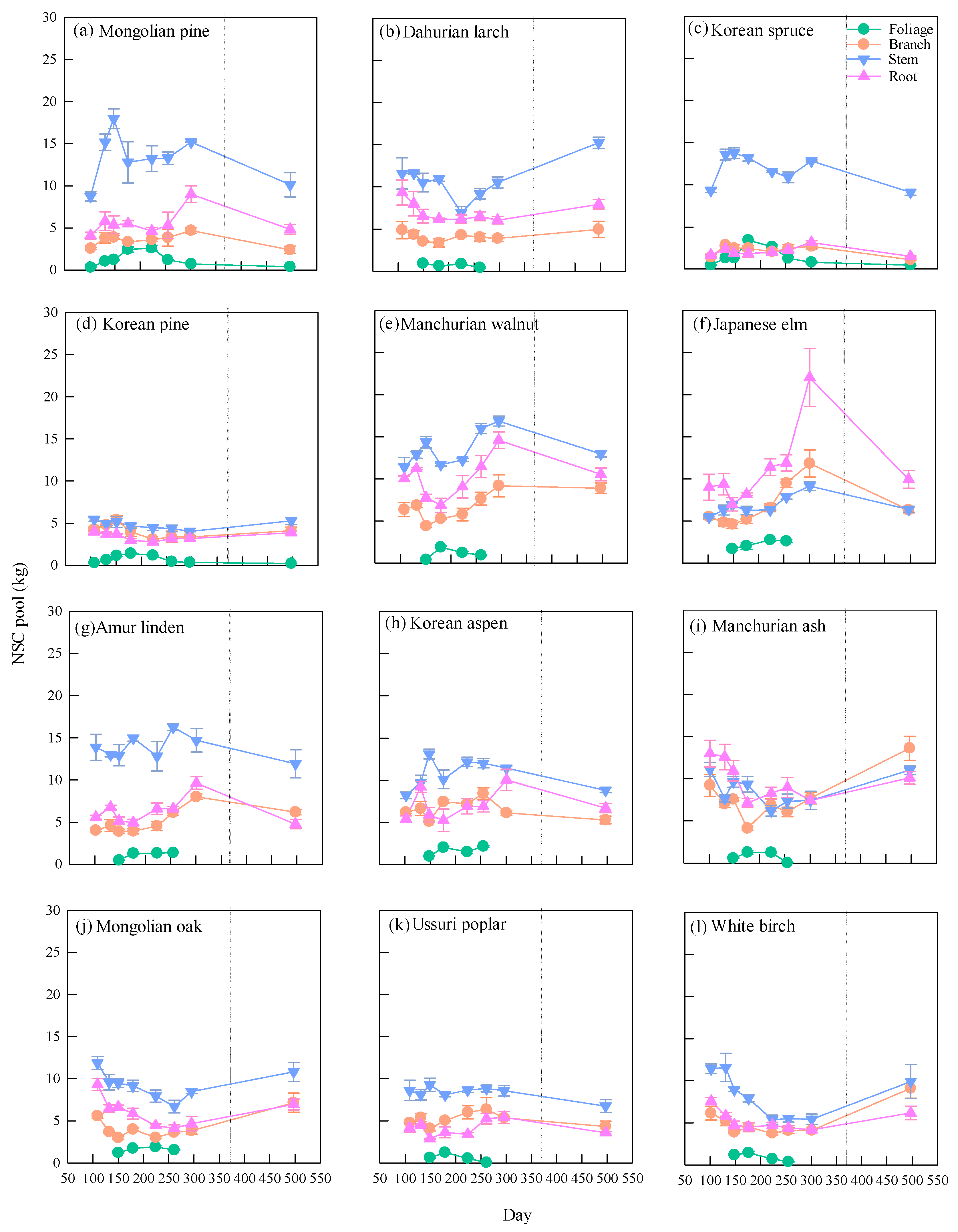
3.1. Whole-Tree NSC Pool and Its Allocation Among Organs
3.2. Seasonal Dynamics of NSC Pool
3.3. Relating Whole-Tree NSC Storage to Annual Biomass Increment
4. Discussion
4.1. The Size of the Whole-Tree NSC Pool
4.2. The Seasonality of Whole-Tree NSC Pools: Storage–Growth Trade-Offs in the Short Term
4.3. Coordination Between NSC Storage and Biomass Production: Carbon Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCs | Nonstructural carbohydrates |
| ΔNSC/ABI | The ratio of the seasonal variation in the whole-tree NSC pool to the annual biomass increment |
References
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world–Challenges and progress: Introduction to a Virtual Issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Change Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Landhäusser, S.; Adams, H. Getting to the root of carbon reserve dynamics in woody plants: Progress, challenges, and goals. Tree Physiol. 2024, 44, 1–10. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L. Source vs sink limitations on tree growth: From physiological mechanisms to evolutionary constraints and terrestrial carbon cycle implications. New Phytol. 2025, 245, 966–981. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Zweifel, R. Beyond source and sink control–toward an integrated approach to understand the carbon balance in plants. New Phytol. 2024, 242, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Agossou, C.; Kim, E.; Lim, J.H.; Hwang, T.; Kang, S. Recent field findings and modeling on non-structural carbohydrates (NSCs). Ecol. Inform. 2022, 70, 101695. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Huggett, B.A.; Furze, M.E.; Czimczik, C.I.; Walker, J.C.; Xu, X.; Schaberg, P.G.; Murakami, P. Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol. 2015, 206, 590–597. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Carbone, M.S.; Enright, M.; Marshall, M.C.; Trowbridge, A.M.; LeMoine, J.; Koch, G.; Richardson, A.D. Old reserves and ancient buds fuel regrowth of coast redwood after catastrophic fire. Nat. Plants 2023, 9, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Blumstein, M.; Gersony, J.; Martínez-Vilalta, J.; Sala, A. Global variation in nonstructural carbohydrate stores in response to climate. Glob. Change Biol. 2023, 29, 1854–1869. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Schulze, E.D.; Mooney, H.A. The Ecology and Economics of Storage in Plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Cabon, A.; Kannenberg, S.A.; Arain, A.; Babst, F.; Baldocchi, D.; Belmecheri, S.; Delpierre, N.; Guerrieri, R.; Maxwell, J.T.; McKenzie, S.; et al. Cross-biome synthesis of source versus sink limits to tree growth. Science 2022, 376, 758–761. [Google Scholar] [CrossRef]
- Green, J.K.; Keenan, T.F. The limits of forest carbon sequestration. Science 2022, 376, 692–693. [Google Scholar] [CrossRef]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
- Palacio, S.; Hoch, G.; Sala, A.; Körner, C.; Millard, P. Does carbon storage limit tree growth? New Phytol. 2014, 201, 1096–1100. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Blumstein, M.; Sala, A.; Weston, D.J.; Holbrook, N.M.; Hopkins, R. Plant carbohydrate storage: Intra- and inter-specific trade-offs reveal a major life history trait. New Phytol. 2022, 235, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [PubMed]
