Response of Growth and Non-Structural Carbohydrates’ Allocation in Pinus yunnanensis Seedlings to Simulated Sunflecks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Experimental Design
2.3. Indicator Measurement
2.4. Statistical Analysis
3. Results
3.1. Effects of Different Shading and Simulated Sunfleck Treatments on Seedling Height and Ground Diameter of P. yunnanensis Seedlings
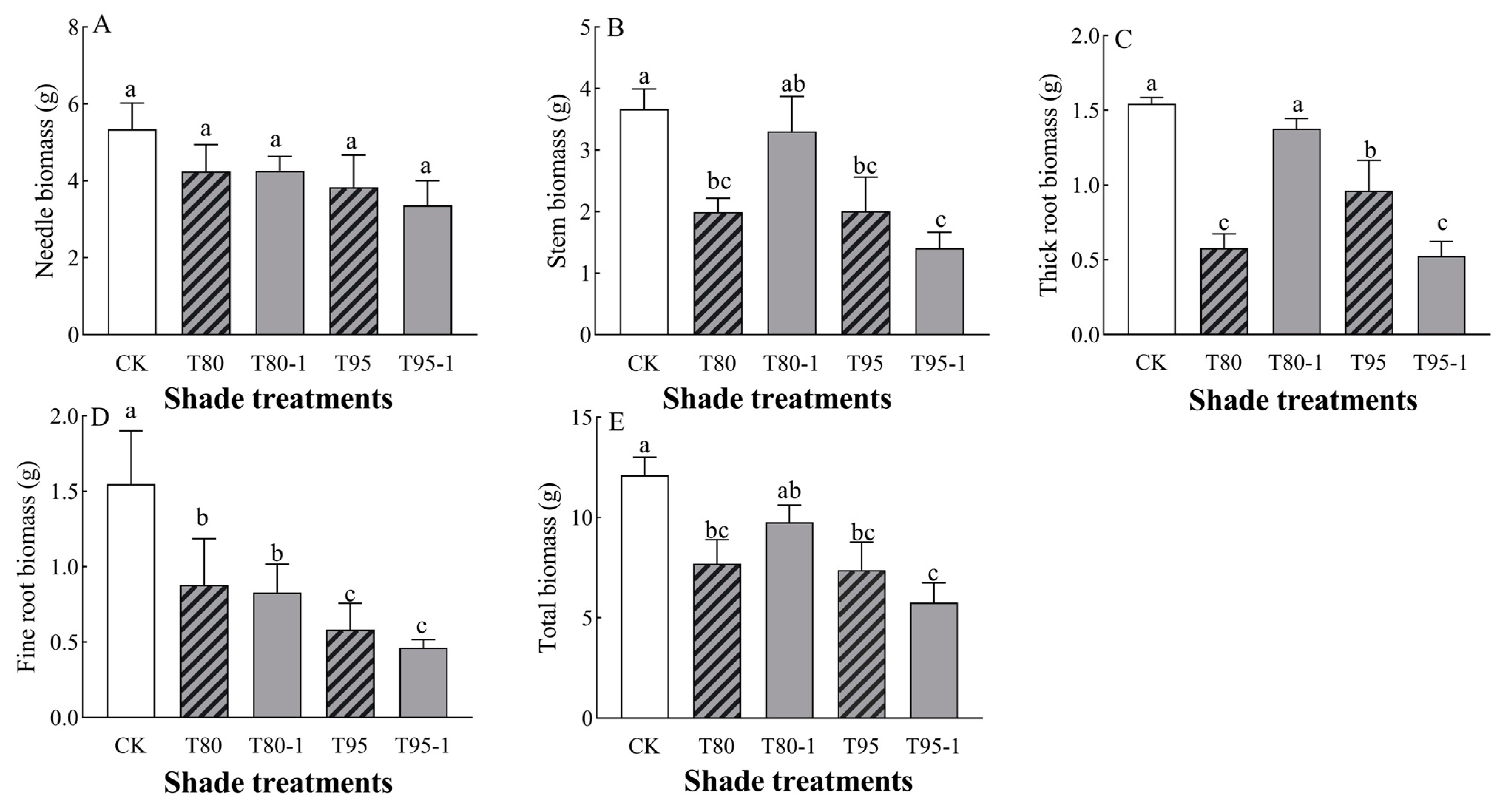
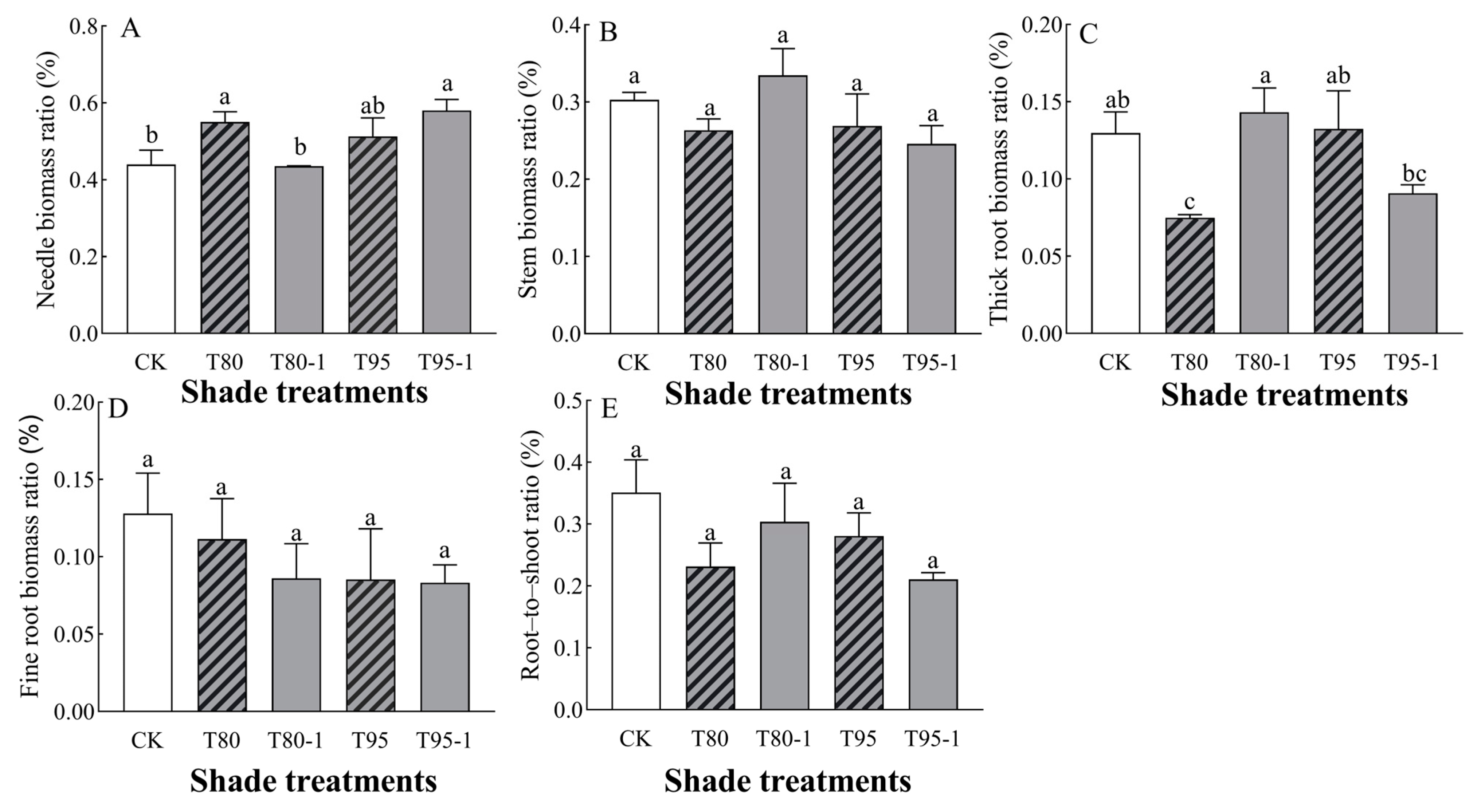
3.2. Effects of Different Shade and Simulated Sunfleck Treatments on Biomass of P. yunnanensis Seedlings
3.2.1. Biomass Changes
3.2.2. Changes in Biomass Allocation Ratio
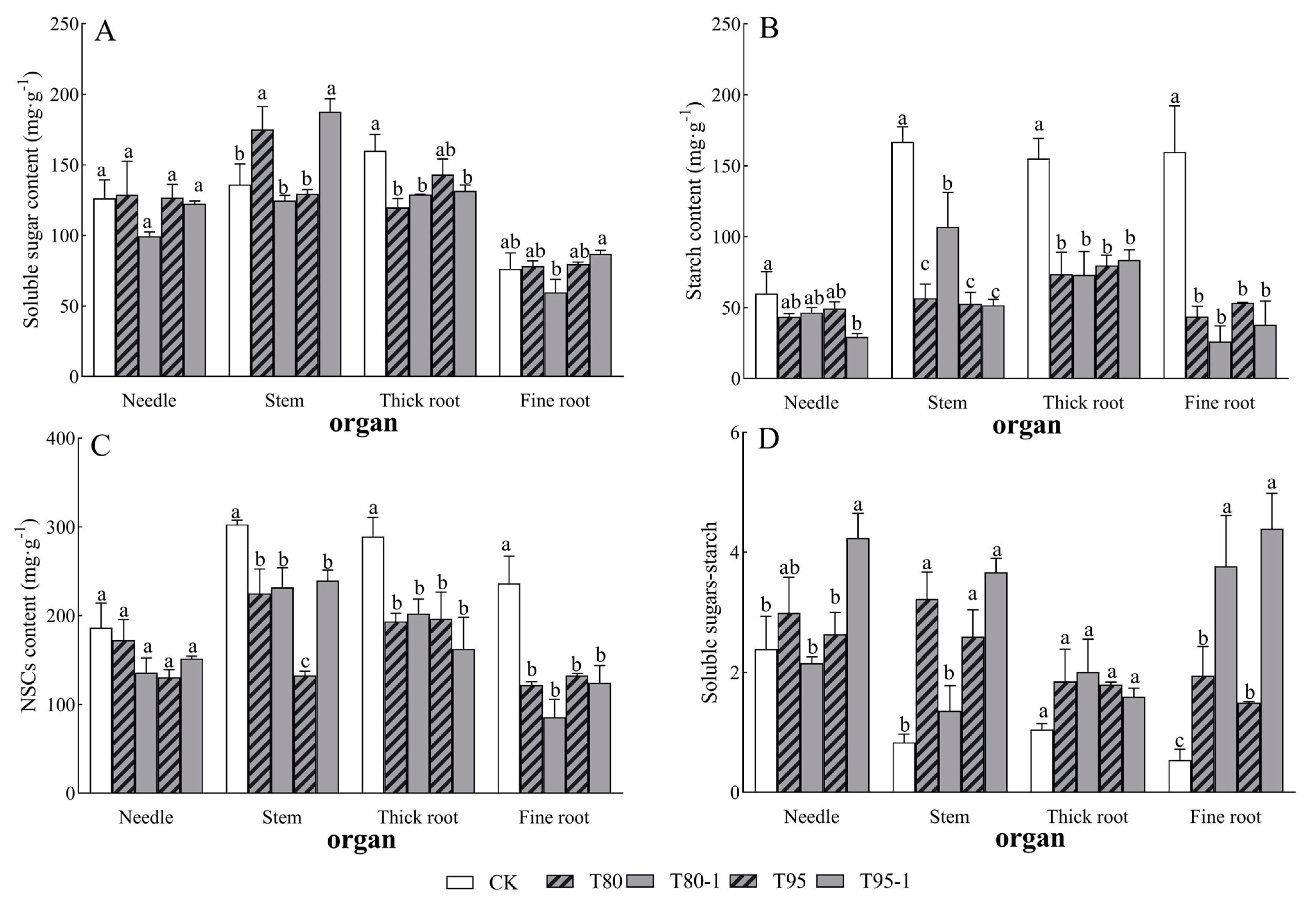
3.3. Effects of Different Shading and Simulated Sunfleck Treatments on P. yunnanensis Non-Structural Carbohydrates
3.4. Distribution Pattern of Non-Structural Carbohydrates Under Shading and Simulated Sunfleck Treatments
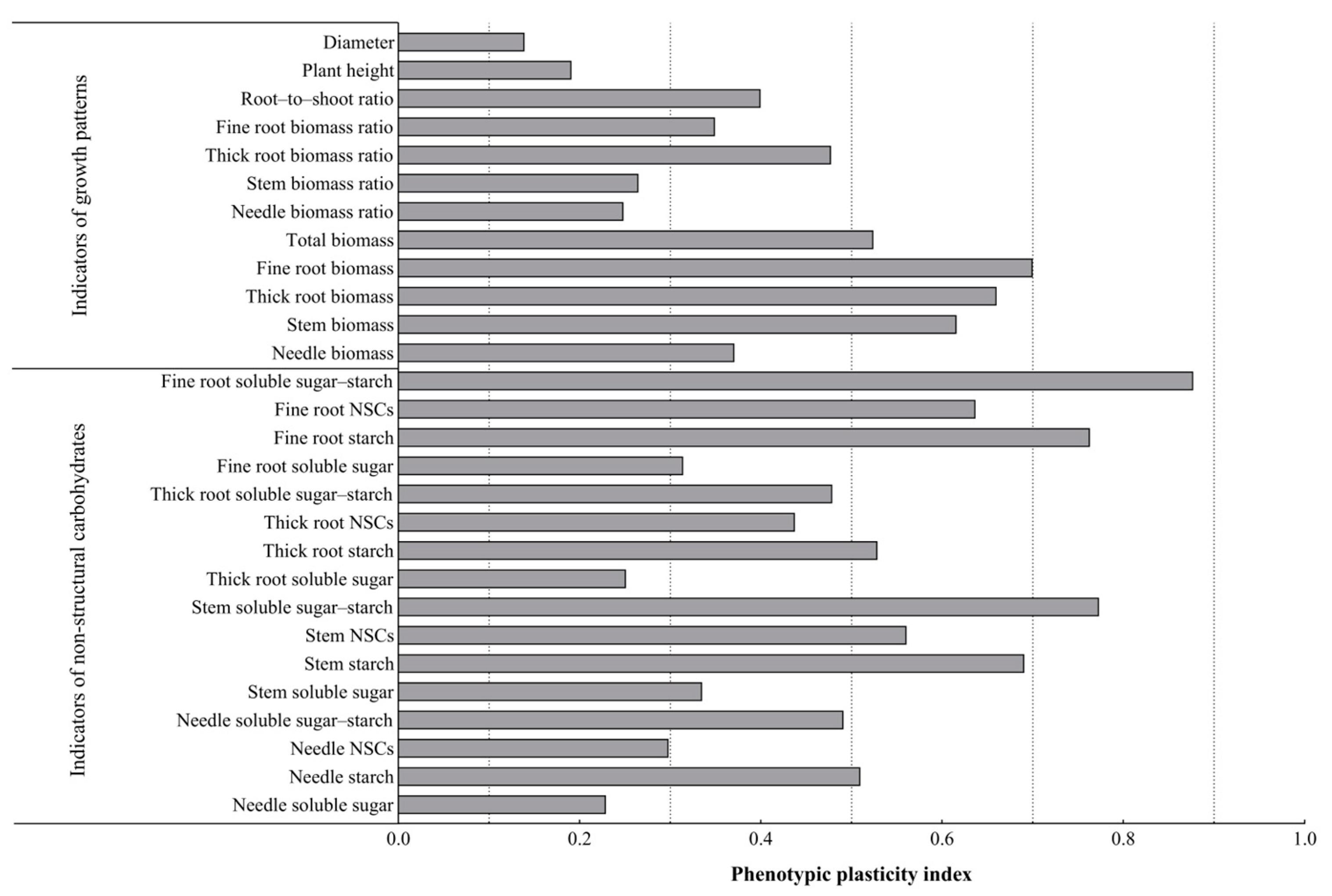
3.5. Phenotypic Plasticity Analysis of P. yunnanensis Seedling Growth and NSCs of Each Organ Under Different Light Conditions
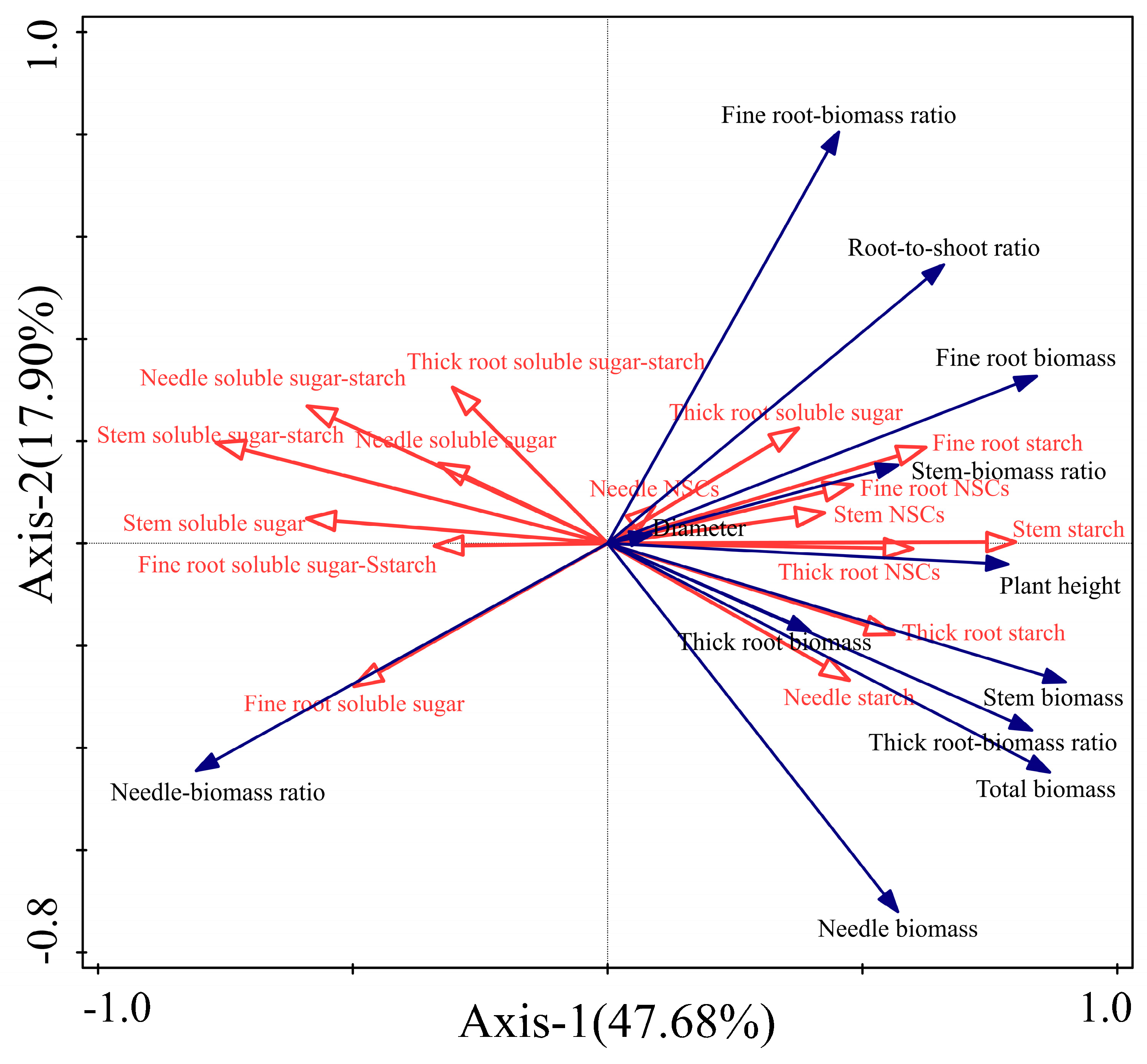
3.6. Relationship Between Non-Structural Carbohydrate Characteristics and Morphological Characteristics of P. yunnanensis Seedlings
4. Discussion
4.1. Growth and Biomass Allocation of P. yunnanensis Seedlings in Response to Shading and Simulated Sunfleck
4.2. Response of Non-Structural Carbohydrates in Various Organs of P. yunnanensis Seedlings to Shading and Simulated Sunfleck
