Water Conservation Capacity of Soil and Litter Layers of Five Magnoliaceae Plants in Hainan Island, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Litter and Soil Sample Collection
2.3. Laboratory Analyses
2.4. Synthetic Assessment of Water Conservation Capacity
2.5. Statistical Analysis
3. Results
3.1. The Litter Thickness and Mass Associated with the Five Magnoliaceae Plants
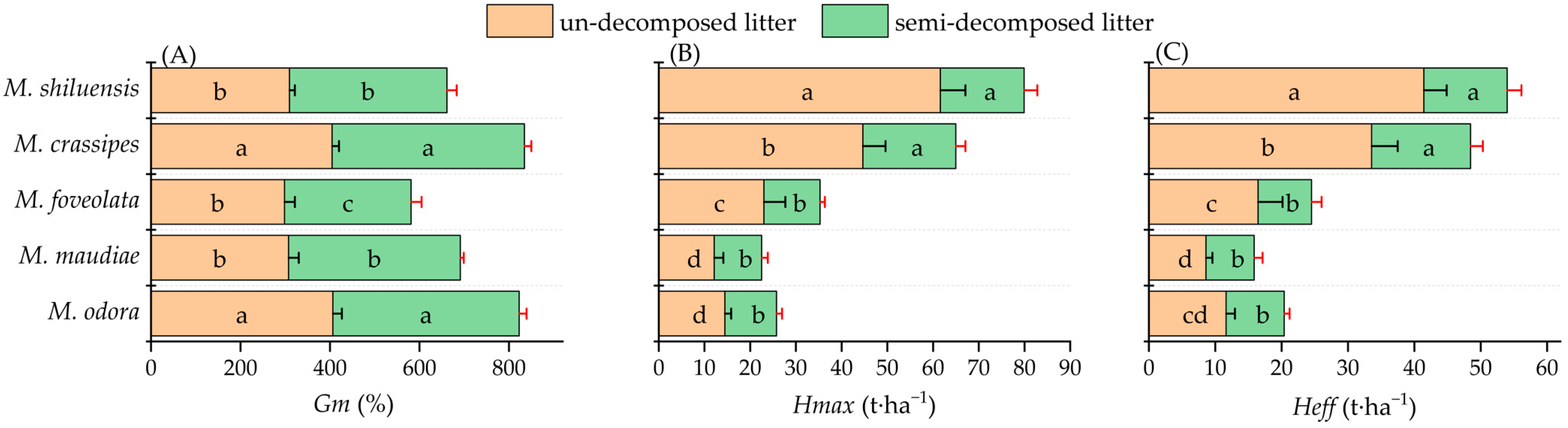
3.2. Gm, Heff, and Hmax
3.3. Litter Water-Holding Ratio
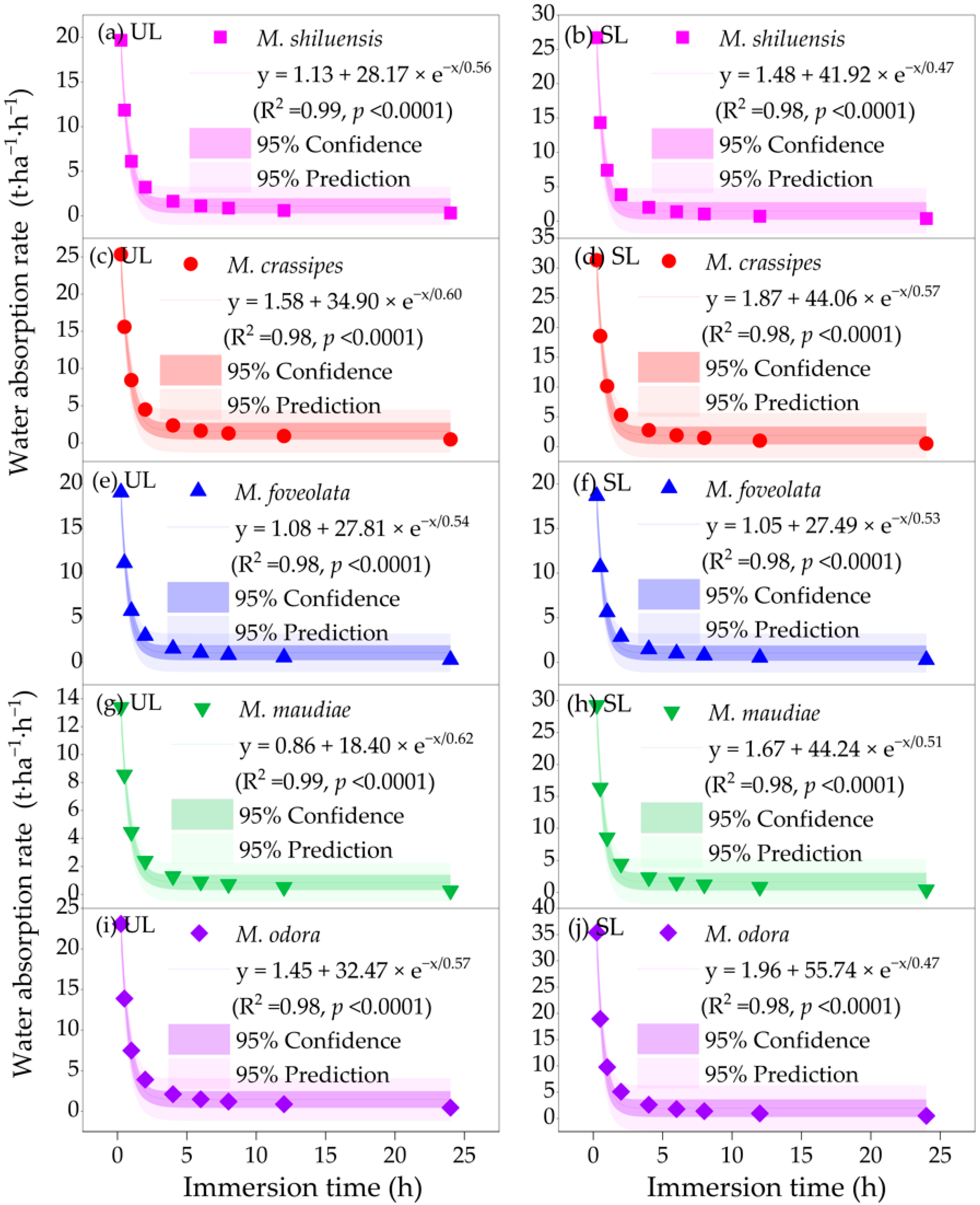
3.4. Litter Water Absorption Rates
3.5. Soil Water-Holding Capacity
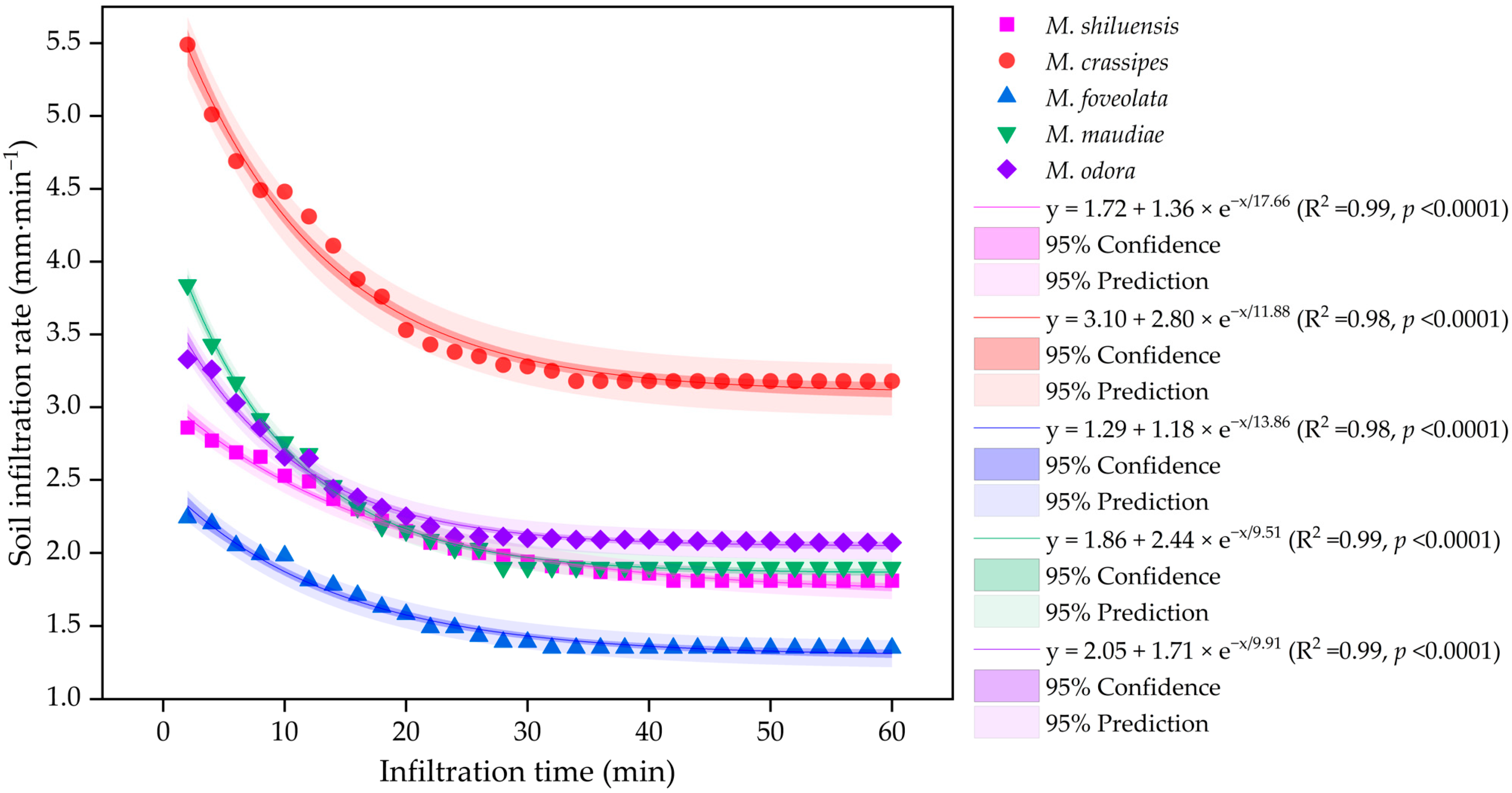
3.6. Variations in Soil Infiltration
3.7. Water Conservation Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, W.; Fan, Y.; Wang, Z.; Liu, D.; Ding, Z.; Ou, J. Diversity and geographic distribution patterns of wild Magnoliaceae Species in China. Sustainability 2024, 16, 9448. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y. Intraspecific variation of floral scent chemistry in Magnolia kobus DC. (Magnoliaceae). J. Plant Res. 2001, 114, 411–422. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Fu, J.; Chi, X.; Du, W.; Dimitrov, D.; Liu, J.; Xi, Z.; Wu, J.; Xu, X. Diversity patterns and conservation gaps of Magnoliaceae species in China. Sci. Total Environ. 2022, 813, 152665. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China; Chinese Academy of Sciences. (Eds.) Flora of China; Science Press: Beijing, China, 1996; Volume 30, pp. 82–85. [Google Scholar]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y. Prediction of Potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Gong, X.; Shi, S.; Pan, Y.; Huang, Y.; Yin, Q. An observation on the main taxonomic characters of subfamiliy Magnolioideae in China. Acta Bot. Yunnanica 2003, 25, 447–456. [Google Scholar]
- Mu, N.; Zhu, K.; Li, S.; Dong, J.; Dong, Z.; Xiao, N. Discovery of Magnoliaceae taxa and its research progress in China. J. Anhui Agric. Sci. 2022, 50, 222–226. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, B. Floral analysis of Magnoliaceae. Guihaia 2001, 21, 315–320. [Google Scholar]
- Wu, Z.; Lu, A.; Tang, Y.; Chen, Z.; Li, D. The Families and Genera of Angiosperms in China a Comprehensive Analysis; Science Press: Beijing, China, 2003; pp. 57–68. [Google Scholar]
- Sima, Y.; Wang, J.; Cao, L.; Wang, B.; Wang, Y. Prefoliation Features of the Magnoliaceae and their systematic significance. J. Yunnan Univ. Nat. Sci. Ed. 2001, 23, 71–78. [Google Scholar]
- Yang, F.; Cai, L.; Dao, Z.; Sun, W. Genomic data reveals population genetic and demographic history of Magnolia fistulosa (Magnoliaceae), a plant species with extremely small populations in Yunnan Province, China. Front. Plant Sci. 2022, 13, 811312. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, X.; Lu, C.; Liu, J.; Diao, S.; Jiang, J. Genetic Diversity and population structure analysis in the Chinese endemic species Michelia crassipes based on SSR markers. Forests 2023, 14, 508. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Li, R.; Movahedi, A.; Chen, J.; Yang, L. Comprehensive bioinformatics and expression analysis of TCP transcription factors in Liriodendron chinense reveals putative abiotic stress regulatory roles. Forests 2022, 13, 1401. [Google Scholar] [CrossRef]
- Deng, S.; Shi, K.; Ma, J.; Zhang, L.; Ma, L.; Jia, Z. Effects of fertilization ratios and frequencies on the growth and nutrient uptake of Magnolia wufengensis (Magnoliaceae). Forests 2019, 10, 65. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, L.; Zhu, Z.; Sang, Z.; Ma, L.; Jia, Z. Growth and physiological responses of Magnoliaceae to NaCl Stress. Plants 2024, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ma, D.; Song, Z.; Yang, M.; Xu, Y. The Composition, antioxidant and antibacterial activity of essential oils from five species of the Magnoliaceae Family. Molecules 2024, 29, 5182. [Google Scholar] [CrossRef]
- Fang, X.C.; Wang, P.; Zhang, X.Y.; Kong, L.L.; Wen, W.; Wang, L.Y.; Ye, D. Resources and evaluation of wild ornamental plants of Magnoliaceae in Zhejiang Province. Chin. J. Trop. Crops 2018, 39, 1513–1518. [Google Scholar] [CrossRef]
- Ma, B. Comprehensive Evaluation of Ornamental and Drought Resistance Characteristics of Five Magnoliaceae Plants. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2020. [Google Scholar] [CrossRef]