- Thompson, R.A. A neutral theory of plant carbon allocation. Tree Physiol. 2024, 44, tpad151. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Fermaniuk, C.; Fleurial, K.G.; Wiley, E.; Landhäusser, S.M. Large seasonal fluctuations in whole-tree carbohydrate reserves: Is storage more dynamic in boreal ecosystems. Ann. Bot. 2021, 128, 943–957. [Google Scholar] [CrossRef]
- Piper, F.I. Decoupling between growth rate and storage remobilization in broadleaf temperate tree species. Funct. Ecol. 2020, 34, 1180–1192. [Google Scholar] [CrossRef]
- Piper, F.I.; Paula, S. The role of nonstructural carbohydrates storage in forest resilience under climate change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree responses to drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Barbaroux, C.; Bréda, N.; Dufrêne, E. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol. 2003, 157, 605–615. [Google Scholar]
- Zhang, H.; Wang, C.; Wang, X. Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees-Struct. Funct. 2014, 28, 77–89. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, C.K.; Wang, X.C. Comparison of concentrations of non-structural carbohydrates new twigs and old branches for 12 temperate species. Acta Ecol. Sin. 2013, 33, 5675–5685. [Google Scholar]
- Guo, X.; Liu, S.; Wang, H.; Chen, Z.; Zhang, J.; Chen, L.; Nie, X.; Zheng, L.; Cai, D.; Jia, H.; et al. Divergent allocations of nonstructural carbohydrates shape growth response to rainfall reduction in two subtropical plantations. Forest Ecosyst. 2022, 9, 100021. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Zhou, Z. Does the net primary production converge across six temperate forest types under the same climate? For. Ecol. Manag. 2019, 448, 535–542. [Google Scholar] [CrossRef]
- Dong, L.H.; Liu, Y.S.; Zhang, L.Z.; Xie, L.F.; Li, F.R. Variation in carbon concentration and allometric equations for estimating tree carbon contents of 10 broadleaf species in natural forests in northeast China. Forests 2019, 10, 928. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, C.K.; Wang, X.C.; Cheng, F.Y. Spatial variation of non-structural carbohydrates in Betula platyphylla and Tilia amurensis stems. Chin. J. Appl. Ecol. 2013, 24, 3050–3056. [Google Scholar]
- Cheng, F.; Wang, C. Estimating nonstructural carbon content of tree crown considering its spatial variability: A case study on Juglans mandshurica and Ulmus japonica. Chin. J. Appl. Ecol. 2015, 26, 2253–2264. [Google Scholar]
- Zhang, H.Y.; Wang, C.K.; Wang, X.C. Within-crown variation in concentrations of non-structural carbohydrates of five temperate tree species. Acta Ecol. Sin. 2015, 35, 6496–6506. [Google Scholar]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]
- Dong, L.H.; Zhang, L.J.; Li, F.R. Developing two additive biomass equations for three coniferous plantation species in northeast China. Forests 2016, 7, 136. [Google Scholar] [CrossRef]
- Dong, L.H.; Zhang, L.J.; Li, F.R. A compatible system of biomass equations for three conifer species in Northeast, China. For. Ecol. Manag. 2014, 329, 306–317. [Google Scholar] [CrossRef]