4.3. Effects of Non-Structural Carbohydrates Characteristics of Various Organs of P. yunnanensis Seedlings on Morphological Characteristics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Poorter, L. Growth responses of 15 rain-forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 2002, 13, 396–410. [Google Scholar] [CrossRef]
- Walters, M.B.; Reich, P.B. Are Shade Tolerance, Survival, and Growth Linked? Low Light and Nitrogen Effects on Hardwood Seedlings. Ecology 1996, 77, 841–853. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Change Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Ouden, F. Dynamics of non-structural carbohydrates in two Ficus species after transfer to deep shade. Environ. Exp. Bot. 2005, 54, 148–154. [Google Scholar] [CrossRef]
- Li, W.; Chen, G.; Fang, Y.; Wang, T.; Wu, Y.; Wu, Y.; Liu, X.; Jiang, B. Hydrogen peroxide as a systemic messenger in the photosynthetic induction of mulberry leaves. J. For. Res. 2021, 32, 945–952. [Google Scholar] [CrossRef]
- O’Brien, M.; Leuzinger, S.; Philipson, C.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Mcdowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Reichenbacker, R.R.; Schultz, R.C.; Elwood, R.; Hart, E.R. Artificial defoliation effect on Populus growth, biomass production, and total nonstructural carbohydrate concentration. Environ. Entomol. 1996, 25, 632–642. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczi, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, D. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar]
