Cespitose Population Structure and Dynamics of Rare Fraxinus sogdiana in the Yili River Valley, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Research Methods
2.2.1. Sample Plot Setting and Survey
2.2.2. Division of Population Age Structure
2.2.3. Compilation of Population Static Life Table and Fitting of the Survival Curve
2.2.4. Dynamic Quantitative Analysis
2.2.5. Spectral Analysis Methods
3. Results
3.1. Situation of F. sogdiana Population Cespitose
3.2. Population Age Structure
3.3. Population Static Life Table, Survival Curve, and Mortality Curve
3.3.1. Static Life Table
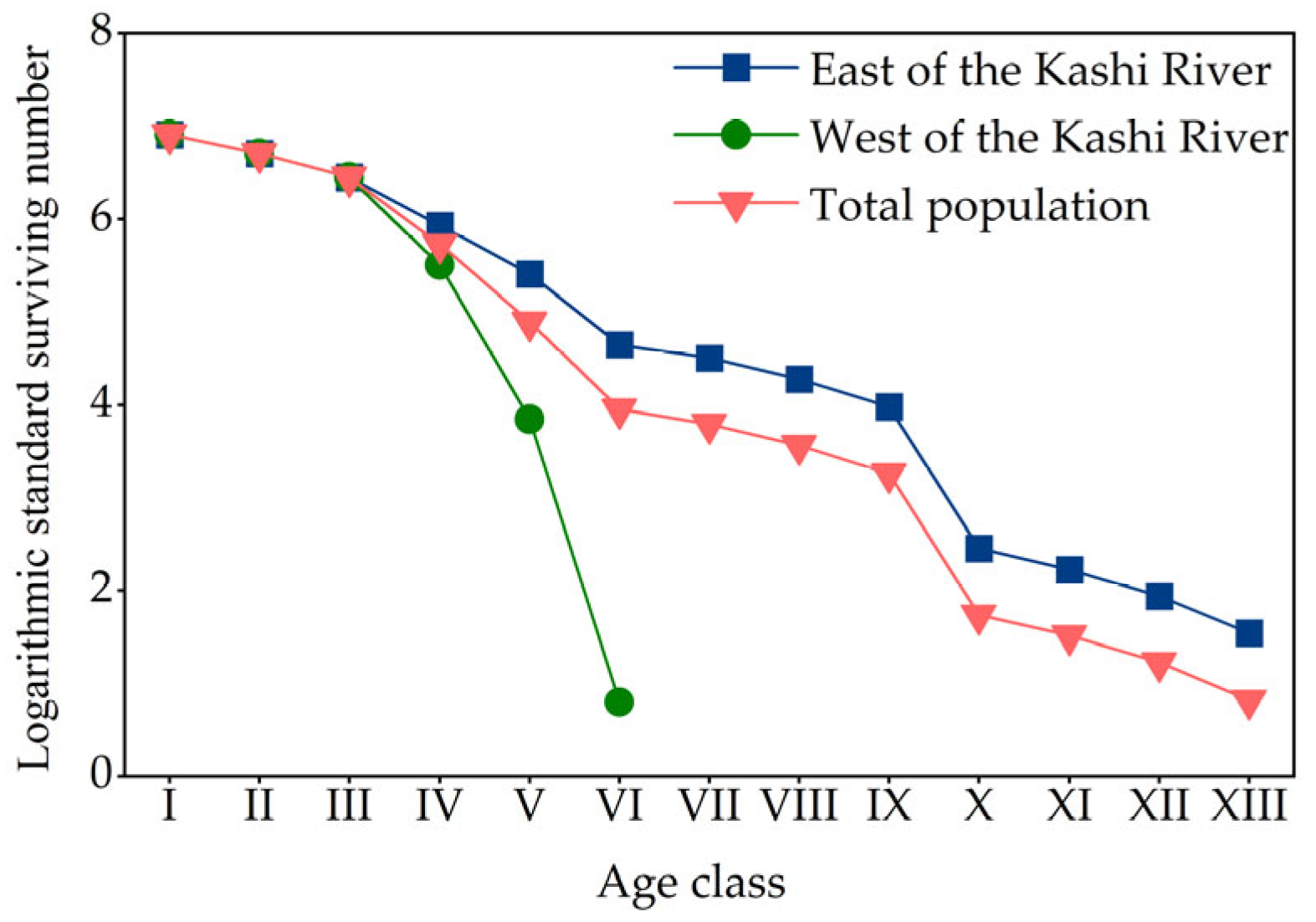
3.3.2. Survival Curve
3.3.3. Mortality Curve
3.4. Population Dynamic Quantitative Analysis
3.5. Spectral Analysis
4. Discussion
4.1. Strategies for Maintaining and Renewing the F. sogdiana Population
4.2. Age Structure and Distributional Variability of Cespitose F. sogdiana Population
4.3. Dynamics and Development Trend of Cespitose F. sogdiana Population
4.4. Conservation Countermeasures for Cespitose F. sogdiana Population
4.5. Discussion on the Dynamic Analysis Method of Cespitose F. sogdiana Population
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Del, T. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar]
- Bellingham, P.J.; Spaow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [PubMed]
- Bond, W.J.; Midgley, J.J. The evolutionary ecology of sprouting in woody plants. Int. J. Plant Sci. 2003, 164, S103–S114. [Google Scholar]
- Chamberlin, E.A.; Aarsen, L.W. The cost of apical dominance in white pine (Pinus strobus L.): Growth in multistemmed versus single-stemmed trees. Bull. Torrey Bot. Club. 1996, 123, 268–272. [Google Scholar]
- Fujiki, D.; Kikuzawa, K. Stem turnover strategy of multiple-stemmed woody plants. Ecol. Res. 2006, 21, 380–386. [Google Scholar]
- Wang, X.; Kent, M.; Fang, X. Evergreen broad-leaved forest in Eastern China: Its ecology and conservation and the importance of resprouting in forest restoration. For. Ecol. Manag. 2007, 245, 76–87. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J. Resprouting as a key functional trait in woody plants—Challenges to developing new organizing principles. New Phytol. 2010, 188, 651–654. [Google Scholar]
- Liu, D.; Guo, Z.; Cui, X.; Fan, C. Estimation of the population dynamics of Taxus cuspidata by using a static life table for its conservation. Forests 2023, 14, 2194. [Google Scholar] [CrossRef]