- He, H.; Zhang, C.; Zhao, X.; Fousseni, F.; Wang, J.; Dai, H.; Yang, S.; Zuo, Q. Allometric biomass equations for 12 tree species in coniferous and broadleaved mixed forests, Northeastern China. PLoS ONE 2018, 13, e0186226. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.C.; Wang, C.K.; Zhang, Q.Z.; Guo, Q.X. Filling the “vertical gap” between canopy tree species and understory shrub species: Biomass allometric equations for subcanopy tree species. J. For. Res. 2022, 34, 903–913. [Google Scholar] [CrossRef]
- Wang, C.K. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, Y.; Gu, J.; Wang, Z.; Guo, D. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 177, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Harvard Forest 2014. Harvard Forest Data Archive: HF308. 2018. Available online: https://harvardforest1.fas.harvard.edu/exist/apps/datasets/showData.html?id=hf308 (accessed on 2 July 2018).
- Ramirez, J.A.; Craven, D.; Herrera, D.; Posada, J.M.; Reu, B.; Sierra, C.A.; Hoch, G.; Handa, I.T.; Messier, C. Non-structural carbohydrate concentrations in tree organs vary across biomes and leaf habits, but are independent of the fast-slow plant economic spectrum. Front. Plant Sci. 2024, 15, 1375958. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Starch: A flexible, adaptable carbon store coupled to plant growth. Annu. Rev. Plant Biol. 2020, 71, 217–245. [Google Scholar] [CrossRef]
- Oswald, S.W.; Aubrey, D.P. Modeling starch dynamics from seasonal variations of photosynthesis, growth and respiration. Tree Physiol. 2024, 44, 11–27. [Google Scholar] [CrossRef]
- Ei Ombri, B. Accumulation versus storage of total non-structural carbohydrates in woody plants. Trees 2022, 36, 869–881. [Google Scholar] [CrossRef]
- Barbaroux, C.; Bréda, N. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol. 2002, 22, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Schoonmaker, A.L.; Hillabrand, R.M.; Lieffers, V.J.; Chow, P.S.; Landhäusser, S.M. Seasonal dynamics of non-structural carbon pools and their relationship to growth in two boreal conifer tree species. Tree Physiol. 2021, 41, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-L.; Liu, H.; Zhao, K.-P.; Wang, J.; Miao, Y.; Lin, H.-C.; Hao, G.-Y.; Han, S.-J. Seasonal patterns of nonstructural carbohydrate storage and mobilization in two tree species with distinct life-history traits. Tree Physiol. 2024, 44, tpae042. [Google Scholar] [CrossRef]
- Long, R.W.; Dudley, T.L.; D’Antonio, C.M.; Grady, K.C.; Bush, S.E.; Hultine, K.R. Spenders versus savers: Climate-induced carbon allocation trade-offs in a recently introduced woody plant. Funct. Ecol. 2021, 35, 1640–1654. [Google Scholar] [CrossRef]
- D’Orangeville, L.; Itter, M.; Kneeshaw, D.; Munger, J.W.; Richardson, A.D.; Dyer, J.M.; Orwig, D.A.; Pan, Y.; Pederson, N. Peak radial growth of diffuse-porous species occurs during periods of lower water availability than for ring-porous and coniferous trees. Tree Physiol. 2022, 42, 304–316. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Cufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gricar, J.; Gruber, A.; Huang, J.-G.; et al. Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere. Glob. Change Biol. 2016, 22, 3804–3813. [Google Scholar] [CrossRef]
- Chen, Y.; Rademacher, T.; Fonti, P.; Eckes-Shephard, A.H.; LeMoine, J.M.; Fonti, M.V.; Richardson, A.D.; Friend, A.D. Inter-annual and inter-species tree growth explained by phenology of xylogenesis. New Phytol. 2022, 235, 939–952. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Interspecific differences of stem diameter variations in response to water conditions for six tree species in Northeast China. Forests 2023, 14, 805. [Google Scholar] [CrossRef]