- Koch, K. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Chapin, F.S.I.; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Jacquet, J.S.; Bosc, A.; O’Grady, A.; Jacte, H. Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Adv. Eng. Softw. 2014, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Shi, C.; Silva, L.C.R.; Zhang, H.; Zheng, Q.; Xiao, B.; Wu, N.; Sun, G. Climate warming alters nitrogen dynamics and total non-structural carbohydrate accumulations of perennial herbs of distinctive functional groups during the plant senescence in autumn in an alpine meadow of the Tibetan Plateau, China. Agric. For. Meteorol. 2015, 200, 21–29. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Lang, X.; Huang, X.; Zhang, Z. Variation of Non-Structural Carbohydrates for the Dominant Species in a Monsoon Broad-Leaved Evergreen Forest in Pu’Er, Yunnan Province. Sci. Silvae Sin. 2017, 53, 1–9. [Google Scholar]
- Liu, Y.; Xiao, J.; Sun, J.; Zhao, Z.; Deng, X.; Wu, J.; Zhang, D.; Bao, Y. Seasonal variation in C:N:P stoichiometry, nonstructural carbohydrates, and carbon isotopes of two coniferous pioneer tree species in subtropical China. Front. Plant Sci. 2023, 27, 1225436. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Gao, C.; Li, S.; Huang, X.; Lang, X.; Su, J. Radial growth response of Pinus yunnanensis to rising temperature and drought stress on the Yunnan Plateau, southwestern China. For. Ecol. Manag. 2020, 474, 118357. [Google Scholar] [CrossRef]
- Xu, Y.; Woeste, K.; Cai, N.; Kang, X.; Li, G.; Chen, S.; Duan, A. Variation in needle and cone traits in natural populations of Pinus yunnanensis. J. For. Res. 2016, 27, 41–49. [Google Scholar] [CrossRef]