- Kong, Y.Y. Resources Evaluation of Magnoliaceae in Hainan Island and Construction of Magnoliaceae Plant Garden. Master’s Thesis, Hainan University, Haikou, China, 2018. [Google Scholar]
- GB/T 33027-2016; Methodology for Field Long-Term Observation of Forest Ecosystem. Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- Li, N.; Zhang, Y.; Wang, T.W.; Li, J.W.; Yang, J.W.; Luo, M.Y. Have anthropogenic factors mitigated or intensified soil erosion over the past three decades in South China? J. Environ. Manag. 2022, 302, 114093. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Tanaka, N.; Gao, T.; Zhu, L.; Zou, C. Hydrological properties of litter layers in mixed forests in Mt. Qinling, China. iForest 2018, 11, 243–250. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhang, X.; Chen, J.J.; Zhan, F.D.; Guo, X.H.; Zu, Y.Q. Differential water and soil conservation capacity and associated processes in four forest ecosystems in Dianchi Watershed, Yunnan Province, China. J. Soil Water Conserv. 2015, 70, 198–206. [Google Scholar] [CrossRef]
- Zhou, Q.; Keith, D.M.; Zhou, X.; Cai, M.; Cui, X.; Wei, X.; Luo, Y. Comparing the water-holding characteristics of broadleaved, coniferous, and mixed forest litter layers in a Karst Region. Mt. Res. Dev. 2018, 38, 220–229. [Google Scholar] [CrossRef]
- LY/T 1215-1999; Determination of Forest Soil Water-Physical Properties. State Forestry Administration of the People’s Republic of China: Beijing, China, 1999.