- Xu, F.; Wang, C.K.; Wang, X.C. Intra- and inter-specific variations in stem respiration for 14 temperate tree species in northeastern China. Acta Ecol. Sin. 2011, 31, 3581–3589. [Google Scholar]
- Avila, E.; Herrera, A.; Tezara, W. Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species. Photosynthetica 2014, 52, 3–15. [Google Scholar] [CrossRef]
- Vandegehuchte, M.W.; Bloemen, J.; Vergeynst, L.L.; Steppe, K. Woody tissue photosynthesis in trees: Salve on the wounds of drought? New Phytol. 2015, 208, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G. Cell wall hemicelluloses as mobile carbon stores in non-reproductive plant tissues. Funct. Ecol. 2007, 21, 823–834. [Google Scholar] [CrossRef]
- Schädel, C.; Blöchl, A.; Richter, A.; Hoch, G. Short-term dynamics of nonstructural carbohydrates and hemicelluloses in young branches of temperate forest trees during bud break. Tree Physiol. 2009, 29, 901–911. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Scartazza, A.; Battistelli, A.; Collalti, A.; Proietti, S.; Rezaie, N.; Matteucci, G.; Moscatello, S. Unravelling resilience mechanisms in forests: Role of non-structural carbohydrates in responding to extreme weather events. Tree Physiol. 2021, 41, 1808–1818. [Google Scholar] [CrossRef]
- Zhang, P.P.; Zhou, X.H.; Fu, Y.L.; Shao, J.J.; Zhou, L.Y.; Li, S.S.; Zhou, G.Y.; Hu, Z.H.; Hu, J.Q.; Bai, S.H.; et al. Differential effects of drought on nonstructural carbohydrate storage in seedlings and mature trees of four species in a subtropical forest. For. Ecol. Manag. 2020, 469, 118159. [Google Scholar] [CrossRef]
- Gough, C.M.; Flower, C.E.; Vogel, C.S.; Dragoni, D.; Curtis, P.S. Whole-ecosystem labile carbon production in a north temperate deciduous forest. Agric. For. Meteorol. 2009, 149, 1531–1540. [Google Scholar] [CrossRef]
- Gholz, H.L.; Cropper, W.P. Carbohydrate dynamics in mature Pinus elliottii var. elliottii trees. Can. J. For. Res. 1991, 21, 1742–1747. [Google Scholar]
- Igarashi, S.; Shibata, M.; Masaki, T.; Tayasu, I.; Ichie, T. Mass flowering of Fagus crenata does not depend on the amount of stored carbohydrates in trees. Trees 2019, 33, 1399–1408. [Google Scholar] [CrossRef]
- Blumstein, M.J.; Furze, M.E. Interannual dynamics of stemwood nonstructural carbohydrates in temperate forest trees surrounding drought. J. For. Res. 2023, 34, 77–86. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Dynamics of nonstructural carbohydrates during drought and subsequent recovery: A global meta-analysis. Agric. For. Meteorol. 2025, 363, 110429. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Carbone, M.S.; Ogle, K.; Koch, G.W.; Richardson, A.D. Decades-old carbon reserves are widespread among tree species, constrained only by sapwood longevity. New Phytol. 2025, 245, 1468–1480. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Mäkinen, H.; Prislan, P.; Rossi, S.; del Castillo, E.M.; Campelo, F.; Vavrčík, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 15160. [Google Scholar] [CrossRef]
- Yin, S.D. Compare Study on Nutrient Ecology of Pinus koraiensis and Larix oigensis Plantation Ecosystems; Northeast Forestry University: Harbin, China, 2004. [Google Scholar]
- Zhan, H.Z.; Liu, C.Z.; Liu, J.C. Study on biomass and nutrient content of broad-leaved red pine forest. Sci. Sil. Sin. 1990, 26, 1994–2023. [Google Scholar]