- Su, W.; Yu, J.; Zhang, S.Z.; Wang, L.; Zhao, G.; Zhou, R. Comparison of the canopy and soil seed banks of Pinus yunnanensis in central Yunnan, China. For. Ecol. Manag. 2019, 437, 41–48. [Google Scholar] [CrossRef]
- Wang, X.Z.; Wang, L.F.; Wang, Y.; Huang, Y.; Ding, Z.; Zhou, J.; Gou, D. Identification and genetic analysis of the pinewood nematode Bursaphelenchus xylophilus from Pinus yunnanensis. For. Pathol. 2015, 45, 388–399. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wu, J.W.; Jing, H.Q. Non-structural carbohydrate (NSC) content and C:N:P stoichiometry of Pinus yunnanensis seedling needles in response to shade treatment. Ind. Crop Prod. 2024, 210, 118138. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y. Carbon: Nitrogen stoichiometry in forest ecosystems during stand development. Glob. Ecol. Biogeogr. 2011, 20, 354–361. [Google Scholar] [CrossRef]
- Frey, B.R.; Ashton, M.S. Growth, survival and sunfleck response of underplanted red oaks (Quercus spp.; section Erythrobalanus) along a topographic gradient in southern New England. For. Ecol. Manag. 2018, 30, 419–420. [Google Scholar] [CrossRef]
- Wagner, A.; McGraw, J.B. Sunfleck effects on physiology, growth, and local demography of American ginseng (Panax quinquefolius L.). For. Ecol. Manag. 2013, 291, 220–227. [Google Scholar] [CrossRef]
- Zhao, S.J. The Experimental Guide for Plant Physiology, 3rd ed.; Science Press: Beijing, China, 2002. [Google Scholar]
