1. Introduction
Heat stress is a significant environmental factor that limits plant growth, metabolism, and productivity [
1]. According to the Intergovernmental Panel on Climate Change, global surface temperatures are projected to increase by 0.3–1.7 °C under RCP2.6 scenarios compared to 1986–2005 averages [
2,
3]. These warming trends are anticipated to have profound impacts on agriculture and forestry worldwide [
4,
5]. Among physiological processes, photosynthesis is particularly susceptible to heat stress [
6,
7]. Elevated temperatures can reduce leaf photosynthesis and decouple it from transpiration, resulting in excessive water loss and diminished leaf water potentials [
8,
9,
10]. Prolonged heat exposure may further damage the photosynthetic apparatus in leaves, particularly photosystem II, leading to extended recovery periods for carbon fixation and new growth [
8,
9,
11,
12]. Variability in heat tolerance among species affects photosynthetic efficiency, with heat-tolerant species maintaining higher photosynthetic rates than heat-sensitive ones [
13,
14]. Recent findings highlight that chloroplasts, the central organelles for photosynthesis, are particularly vulnerable to heat-induced damage, which disrupts energy generation and stress adaptation pathways [
7].
Poplar (
Populus spp.) is widely cultivated across the globe and serves as the primary tree species for establishing forest plantations in Northern China. Its distribution spans latitudes from 22° to 70° N and elevations reaching up to 4800 m [
15]. However, with the large-scale promotion and planting of poplars, the prevalence of pests and diseases has intensified. Among these, poplar anthracnose, primarily caused by
Colletotrichum gloeosporioides, is one of the most severe diseases affecting poplars [
16]. This disease significantly reduces poplar productivity and can even result in tree mortality, leading to considerable economic and ecological losses [
17,
18]. Developing climate-resilient plants is crucial to sustaining agricultural productivity, preserving ecosystems, and addressing the challenges posed by emerging plant diseases [
19,
20].
Poplars, known for their rapid growth, hybridization potential, and ecological contributions, play an essential role in global forestry [
21,
22]. Nevertheless, poplar anthracnose remains a significant threat, severely impacting productivity and causing notable economic and ecological damage [
17,
18,
23]. Recent studies have highlighted the effects of
C. gloeosporioides infection on poplar phyllosphere microbial communities, shedding light on disease impacts and resistance mechanisms [
24]. While plant disease resistance mechanisms are well-studied, the interactions between pathogen stress and abiotic factors, such as heat stress, remain poorly understood in trees. This gap is particularly critical in the context of a changing climate.
Our prior work demonstrated the resistance of
Populus × canadensis to
C. gloeosporioides in tissue culture and hydroponic conditions [
24]. However, the response of
P. × canadensis to high temperatures and combined stressors remains largely unexplored. While studies on heat stress in trees have traditionally focused on physiological and cellular responses [
25], molecular mechanisms—particularly those integrating transcriptomic and metabolomic data—are understudied. Recent transcriptome analyses underscore the importance of phenylpropanoid biosynthesis in poplar resistance to anthracnose, particularly under combined stress conditions [
26].
This study builds upon prior research by combining transcriptome and metabolome analyses to investigate the responses of P. × canadensis to high temperatures and C. gloeosporioides infection. We specifically aimed to elucidate the molecular mechanisms underlying chlorophyll degradation, photosynthetic efficiency, and stress signaling pathways under dual stress conditions. By identifying key pathways and regulatory networks, this work provides insights into high-temperature stress adaptation in trees and contributes to breeding programs for anthracnose-resistant and climate-resilient poplar varieties.
2. Materials and Methods
2.1. Plant and Fungal Materials
Populus × canadensis (Pc) branches were collected from Yanqing District, Beijing, and hydroponically cultivated. New branches were sterilized and used as explants for tissue culture. These were grown in a rooting medium and subcultured three times for subsequent experiments.
Colletotrichum gloeosporioides CFCC 80308 (Cg) was provided by the Forest Pathology Laboratory of Beijing Forestry University [
24].
Tissue-cultured
P. × canadensis seedlings were maintained at 25 °C and 60% humidity, with a 16 h light and 8 h dark photoperiod for 5–8 weeks. For high-temperature treatment, plants were transferred to 35 °C for 48 h (H), while the control group was maintained at 25 °C (N). For inoculation,
C. gloeosporioides was cultured on a potato dextrose agar (PDA) medium for 7 days, and conidia were harvested in sterile water to a concentration of 10⁶ conidia/mL. A 30 µL aliquot of conidia suspension was applied to the surface of
P. × canadensis leaves in the respective treatment groups, while sterile water served as a control. All seedlings were maintained in their respective conditions for six days post-inoculation [
19]. Samples were then lyophilized at −40 °C for 24 h; ground into powder (30 Hz; 1 min; MM 400 grinder; Retsch GmbH, Haan, Germany); and stored at −20 °C until analysis.