- Hu, H.Q.; Guo, F.T. Estimation of total carbon emissions from major tree species during forest fires in the Greater Khingan Mountains. Chin. J. Appl. Ecol. 2008, 19, 1884–1890. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Wang, C.K. Carbon density and distribution of six Chinese temperate forests. Sci. China Life Sci. 2010, 53, 831–840. [Google Scholar] [CrossRef]
- Cai, H.Y.; Di, X.Y.; Chang, S.X.; Wang, C.K.; Shi, B.K.; Geng, P.F.; Jin, G.G. Carbon storage, net primary production, and net ecosystem production in four major temperate forest types in northeastern China. Can. J. Forest. Res. 2016, 46, 143–151. [Google Scholar] [CrossRef]




| Scientific Name | Common Name | Leaf Form and Habit | Wood Type | H (m) | DBH (cm) |
|---|---|---|---|---|---|
| Pinus sylvestris var. mongolica Litv. | Mongolian pine | Evergreen conifer | Non-porous | 23.2 (0.5) | 27.3 (1.0) |
| Larix gmelinii (Ruprecht) Kuzeneva | Dahurian larch | Deciduous conifer | Non-porous | 26.5 (0.4) | 31.6 (1.1) |
| Picea koraiensis Nakai | Korean spruce | Evergreen conifer | Non-porous | 18.9 (0.2) | 29.9 (1.3) |
| Pinus koraiensis Siebold et Zuccarini | Korean pine | Evergreen conifer | Non-porous | 20.4 (0.9) | 23.3 (0.4) |
| Juglans mandshurica Maxim. | Manchurian walnut | Deciduous broadleaf | Semi-ring-porous | 21.3 (1.0) | 33.5 (1.5) |
| Ulmus davidiana var. japonica (Rehd.) Nakai | Japanese elm | Deciduous broadleaf | Ring-porous | 24.1 (1.2) | 40.3 (1.9) |
| Tilia amurensis Rupr. | Amur linden | Deciduous broadleaf | Diffuse-porous | 20.8 (2.0) | 46.1 (3.6) |
| Populus davidiana Dode | Korean aspen | Deciduous broadleaf | Diffuse-porous | 25.4 (0.6) | 42.3 (0.9) |
| Fraxinus mandshurica Rupr. | Manchurian ash | Deciduous broadleaf | Ring-porous | 24.7 (2.0) | 33.6 (1.2) |
| Quercus mongolica Fisch. Ex Ledeb. | Mongolian oak | Deciduous broadleaf | Ring-porous | 19.1 (0.3) | 32.5 (0.6) |
| Populus ussuriensis Kom. | Ussuri poplar | Deciduous broadleaf | Diffuse-porous | 23.4 (0.9) | 41.5 (1.5) |
| Betula platyphylla Suk. | White birch | Deciduous broadleaf | Diffuse-porous | 23.0 (1.0) | 33.4 (1.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, G.; Zhang, Q.; Quan, X.; Zhang, H.; Aubrey, D.P.; Wang, C. Annual Tree Biomass Increment Is Positively Related to Nonstructural Carbohydrate Pool Size and Depletion: Evidence for Carbon Limitation? Forests 2025, 16, 619. https://doi.org/10.3390/f16040619
Wang X, Hu G, Zhang Q, Quan X, Zhang H, Aubrey DP, Wang C. Annual Tree Biomass Increment Is Positively Related to Nonstructural Carbohydrate Pool Size and Depletion: Evidence for Carbon Limitation? Forests. 2025; 16(4):619. https://doi.org/10.3390/f16040619
Chicago/Turabian StyleWang, Xingchang, Guirong Hu, Quanzhi Zhang, Xiankui Quan, Haiyan Zhang, Doug P. Aubrey, and Chuankuan Wang. 2025. "Annual Tree Biomass Increment Is Positively Related to Nonstructural Carbohydrate Pool Size and Depletion: Evidence for Carbon Limitation?" Forests 16, no. 4: 619. https://doi.org/10.3390/f16040619
APA StyleWang, X., Hu, G., Zhang, Q., Quan, X., Zhang, H., Aubrey, D. P., & Wang, C. (2025). Annual Tree Biomass Increment Is Positively Related to Nonstructural Carbohydrate Pool Size and Depletion: Evidence for Carbon Limitation? Forests, 16(4), 619. https://doi.org/10.3390/f16040619