- Katahata, S.; Naramoto, M.; Kakubari, Y.; Mukai, Y. Photosynthetic capacity and nitrogen partitioning in foliage of the evergreen shrub Daphniphyllum humile along a natural light gradient. Tree Physiol. 2007, 27, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Q.; Daryanto, S.; Guo, S.; Huang, Z.; Wang, Z.; Wang, L.; Ma, X. Responses of Chinese fir and Schima superba seedlings to light gradients: Implications for the restoration of mixed broadleaf-conifer forests from Chinese fir monocultures. For. Ecol. Manag. 2018, 419–420, 51–57. [Google Scholar] [CrossRef]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Pires, M.V.; Almeida, A.A.F.; Figueiredo, A.L. Photosynthetic characteristics of ornamental passion flowers grown under different light intensities. Photosynthetica 2011, 49, 593–602. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar]
- Poorter, L.; Hayashida-Oliver, Y. Effects of seasonal drought on gap and understorey seedlings in Bolivian moistforest. J. Trop. Ecol. 2000, 16, 481–498. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Differences in biomass allo-cation patterns between saplings of two co-occurring Mediterranean oaks as reflecting different strategies in the use of light and water. Eur. J. For. Res. 2010, 129, 697–706. [Google Scholar] [CrossRef]
- Portsmuth, A.; NiinemetsI, Ü. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Deng, X.X.; Xiao, W.F.; Shi, Z.; Zeng, L.X.; Lei, L. Combined effects of drought and shading on growth and Non-Structural carbohydrates in Pinus massoniana Lamb. Seedlings. Forests 2019, 11, 18. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Huang, Z.J.; Wang, Z.N.; Chen, Y.F.; Wen, Z.M.; Liu, B.; Mulualem, T. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Bllaré, C.L.; Scopel, A.L.; Sànchez, R.A. Foraging for light: Photosensory ecology and agricultural implications. Plant Cell Environ. 1997, 20, 820–825. [Google Scholar] [CrossRef]
- Wei, C.; Li, Y.; Jin, Z.; Luo, G.; Chen, C.; Shan, F. Effects of Shading on Photosynthetic Characteristics and Non-structural Carbohydrate Content of Heptacodium miconioides Seedlings. Bull. Bot. Res. 2022, 42, 10. [Google Scholar]
- Zhang, L.; Zhang, D. Gender Differences in Growth and Physiological Respond of Phellodendron amurense Rupr. in Condition of Overshadow. Bull. Bot. Res. 2020, 40, 735–742. [Google Scholar]
- Yin, D.; Shen, H.; Wei, X. Effects of Shading on Physocarpus amurensis Seedlings Photosynthetic Ability and Carbohydrate Accumulation. Bull. Bot. Res. 2017, 37, 841–847. [Google Scholar]
- Piper, F.I. Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann. For. Sci. 2011, 68, 415–424. [Google Scholar] [CrossRef]
- Petrussa, E.; Boscutti, F.; Vianello, A.; Casolo, V. “Last in-first out”: Seasonal variations of non-structural carbohydrates, glucose-6-phospate and ATP, in tubers of two Arum species. Plant Biol. 2017, 20, 346–356. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Brutnell, T.P.; Finlayson, S.A. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ. 2010, 33, 48–58. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Unravelling phenotypic plasticity why should we bother. New Phytol. 2006, 170, 644–648. [Google Scholar] [CrossRef]