- Farahat, E.A. Age structure and static life tables of the endangered Juniperus phoenicea L. in North Sinai Mountains, Egypt: Implication for conservation. J. Mt. Sci. 2020, 17, 2170–2178. [Google Scholar]
- Aubin, I.; Messier, C.; Kneeshaw, D. Population structure and growth acclimation of mountain maple along a successional gradient in the southern boreal forest. Ecoscience 2005, 12, 540–548. [Google Scholar] [CrossRef]
- Kang, D.; Guo, Y.; Ren, C.; Zhao, F.; Feng, Y.; Han, X.; Yang, G. Population structure and spatial pattern of main tree species in secondary Betula platyphylla forest in Ziwuling Mountains, China. Sci. Rep. 2014, 4, 6873. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.B.; Buckland, S.T.; Morgan, B.; King, R.; Borchers, D.L.; Cole, D.J.; Besbeas, P.; Gimenez, O.; Thomas, L. Modelling population dynamics: Model formulation, fitting and assessment using state-space methods. In Methods in Statistical Ecology; Springer: New York, NY, USA, 2014. [Google Scholar]
- Yang, Y.; Ke, X.; Ji, Q.; Lang, T.; Lai, Z.; Guan, Y. Conservation Implications of Population Structure and Dynamics in Medicinal Arbor Albizia odoratissima on Hainan Island, China. Forests 2024, 15, 2227. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Deng, C.; Liu, G.; Li, H. Characteristics and dynamics analysis of Populus euphratica populations in the middle reaches of Tarim River. J. Ari. Land 2010, 2, 250–256. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G. Population structure and spatial pattern of the endemic and endangered subtropical tree Parrotia subaequalis (Hamamelidaceae). Flora 2015, 212, 10–18. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Li, J. Dynamics of the population quantity of Juglans mandshurica Maxim. in different habitats in Xinjiang, China. Pak. J. Bot. 2015, 47, 911–918. [Google Scholar]
- Omelko, A.; Ukhvatkina, O.; Zhmerenetsky, A.; Sibirina, L.; Petrenko, T.; Bobrovsky, M. From young to adult trees: How spatial patterns of plants with different life strategies change during age development in an old-growth Korean pine-broadleaved forest. For Ecol. Manag. 2018, 411, 46–66. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiao, J.; Li, Y.; Li, Y.; Zhang, S. Ramet age structure and its dynamics of Nitraria tangutorum clonal populations in the Southern Margin Area of Tengger Desert. Sci. Silv. Sin. 2013, 49, 1–9. [Google Scholar]
- Chen, G.; Zhao, W. Age structure and dynamics of Sali psammophila branches in southern edge of the Mu Us Sandy Land. J. Desert. Res. 2015, 35, 1520–1526. [Google Scholar]