2.2. RNA Isolation and Library Preparation
Total RNA was extracted using the TRIzol reagent (Invitrogen; Waltham, MA, USA), according to the manufacturer’s protocol. RNA purity and quantification were assessed using a NanoDrop 2000 spectrophotometer (Thermo Scientific; Waltham, MA, USA), while integrity was determined with an Agilent 2100 Bioanalyzer (Agilent Technologies; Santa Clara, CA, USA) (
Table S1). Library construction was performed using the VAHTS Universal V6 RNA-seq Library Prep Kit (Vazyme, Nanjing, China). Transcriptome sequencing was outsourced to OE Biotech Co., Ltd. (Shanghai, China). To ensure reproducibility, three biological replicates were prepared for each treatment group.
2.3. RNA Sequencing and Data Processing
Libraries were sequenced on an Illumina NovaSeq 6000 platform (Illumina; San Diego, CA, USA), generating 150 bp paired-end reads.
Clean reads were obtained using fastp v0.20.0 [
27], removing low-quality reads and adaptors. Reads were aligned to the reference genome using HISAT2 v2.2.1 [
28], and gene expression was quantified with FPKM [
29], and read counts were obtained via HTSeq-count [
30]. Principal Component Analysis (PCA) was performed using R (v3.2.0) to assess biological replicates and sample clustering.
2.4. Differential Gene Expression Analysis
Differentially expressed genes (DEGs) were identified using DESeq2 [
31], with significance thresholds set at q-value < 0.05 and fold change > 2 (upregulated) or <0.5 (downregulated). Hierarchical clustering and radar plots of the top 30 genes were generated using R (v 3.2.0). Functional enrichment analysis for Gene Ontology (GO), KEGG, Reactome, and WikiPathways was conducted using R packages. Gene Set Enrichment Analysis (GSEA) was performed to identify enriched pathways using predefined gene sets ranked by differential expression levels [
32,
33].
2.5. Metabolite Extraction and Analysis
Metabolites were extracted by combining 60 mg of the sample powder with 600 µL of methanol–water (7:3, v/v) containing a mixed internal standard (4 µg/mL). Samples were homogenized (60 Hz; 2 min); sonicated on ice for 30 min; and centrifuged at 13,000 rpm for 20 min at 4 °C. Supernatants were transferred to LC injection vials and stored at −80 °C. High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS/MS) was performed using an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm; 1.8 µm; Waters Corp., Milford, MA, USA) with mobile phases of 0.1% formic acid in water (A) and acetonitrile (B). Data acquisition was performed in both positive and negative ionization modes using a Q Exactive Orbitrap MS (Thermo Scientific, Waltham, MA, USA).
2.6. Chlorophyll Content Analysis
The chlorophyll content was determined using the method of Xie et al. [
34]. Powdered leaf samples (0.05 g) were mixed with 1 mL of 80% acetone and incubated on ice for 30 min in the dark. After centrifugation (12,000×
g; 5 min; 4 °C), supernatants were pooled, adjusted to 3 mL, and measured for absorbance at 663.2 nm, 646.8 nm, and 470 nm using a spectrophotometer. Chlorophyll
a and
b and the total chlorophyll content were calculated using Wellburn’s formula (1994).
2.7. Statistical Analysis
All experiments were performed in triplicate. Data were analyzed using one-way ANOVA with Duncan’s post hoc test for significance (p < 0.05) using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Plots were generated with Origin 8.0 (OriginLab) and R (v3.2.0).
3. Results
3.1. Transcriptome Sequencing and Data Quality
This study hypothesizes that elevated temperatures exacerbate the transcriptomic responses of P. × canadensis (Pc) to C. gloeosporioides (Cg) infection, with a particular focus on pathways related to photosynthetic efficiency, chlorophyll degradation, and stress signaling. Through the analysis of transcriptome data under individual and combined stress conditions, we aimed to uncover key regulatory networks and molecular mechanisms that drive poplar adaptation to dual stress factors.