- Se, H.P.; Jae, H.L.; Sang, Y.N. An Analysis of the Growth and Photosynthetic Responses of Potted Veronica pusanensis Y.N. Lee according to the Shading Levels. J. People Plants Environ. 2023, 26, 219–231. [Google Scholar]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Plant Height/cm | Diameter/mm | ||||
|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Increment | Before Treatment | After Treatment | Increment | |
| CK | 6.79 ± 0.06 a | 10.43 ± 0.02 a | 3.65 ± 0.06 a | 8.72 ± 0.09 a | 12.21 ± 0.16 a | 3.49 ± 0.15 a |
| T80 | 6.32 ± 0.07 c | 8.89 ± 0.03 b | 2.57 ± 0.04 c | 8.77 ± 0.03 a | 10.87 ± 0.12 b | 2.10 ± 0.10 c |
| T80-1 | 6.06 ± 0.03 d | 8.86 ± 0.02 b | 2.80 ± 0.04 b | 8.50 ± 0.05 ab | 10.86 ± 0.17 b | 2.36 ± 0.21 bc |
| T95 | 6.51 ± 0.05 b | 8.43 ± 0.02 c | 1.92 ± 0.06 e | 8.61 ± 0.06 a | 10.45 ± 0.08 c | 1.84 ± 0.10 c |
| T95-1 | 6.61 ± 0.04 b | 8.82 ± 0.02 b | 2.21 ± 0.06 d | 8.06 ± 0.29 b | 10.89 ± 0.05 b | 2.83 ± 0.27 b |
| Factor | F | p |
|---|---|---|
| Needle soluble sugar | 0.898 | 0.501 |
| Needle starch | 2.153 | 0.148 |
| Needle NSCs | 1.707 | 0.225 |
| Needle soluble sugar–starch | 3.505 | 0.049 |
| Stem soluble sugar | 7.094 | 0.006 |
| Stem starch | 14.362 | 0.000 |
| Stem NSCs | 12.861 | 0.001 |
| Stem soluble sugar–starch | 11.168 | 0.001 |
| Thick root soluble sugar | 3.834 | 0.039 |
| Thick root starch | 7.496 | 0.005 |
| Thick root NSCs | 3.753 | 0.041 |
| Thick root soluble sugar–starch | 1.144 | 0.390 |