- Wei, H.; Li, Y.; Lai, X.; Zhang, W. Age structure and quantitative dynamics of cespitose Juglans regia in different slope directions in Xinjiang Wild Walnut Nature Reserve. J. Trop. Subtrop. Bot. 2023, 31, 163–172. [Google Scholar]
- Li, J.; Liu, S.; Wang, Q.; Wang, F.; Wang, W. Study of Fraxinus mandshurica asexual regeneration in the Hills Area of Sanjiang Plain. Bull. Bot. Res. 2000, 20, 215–220. [Google Scholar]
- Li, J.; Nie, S.; An, B. Stump Sprouting of the Main Broad-Leaved Tree Species of Secondary Forest in Eastern Area of Northeast China. Sci. Silv. Sin. 2005, 41, 75–80. [Google Scholar]
- Tian, M.; Ke, X.; Li, M.; Deng, K.; Yang, Y.; Fang, Z.; Zhong, C.; Li, S.; Zhu, Z.; Tam, N.F.; et al. Population Status of the Endangered Semi-Mangrove Dolichandrone spathacea on Hainan Island, China. Forests 2024, 15, 865. [Google Scholar] [CrossRef]
- Nuer, M.; Zhang, W.; Yang, X.; Jia, N. Phenotypic plasticity on compnent size of compound leaves of Fraxinus sogdiana in Yili, Xinjiang. J. North. Forest. Univ. 2014, 42, 31–34+61. [Google Scholar]
- Li, D.; Yu, Y.; Xu, S.; Wen, B.; Shi, R.; Han, D. Growth regulation characteristics of current-year shoots of Fraxinus sogdiana in Yili River Valley. J. Zhejiang A F Univ. 2023, 40, 382–389. [Google Scholar]
- Zhang, W.; Yang, X.; Jia, N.; Yang, Y. Biomass Allocation among Components of Compound Leaves of Fraxinus sogdiana in Yili River Reaches, Xinjiang. Arid. Zone Res. 2016, 33, 114–119. [Google Scholar]
- Liu, Y.; Xiong, J.; Sidikejiang, M.; Liu, P. Study on the flora of seed plants in Fraxinus sogdiana Nature Reserve in Yili, Xinjiang. Cent. South. For. Invent. Plan. 2014, 33, 41–46. [Google Scholar]
- Li, C.; Du, Y.; Shi, B.; Liu, J.; Xiang, B. The identification and sequence analysis of 16S rRNA gene of Fraxinus sogdiana Bunge witches’ broom phytoplasma. Microbiol. China 2014, 41, 601–606. [Google Scholar]
- Diaz, S.; Mercado, C.; Alvarez-Cardenas, S. Structure and population dynamics of Pinus lagunae MF. Passini. For. Ecol. Manag. 2000, 134, 249–256. [Google Scholar] [CrossRef]
- Frost, I.; Rydin, H. Spatial pattern and size distribution of the animal-dispersed tree Quercus robur in two spruce-dominated forests. Ecosci 2000, 7, 38–44. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, Y.; Wang, Y.; Ji, L.; Wu, P. Population structure of Fraxinus mandshurica on Changbai Mountain. Acta. Ecol. Sin. 2015, 35, 91–97. [Google Scholar]
- Deevey, J. Life tables for natural populations of animals. Q. Rev. Biol. 1947, 22, 283–314. [Google Scholar] [PubMed]