Transcriptomes from Pc-Cg-N, Pc-No-N, Pc-Cg-H, and Pc-No-H samples were sequenced to examine gene expression changes under high temperature (H) and
C. gloeosporioides inoculation (Cg) conditions. The sample code indicates the poplar species
Populus ×
canadensis (Pc) and whether it was infected by
C. gloeosporioides (-Cg) or mock infected (-No) and incubated at a normal (-N) or a high (-H) temperature. Each sample generated an average of 45 million raw reads, with clean reads exceeding 6.12 Gb per sample. Over 98.61% of the data achieved a quality score of Q30, and the mapping rate to the reference genome ranged from 78.26% to 79.76% (
Table 1). These high-quality data confirm the reliability of sequencing for downstream analyses. The Principal Component Analysis (PCA) revealed a clear clustering of biological replicates, confirming the reproducibility of the experimental groups.
3.2. Differentially Expressed Genes (DEGs)
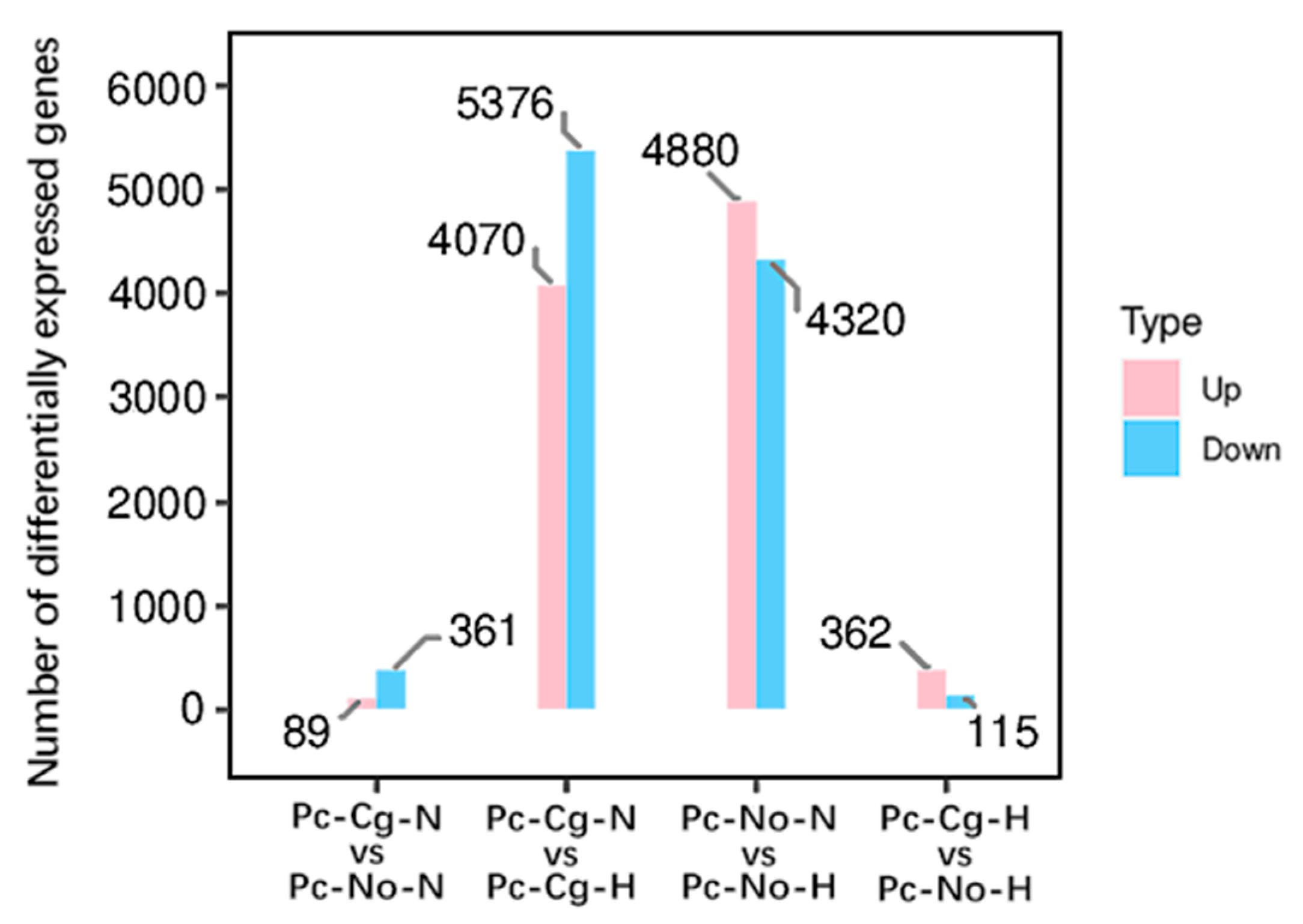
Differentially expressed gene (DEG) analysis revealed extensive transcriptional changes across the conditions, with high temperature inducing the most significant differences. The Pc-Cg-N vs. Pc-No-N comparison identified 450 DEGs, whereas over 9000 DEGs were detected in both Pc-Cg-N vs. Pc-Cg-H and Pc-No-N vs. Pc-No-H comparisons, highlighting the strong impact of heat stress. Under dual stress (Pc-Cg-H vs. Pc-No-H), 477 DEGs were identified, with upregulated genes outnumbering downregulated ones (
Figure 1).