| Fine root soluble sugar | 2.167 | 0.147 |
| Fine root starch | 9.768 | 0.002 |
| Fine root NSCs | 9.126 | 0.002 |
| Fine root soluble sugar–starch | 1.243 | 0.354 |
| Needle biomass | 1.216 | 0.363 |
| Stem biomass | 5.420 | 0.014 |
| Thick root biomass | 15.791 | 0.000 |
| Fine root biomass | 3.068 | 0.068 |
| Total biomass | 5.079 | 0.017 |
| Needle–biomass ratio | 3.972 | 0.035 |
| Stem–biomass ratio | 1.658 | 0.235 |
| Thick root–biomass ratio | 4.089 | 0.032 |
| Fine root–biomass ratio | 0.650 | 0.639 |
| Root-to-shoot ratio | 1.644 | 0.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, W.; Dai, C.; Wu, J.; Li, C. Response of Growth and Non-Structural Carbohydrates’ Allocation in Pinus yunnanensis Seedlings to Simulated Sunflecks. Forests 2025, 16, 522. https://doi.org/10.3390/f16030522
Liu Y, Zhu W, Dai C, Wu J, Li C. Response of Growth and Non-Structural Carbohydrates’ Allocation in Pinus yunnanensis Seedlings to Simulated Sunflecks. Forests. 2025; 16(3):522. https://doi.org/10.3390/f16030522
Chicago/Turabian StyleLiu, Yuanxi, Weisong Zhu, Cefeng Dai, Junwen Wu, and Chaojun Li. 2025. "Response of Growth and Non-Structural Carbohydrates’ Allocation in Pinus yunnanensis Seedlings to Simulated Sunflecks" Forests 16, no. 3: 522. https://doi.org/10.3390/f16030522
APA StyleLiu, Y., Zhu, W., Dai, C., Wu, J., & Li, C. (2025). Response of Growth and Non-Structural Carbohydrates’ Allocation in Pinus yunnanensis Seedlings to Simulated Sunflecks. Forests, 16(3), 522. https://doi.org/10.3390/f16030522