- Hett, J.; Loucks, O. Age structure models of balsam fir and eastern hemlock. J. Ecol. 1976, 64, 1029–1044. [Google Scholar]
- Shen, S.; Ma, H.; Wang, Y.; Wang, B.; Shen, G. Structure and dynamics of natural populations of the endangered plant Euryodendron excelsum H.T. Chang. Front. Forest. China 2009, 4, 14–20. [Google Scholar]
- Zhang, J.; Ge, S.; Liang, J.; Li, J. Population age structure and dynamics of Pinus koraiensis in a broadleaved Korean pine forest in Changbai Mountain, China. Chin. J. Plant. Ecol. 2022, 46, 667–677. [Google Scholar] [CrossRef]
- Zhang, W.; Jiao, Z.; Shang, T.; Yang, Y. Demography and spectrum analysis of Juglans cathayensis populations at different altitudes in the west Tianshan valley in Xinjiang, China. Chin. J. Appl. Ecol. 2015, 26, 1091–1098. [Google Scholar]
- Liu, W.; Zhang, J.; Liu, Q. Dominant population structure and dynamic characteristics of Benula plalyphylla and Larx olgensis foret on the eastem slope of Changbai Mountain, Northeast China. Acta Ecol. Sin. 2023, 43, 7462–7473. [Google Scholar]
- Bellingham, P.; Sparrow, A. Multi-stemmed trees in montane rain forests: Their frequency and demography in relation to elevation, soil nutrients and disturbance. J. Ecol. 2009, 97, 472–483. [Google Scholar]
- Ye, J.; Hao, Z.; Wang, X.; Bai, X.; Xing, D.; Yuan, Z. Local-seale drivers of multi-stemmed tree formation in Acer.in a temperate forest of Northeast China. Chin. Sci. Bull. 2014, 59, 320–325. [Google Scholar]
- Dang, H.; Zhang, Y.; Zhang, K.; Jiang, M.; Zhang, Q. Age structure and regeneration of subalpine fir (Abies fargesii) forests across an altitudinal range in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 547–554. [Google Scholar]
- Li, W.; Li, H.; Gan, X.; Zhang, X.; Fan, Z. Population structure and dynamics of the endangered tree Tetracentron sinense Oliver. Pak. J. Bot. 2020, 52, 613–619. [Google Scholar]
- Bazzaz, F.; Chiariello, N.; Coley, P.; Pitelka, L. Allocating resources to reproduction and defense: New assessments of the costs and benefits of allocation patterns in plants are relating ecological roles to resource use. BioScience 1987, 37, 58–67. [Google Scholar]
- Zhang, W.; Yang, Y.; Li, J. Static life table and growth analysis of seedlings of Juglans mandshurica in Xinjiang, China. Austrian J. For. Sci. 2015, 132, 131–144. [Google Scholar]
- Thippawan, S.; Chowtiwuttakorn, K.; Pongpattananurak, N.; Kraichak, E. Allometric Models to Estimate the Biomass of Tree Seedlings from Dry Evergreen Forest in Thailand. Forests 2023, 14, 725. [Google Scholar] [CrossRef]