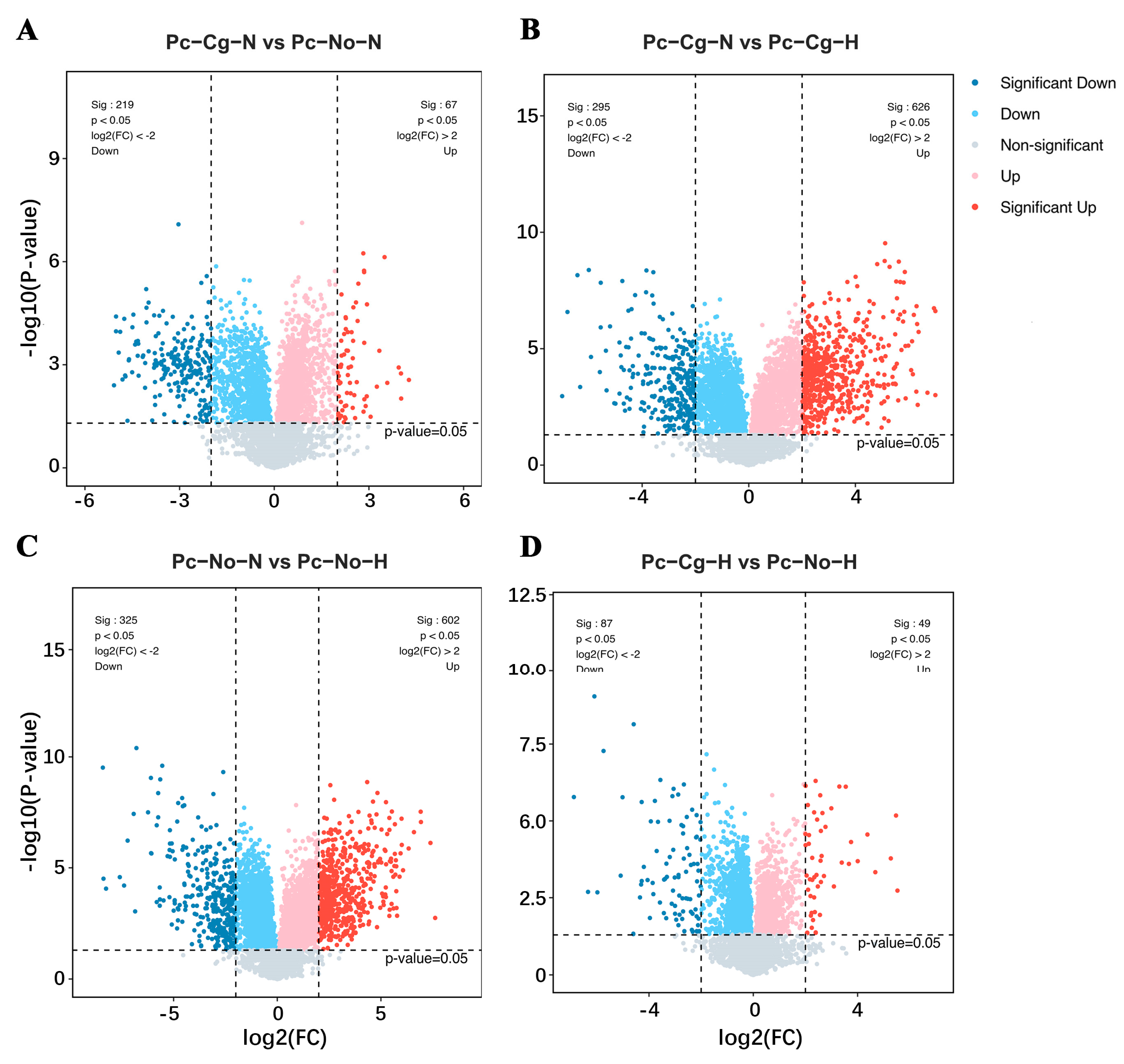
Volcano plots (
Figure S1) illustrate the distribution of upregulated and downregulated genes in each comparison. In Pc-Cg-N vs. Pc-No-N, inoculation with
C. gloeosporioides at a normal temperature triggered defense responses, as evidenced by the upregulation of defense-related genes. High-temperature treatment (Pc-Cg-N vs. Pc-Cg-H) intensified the expression of several defense-related genes while suppressing others, demonstrating the complex impact of heat stress on transcriptional regulation.
In the Pc-Cg-N vs. Pc-No-N comparison, inoculation with
C. gloeosporioides at a normal temperature resulted in a significant upregulation of multiple defense-related genes, such as At1g17230 (Pag.B11G000332.v3.1), a putative leucine-rich repeat receptor-like serine/threonine-protein kinase. Other genes, including the putative jasmonate catabolic enzyme JOX2 (Pag.B16G000308.v3.1), were downregulated. These DEGs may be associated with the poplar’s defense mechanisms, indicating that the pathogen triggers an innate immune response in poplar, thereby activating the expression of related genes (
Figure 2A,
Table S2).
In the Pc-Cg-N vs. Pc-Cg-H comparison, high-temperature treatment caused significant changes in gene expression following
C. gloeosporioides inoculation. In accordance with the heat treatment, the small heat shock protein HSP18.1 (Pag.A01G002756.v3.1) was strongly induced, whereas other defense-related enzymes, including the linoleate 13S-lipoxygenase LOX2.1 (Pag.B01G000229.v3.1), were downregulated. Some defense-related genes showed higher expression levels at elevated temperatures, while others were inhibited, suggesting that temperature modulates the expression of disease-resistance genes. These results indicate that temperature fluctuations significantly impact gene expression during pathogen infection (
Figure 2B and
Table S3).
In the Pc-No-N vs. Pc-No-H comparison, high-temperature treatment alone altered gene expression in the absence of
C. gloeosporioides. The heat stress transcription factor HSFB2B (Pag.A01G001259.v3.1), for example, was strongly upregulated, whereas the putative dehydration-responsive element-binding protein 3 DREB3 (Pag.A01G001025.v3.1) was downregulated, reflecting the adaptive mechanisms of poplar in response to heat stress. This includes the activation of heat-responsive and metabolic regulation pathways, even without pathogen interaction (
Figure 2C and
Table S4).
In the Pc-Cg-H vs. Pc-No-H comparison,
C. gloeosporioides inoculation under high-temperature conditions caused significant gene expression changes compared to uninoculated plants. Under these conditions the pathogenesis-related protein 1, PRB1 (Pag.B01G003051.v3.1) was upregulated, whereas the putative defense related WRKY transcription factor WRKY33 (Pag.B16G000189.v3.1) was downregulated. However, defense-related genes were both up- and downregulated, suggesting that dual stress conditions activated specific mechanisms to counteract the combined effects of heat and pathogen stress (
Figure 2D and
Table S5).
3.3. GO Enrichment Analysis of DEGs
A GO enrichment analysis provided insights into the biological processes (BPs), cellular components (CCs), and molecular functions (MFs) affected under different stress conditions. The results for each comparison group are detailed below and illustrated in
Figure 3.
In Pc-Cg-N vs. Pc-No-N, significant enrichment was observed in BP categories related to defense, such as “positive regulation of defense response to insect”, “defense response”, and “response to wounding” (
Figure 3A). These results suggest an upregulation of stress-resistance pathways under normal temperature conditions following
C. gloeosporioides inoculation. The CC categories were enriched in extracellular components, including “extracellular region” and “extracellular matrix”, highlighting the involvement of cell surface-related defense mechanisms. In the MF category, catalytic activity and binding functions, such as “calcium ion binding”, were significant, reflecting the metabolic and signaling changes triggered by pathogen infection.
In Pc-Cg-N vs. Pc-Cg-H, a high temperature caused notable changes in BP categories, including “mitotic spindle assembly”, “mitotic cell cycle phase transition”, and “regulation of cyclin-dependent protein serine/threonine kinase activity”, indicating disruptions to the cell cycle and division processes under stress (
Figure 3B). Enriched CC categories, such as “condensed chromosome”, “kinesin complex”, and “thylakoid”, suggest impacts on chromosome organization and photosynthesis. MF categories included protein activities such as “protein kinase activity” and “microtubule binding”, emphasizing the regulation of protein dynamics and structural components under heat stress.
In Pc-No-N vs. Pc-No-H, BP enrichment was observed in pathways like “glutathione metabolic process” and “defense response”, indicating that heat stress alone activates mechanisms to counteract oxidative damage and maintain cellular stability (
Figure 3C). CC categories included structural components such as “spindle microtubule” and “membrane”, while MF categories highlighted enzymatic activities like “lipase activity” and “hydrolase activity”, reflecting enhanced metabolic regulation under heat stress.