- Sánchez-Coronado, M.E.; Coates, R.; Castro-Colina, L.; de Buen, A.G.; Paez-Valencia, J.; Barradas, V.L.; Orozco-Segovia, A. Improving seed germination and seedling growth of Omphalea oleifera (Euphorbiaceae) for restoration projects in tropical rain forests. For. Ecol. Manag. 2007, 243, 144–155. [Google Scholar]
- Long, T.; Wu, X.; Wang, Y.; Chen, J.; Xu, C.; Li, J.; Li, J.Q.; Zang, R. The population status and threats of Taxus cuspidata, a plant species with extremely small populations in China. Glob. Ecol. Conserv. 2021, 26, e01495. [Google Scholar]
- Chang, B.; Yu, S.; Chen, W.; He, X.; Huang, Y.; Zhang, Y. Population structure and dynamic characteristics of Taxus cuspidata in Baishilazi National Nature Reserve, China. Glob. Ecol. Conserv. 2024, 56, e03263. [Google Scholar]
- Zacharias, M.; Pampuch, T.; Heer, K.; Avanzi, C.; Wuerth, D.G.; Trouillier, M.; Bog, M.; Wilmking, M.; Schnittler, M. Population structure and the influence of microenvironment and genetic similarity on individual growth at Alaskan white spruce treelines. Sci. Total Environ. 2021, 798, 149267. [Google Scholar]
- He, J.; Ning, C.; Zhang, W.; Halik, Ü.; Shen, Z. The Effect of Elevation on the Population Structure, Spatial Patterning and Intraspecific Interactions of Picea schrenkiana in the Eastern Tianshan Mountains: A Test of the Stress Gradient Hypothesis. Forests 2023, 14, 2092. [Google Scholar] [CrossRef]
- He, C.; Jia, S.; Luo, Y.; Hao, Z.; Yin, Q. Spatial distribution and species association of dominant tree species in Huangguan Plot of Qinling Mountains, China. Forests 2022, 13, 866. [Google Scholar] [CrossRef]
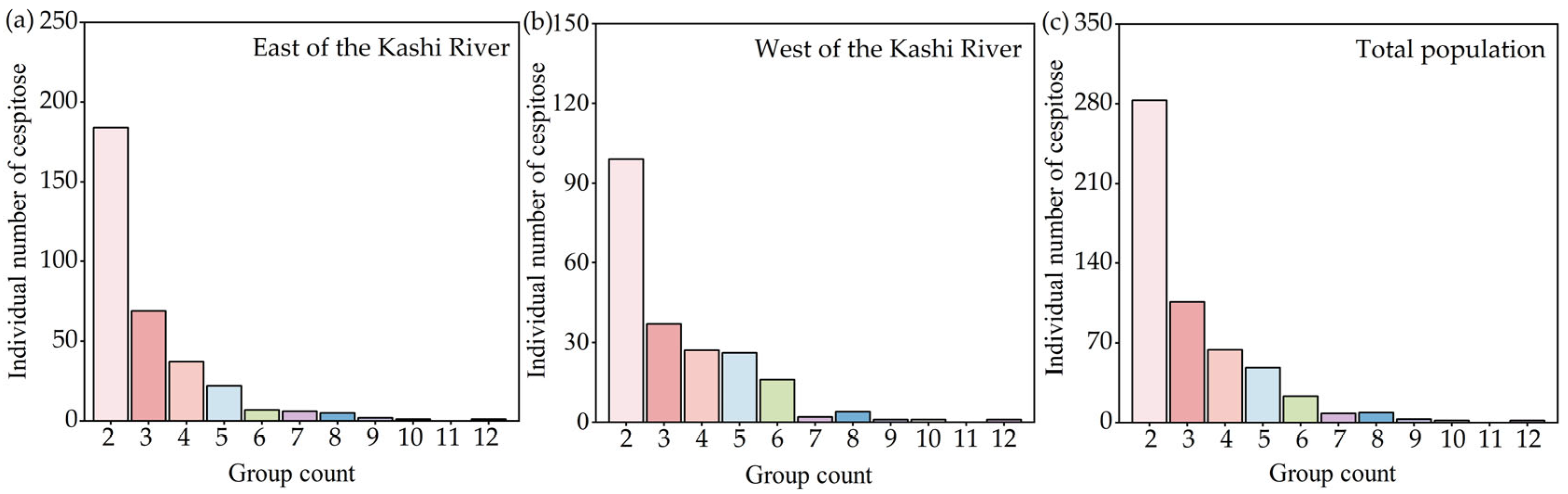


| Population Habitat | East of the Kashi River | West of the Kashi River |
|---|---|---|
| Vegetation coverage (%) | 87.26 ± 6.82 | 77.31 ± 13.49 |
| Crown density (%) | 91.02 ± 7.37 | 78.97 ± 18.51 |
| Population DBH (cm) | 24.96 ± 18.05 | 17.53 ± 8.43 |
| Population height (m) | 11.69 ± 4.43 | 10.87 ± 5.73 |
| Population crown width (m) | 4.92 ± 1.95 | 3.65 ± 1.82 |
| Soil moisture content (%) | 24.86 ± 1.40 | 11.62 ± 1.67 |
| Soil organic matter content (g∙kg−1) | 34.91 ± 1.64 | 23.53 ± 1.33 |
| Dominant species | Fraxinus sogdiana, Morus alba, Ulmus pumila | Fraxinus sogdiana, Ulmus pumila, Populus talassica |
| DBH (cm) | ≤5 | 5–15 | 15–25 | 25–35 | 35–45 | 45–55 | 55–65 | 65–75 | 75–85 | 85–95 | 95–105 | 105–115 | >115 |
| Age class | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII |
| Habitat | Age Class | Ax | ax | lx | lnlx | dx | qx | Lx | Tx | ex | Kx | Sx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| East of the Kashi River | I | 35 | 430 | 1000 | 6.908 | 184 | 0.184 | 908 | 2900 | 2.900 | 0.203 | 0.816 |
| II | 311 | 351 | 816 | 6.705 | 184 | 0.225 | 724 | 1992 | 2.440 | 0.255 | 0.775 | |
| III | 272 | 272 | 633 | 6.450 | 256 | 0.404 | 505 | 1267 | 2.004 | 0.518 | 0.596 | |
| IV | 162 | 162 | 377 | 5.932 | 153 | 0.407 | 300 | 763 | 2.025 | 0.523 | 0.593 | |
| V | 96 | 96 | 223 | 5.408 | 119 | 0.531 | 164 | 463 | 2.073 | 0.758 | 0.469 | |
| VI | 45 | 45 | 105 | 4.651 | 14 | 0.133 | 98 | 299 | 2.856 | 0.143 | 0.867 | |
| VII | 39 | 39 | 91 | 4.508 | 19 | 0.205 | 81 | 201 | 2.218 | 0.230 | 0.795 | |
| VIII | 12 | 31 | 72 | 4.278 | 19 | 0.258 | 63 | 120 | 1.661 | 0.298 | 0.742 | |
| IX | 14 | 23 | 53 | 3.979 | 42 | 0.783 | 33 | 57 | 1.065 | 1.526 | 0.217 | |
| X | 5 | 5 | 12 | 2.453 | 2 | 0.200 | 10 | 24 | 2.100 | 0.223 | 0.800 | |
| XI | 1 | 4 | 9 | 2.230 | 2 | 0.250 | 8 | 14 | 1.500 | 0.288 | 0.750 | |
| XII | 3 | 3 | 7 | 1.943 | 2 | 0.333 | 6 | 6 | 0.833 | 0.405 | 0.667 | |
| XIII | 2 | 2 | 5 | 1.537 | – | – | – | – | – | – | – | |
| West of the Kashi River | I | 39 | 450 | 1000 | 6.908 | 182 | 0.182 | 909 | 2221 | 2.221 | 0.201 | 0.818 |
| II | 263 | 368 | 818 | 6.707 | 182 | 0.223 | 727 | 1312 | 1.605 | 0.252 | 0.777 | |
| III | 286 | 286 | 636 | 6.454 | 391 | 0.615 | 440 | 586 | 0.921 | 0.956 | 0.385 | |
| IV | 110 | 110 | 244 | 5.499 | 198 | 0.809 | 146 | 170 | 0.695 | 1.656 | 0.191 | |
| V | 21 | 21 | 47 | 3.843 | 44 | 0.952 | 24 | 24 | 0.524 | 3.045 | 0.048 | |
| VI | 1 | 1 | 2 | 0.799 | – | – | – | – | – | – | – | |
| Total population | I | 74 | 880 | 1000 | 6.908 | 183 | 0.183 | 909 | 2566 | 2.566 | 0.202 | 0.817 |
| II | 574 | 719 | 817 | 6.706 | 183 | 0.224 | 726 | 1657 | 2.029 | 0.254 | 0.776 | |
| III | 558 | 558 | 634 | 6.452 | 325 | 0.513 | 472 | 932 | 1.470 | 0.719 | 0.487 | |
| IV | 272 | 272 | 309 | 5.734 | 176 | 0.570 | 221 | 460 | 1.489 | 0.844 | 0.430 | |
| V | 117 | 117 | 133 | 4.890 | 81 | 0.607 | 93 | 239 | 1.799 | 0.934 | 0.393 | |
| VI | 46 | 46 | 52 | 3.956 | 8 | 0.152 | 48 | 147 | 2.804 | 0.165 | 0.848 | |
| VII | 39 | 39 | 44 | 3.791 | 9 | 0.205 | 40 | 98 | 2.218 | 0.230 | 0.795 | |
| VIII | 12 | 31 | 35 | 3.562 | 9 | 0.258 | 31 | 59 | 1.661 | 0.298 | 0.742 | |
| IX | 14 | 23 | 26 | 3.263 | 20 | 0.783 | 16 | 28 | 1.065 | 1.526 | 0.217 | |
| X | 5 | 5 | 6 | 1.737 | 1 | 0.200 | 5 | 12 | 2.100 | 0.223 | 0.800 | |
| XI | 1 | 4 | 5 | 1.514 | 1 | 0.250 | 4 | 7 | 1.500 | 0.288 | 0.750 | |