Under dual stress (Pc-Cg-H vs. Pc-No-H), pathways related to secondary metabolism, such as “lignan biosynthetic process”, and stress-related components like “phagocytic vesicle”, were enriched, underscoring the synergistic impact of heat and pathogen stress (
Figure S2). Enriched CC categories, such as “contractile ring” and “phagocytic vesicle”, suggest cellular reorganization to combat stress. MF categories included “glutathione transferase activity” and “chitinase activity”, indicating robust antioxidant and antimicrobial defenses activated under combined heat and pathogen stress (
Figure 3D).
Overall, these results demonstrate the distinct yet overlapping GO categories impacted by C. gloeosporioides inoculation, high temperatures, and their combined effects, emphasizing the complexity of stress responses in P. × canadensis.
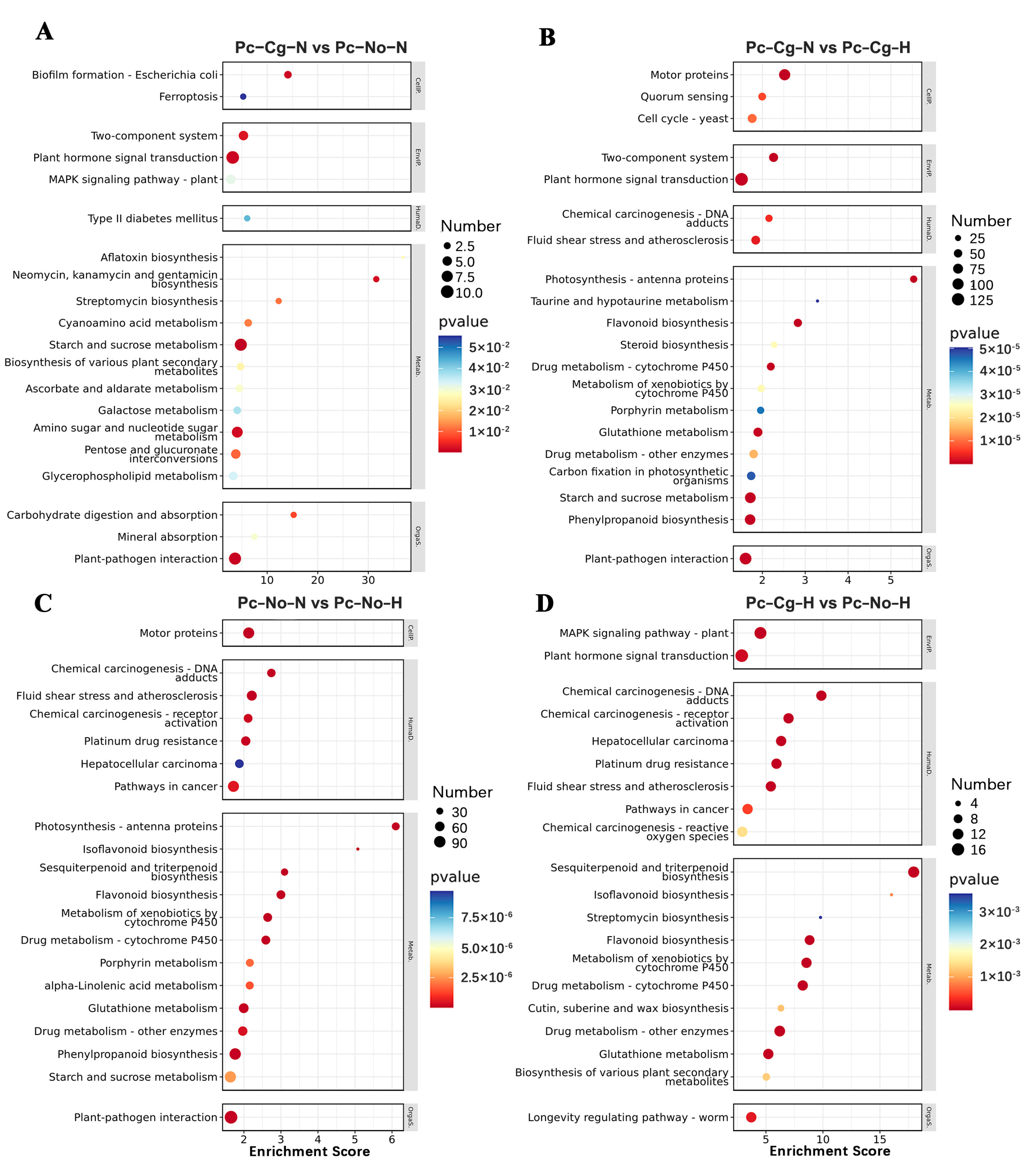
3.4. KEGG Pathway Enrichment Analysis of DEGs
The KEGG pathway enrichment analysis identified critical pathways affected by
C. gloeosporioides inoculation, high temperatures, and their combined effects. The enriched pathways for each comparison group are described below and illustrated in
Figure S3 and
Figure 4.
In Pc-Cg-N vs. Pc-No-N, enriched pathways included ferroptosis, a two-component system, plant hormone signaling, and MAPK signaling pathways (
Figure 4A). These results suggest that under normal temperature conditions,
C. gloeosporioides inoculation activates defense-related mechanisms, such as ferroptosis, which is involved in oxidative stress management, and MAPK signaling, which mediates stress-responsive gene expression.
In Pc-Cg-N vs. Pc-Cg-H, significant pathways included cell cycle regulation, photosynthesis, and plant hormone signaling (
Figure 4B). These findings indicate that a high temperature exacerbates the effects of
C. gloeosporioides infection, particularly through alterations in photosynthetic efficiency and hormonal regulation. The enrichment of hormone signaling pathways suggests their role in modulating poplar’s defense responses under dual stress conditions.
In Pc-No-N vs. Pc-No-H, heat stress alone significantly impacted photosynthesis; metabolic pathways; and pathways associated with stress responses, including cancer-related pathways (
Figure 4C). These results reflect the adaptive responses of
P. × canadensis to high temperatures, such as activating metabolic adjustments to maintain cellular stability and mitigate heat-induced damage.
In Pc-Cg-H vs. Pc-No-H, dual stress conditions enriched pathways related to MAPK signaling, plant hormone signaling, plant–pathogen interactions, and chemical carcinogenesis (
Figure 4D). These results suggest that the combined stressors trigger more complex and robust defense mechanisms, including crosstalk between biotic and abiotic stress pathways. The enrichment of MAPK signaling and plant–pathogen interaction pathways highlights their importance in coordinating rapid stress responses.
Overall, the KEGG pathway enrichment analysis underscored the dynamic interplay between metabolic regulation, signaling, and defense pathways under C. gloeosporioides infection, high temperatures, and their combined stress, illustrating the multifaceted nature of stress adaptation in P. × canadensis.
3.5. Comparison of Differentially Accumulated Metabolites
An analysis of differentially accumulated metabolites (DAMs) revealed significant metabolic adjustments in response to stress conditions. Heat stress induced the most pronounced changes, with over 900 DAMs identified in both the Pc-Cg-N vs. Pc-Cg-H and Pc-No-N vs. Pc-No-H comparisons. In contrast, pathogen infection alone (Pc-Cg-N vs. Pc-No-N) led to 286 DAMs, while the dual stress condition resulted in 136 DAMs, indicating a distinct and attenuated metabolic response pattern (
Figure 5).
Volcano plots illustrate a significant upregulation or downregulation of metabolites, with notable enrichment in pathways associated with secondary metabolism, oxidative stress, and signaling. These results highlight the dynamic metabolic adjustments in P. × canadensis under combined stress conditions.
3.6. Chlorophyll Content and Photosynthetic Characteristics
The chlorophyll content, an indicator of photosynthetic efficiency, was significantly reduced under high temperature and pathogen stress. In Pc-Cg-H, chlorophyll
a and
b levels and that of the total chlorophyll were 1067.57 ± 24.80 µg/g, 432.32 ± 28.13 µg/g, and 1499.88 ± 50.71 µg/g, respectively. These values were substantially lower than those in Pc-Cg-N (chlorophyll
a: 1569.45 ± 45.27 µg/g; chlorophyll
b: 619.56 ± 12.56 µg/g; total chlorophyll: 2189.01 ± 57.52 µg/g) (
Figure 6).
A high temperature alone (Pc-No-H) reduced the chlorophyll content compared to normal conditions (Pc-No-N), and dual stress (Pc-Cg-H) caused the most significant reduction. This suggests that high temperatures and pathogen infection have an additive impact, impairing the stability and synthesis of chlorophyll.
4. Discussion
Plants face multiple environmental challenges, with high temperatures and pathogen infections among the most significant stressors affecting growth and productivity. This study highlights the complex response mechanisms of Populus × canadensis under combined high temperature and Colletotrichum gloeosporioides stress, providing valuable insights into poplar resilience mechanisms.
4.1. Impact of High Temperatures on Photosynthesis
Photosynthesis is one of the most temperature-sensitive physiological processes in plants, and our findings confirm that high temperatures significantly suppress the chlorophyll content and photosynthetic efficiency in
P. × canadensis. Chlorophyll degradation was most pronounced under dual stress, suggesting an additive impact of heat and pathogen infection. These results align with previous studies demonstrating heat-induced disruptions in chloroplast structure and function [
35,
36]. The reduced chlorophyll levels may result from the oxidative stress-induced degradation of pigments, as observed in other species under similar conditions [
37,
38]. Notably, chlorophyll
a showed the greatest reduction, suggesting a disproportionate impact on the primary photosynthetic pigment [
7].