| XII | 3 | 3 | 3 | 1.226 | 1 | 0.333 | 3 | 3 | 0.833 | 0.405 | 0.667 | |
| XIII | 2 | 2 | 2 | 0.821 | – | – | – | – | – | – | – |
| Habitat | Equation | R2 | F | p | Type |
|---|---|---|---|---|---|
| East of the Kashi River | Nx = 9.481e−0.125x | 0.923 | 132.010 | 0.000 | Deevey–II |
| Nx = 10.354x−0.556 | 0.703 | 26.019 | 0.000 | ||
| West of the Kashi River | Nx = 14.628e−0.361x | 0.652 | 7.510 | 0.052 | Deevey–II |
| Nx = 10.460x−0.845 | 0.450 | 3.267 | 0.145 | ||
| Total population | Nx = 10.815e−0.173x | 0.922 | 129.619 | 0.000 | Deevey–II |
| Nx = 12.191x−0.769 | 0.700 | 25.636 | 0.000 |
| Dynamic Index Class | Dynamic Index Value (%) | ||
|---|---|---|---|
| East of the Kashi River | West of the Kashi River | Total Population | |
| V1 | −88.75 | −85.17 | −87.11 |
| V2 | 12.54 | −8.04 | 2.79 |
| V3 | 40.44 | 61.54 | 51.25 |
| V4 | 40.74 | 80.91 | 56.99 |
| V5 | 53.13 | 95.24 | 60.68 |
| V6 | 13.33 | – | 15.22 |
| V7 | 69.23 | – | 69.23 |
| V8 | −14.29 | – | −14.29 |
| V9 | 64.29 | – | 64.29 |
| V10 | 80.00 | – | 80.00 |
| V11 | −66.67 | – | −66.67 |
| V12 | 33.33 | – | 33.33 |
| Vpi | 28.04 | 32.03 | 29.65 |
| V′pi | 2.16 | 5.34 | 2.28 |
| Pmax | 7.69 | 16.67 | 7.69 |
| Habitat | A0 | A1 | A2 | A3 | A4 | A5 | A6 | A7 |
|---|---|---|---|---|---|---|---|---|
| East of the Kashi River | 3.259 | 2.103 | 0.835 | 0.292 | 0.226 | 0.367 | 0.138 | 0.140 |
| West of the Kashi River | 3.903 | 2.283 | 0.732 | 0.487 | – | – | – | – |
| Total population | 3.474 | 2.378 | 1.013 | 0.329 | 0.194 | 0.384 | 0.211 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Jia, Y.; Xie, X.; Yang, F.; Zhang, W.; Yang, Y. Cespitose Population Structure and Dynamics of Rare Fraxinus sogdiana in the Yili River Valley, China. Forests 2025, 16, 567. https://doi.org/10.3390/f16040567
Liu H, Jia Y, Xie X, Yang F, Zhang W, Yang Y. Cespitose Population Structure and Dynamics of Rare Fraxinus sogdiana in the Yili River Valley, China. Forests. 2025; 16(4):567. https://doi.org/10.3390/f16040567
Chicago/Turabian StyleLiu, Huaqing, Yanyan Jia, Xinran Xie, Fan Yang, Wei Zhang, and Yunfei Yang. 2025. "Cespitose Population Structure and Dynamics of Rare Fraxinus sogdiana in the Yili River Valley, China" Forests 16, no. 4: 567. https://doi.org/10.3390/f16040567
APA StyleLiu, H., Jia, Y., Xie, X., Yang, F., Zhang, W., & Yang, Y. (2025). Cespitose Population Structure and Dynamics of Rare Fraxinus sogdiana in the Yili River Valley, China. Forests, 16(4), 567. https://doi.org/10.3390/f16040567