4.2. Transcriptional and Metabolic Adjustments to Stress
The transcriptome analysis revealed that defense-related pathways, including MAPK signaling and ferroptosis, were significantly activated in response to
C. gloeosporioides infection. High temperatures amplified these responses, modulating pathways related to the cell cycle, phytohormone signaling, and photosynthesis. The enrichment of phytohormone signaling pathways, particularly abscisic acid and ethylene, underscores their role in coordinating responses to combined stressors. These findings are consistent with studies in
Arabidopsis and rice, where hormonal regulation was shown to mediate stress adaptation [
39,
40].
Metabolomic analysis revealed significant changes in secondary metabolism and antioxidant pathways. The upregulation of phenylpropanoid biosynthesis, particularly under dual stress, highlights its role in reinforcing structural defenses and mitigating oxidative damage. This observation aligns with recent findings on phenylpropanoid pathway activation in poplars under stress [
26]. Additionally, the activation of glutathione metabolic pathways suggests that ROS-scavenging mechanisms are central to the heat stress response, as reported in other tree species [
7,
41].
4.3. Synergistic Effects of Combined Stressors
The unique transcriptional and metabolic profiles observed under dual stress conditions underscore the complex interplay between heat and pathogen responses. The significant reduction in chlorophyll content and the activation of MAPK signaling pathways suggest that heat stress exacerbates the detrimental effects of
C. gloeosporioides [
19,
42]. This synergy is likely due to overlapping signaling pathways, such as ROS generation and hormonal crosstalk, as suggested in other studies on combined abiotic and biotic stresses [
20,
26].
Interestingly, dual stress conditions enriched pathways associated with lignan biosynthesis and phagocytic vesicle formation, indicating a structural and cellular reorganization to counteract external stressors. These findings emphasize the importance of secondary metabolic adjustments in adapting to simultaneous challenges, reinforcing previous work on stress-induced accumulation in woody species [
26].
4.4. Implications for Breeding Stress-Resilient Poplars
The findings from this study provide a comprehensive framework for understanding stress tolerance in poplar. By elucidating the molecular mechanisms of defense, these results can guide breeding programs aimed at enhancing resistance to both high temperatures and pathogens. Targeting key pathways, such as MAPK signaling, phenylpropanoid biosynthesis, and chlorophyll metabolism, could yield cultivars with improved resilience to environmental stressors.
4.5. Future Directions
While this study provides critical insights into the dual stress response of P. × canadensis, further research is needed to explore the following:
The specific roles of individual phytohormones in stress signaling.
Long-term effects of dual stress on growth and productivity in field conditions.
Genetic engineering approaches to modulate key pathways identified in this study.
By integrating transcriptomics and metabolomics, this work sets the stage for advanced studies on stress resilience in forest species. The combined approach not only reveals the complexity of stress responses but also highlights actionable targets for improving tree adaptability in the face of climate change.
5. Conclusions
This study provides a comprehensive analysis of the molecular responses of Populus × canadensis to combined high temperature and Colletotrichum gloeosporioides stress using an integrated transcriptomic and metabolomic approach. Our findings reveal that C. gloeosporioides inoculation under ambient temperature conditions activates key defense-related pathways, such as MAPK signaling and ferroptosis. High temperatures amplify these responses, leading to significant transcriptional and metabolic changes, particularly in pathways associated with the cell cycle, photosynthesis, and phytohormone signaling. Additionally, high temperatures exacerbate the decline in chlorophyll content, with the most pronounced reductions observed under dual stress conditions. These findings highlight the complex interplay between biotic and abiotic stress responses in poplar, identifying key regulatory pathways as potential targets for breeding stress-resilient cultivars. Pathways such as MAPK signaling, phenylpropanoid biosynthesis, and chlorophyll metabolism represent promising targets for genetic engineering and marker-assisted selection to enhance stress tolerance. This study underscores the value of combining transcriptomic and metabolomic approaches to unravel the intricate molecular mechanisms underlying tree resilience. However, the experiments were conducted in controlled environments, which may not fully replicate field conditions. Future research should assess the long-term effects of combined stresses on poplar growth and productivity in natural settings, integrating findings from controlled studies into field applications. By linking molecular insights to practical strategies, such as poplar breeding and forest management, this research contributes to sustainable forestry practices that support ecosystem stability and productivity in the face of climate change.