- Bai, Y.X.; Zhou, Y.C.; Zhang, X.Y.; Du, J.J. Water conservation capacity of litter and soil in mixed plantation of Pinus massoniana and Broadleaved Trees. Sci. Silvae Sin. 2021, 57, 24–36. (In Chinese) [Google Scholar] [CrossRef]
- Cheng, H.; Fu, Y.; Dong, H.; Hu, X.; Huang, C.; Liu, Y.; Che, M.; Fu, W.; Gong, Y. Physical and chemical properties of soil and the hydrological effects of different vegetation types in the central Sichuan hilly region. Chinese J. Appl. Environ. Biol. 2019, 25, 845–853. [Google Scholar] [CrossRef]
- Hu, S.J.; Tian, C.Y.; Song, Y.D.; Gan, Y.D. Determination and calculation of soil permeability coefficient. Trans. CSAE 2011, 27, 68. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, S.; Chen, Z.; Ruan, D.; Zhang, W.; Han, Y.; Han, L.; Wang, K.; Huang, Y.; Chen, J. Hydrological properties of soil and litter layers of four forest types restored in the gully erosion area of Latosol in South China. Forests 2023, 14, 360. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, F.; Chen, Y.; Hu, Z. Optimal kinematics design of macpherson suspension: Integrated use of grey relational analysis and improved entropy weight method. J. Harbin Inst. Technol. 2022, 29, 41–51. [Google Scholar]
- Cheng, C.; He, K.N.; Yu, G.F.; Chai, S.X. Comparative study on water conservation capacity of different forest types of artificial forest in arid and semi-arid area. Acta Ecol. Sin. 2021, 41, 1979–1990. (In Chinese) [Google Scholar] [CrossRef]
- Keith, D.M.; Johnson, E.A.; Valeo, C. A hillslope forest floor (duff) water budget and the transition to local control. Hydrol. Process. 2010, 24, 2738–2751. [Google Scholar] [CrossRef]
- Neris, J.; Tejedor, M.; Rodríguez, M.; Fuentes, J.; Jiménez, C. Effect of forest floor characteristics on water repellency, infiltration, runoff and soil loss in Andisols of Tenerife (Canary Islands, Spain). Catena 2013, 108, 50–57. [Google Scholar] [CrossRef]
- Dong, H.; Yang, C.; Su, C.; Cao, H. Litter and soil hydrological effects of five no-commercial forests in Dongguan. J. Soil Water Conserv. 2021, 35, 144–149, 160. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Xu, Z.; Wang, Y.; Peng, H. Effect of vegetation on soil water retention and storage in a semi-arid alpine forest catchment. J. Arid Land 2013, 5, 207–219. [Google Scholar] [CrossRef]
- Dunkerley, D. Percolation through leaf litter: What happens during rainfall events of varying intensity? J. Hydrol. 2015, 525, 737–746. [Google Scholar] [CrossRef]
- Sato, Y.; Kumagai, T.O.; Kume, A.; Otsuki, K.; Ogawa, S. Experimental analysis of moisture dynamics of litter layers—The effects of rainfall conditions and leaf shapes. Hydrol. Process. 2004, 18, 3007–3018. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Wang, H.; Jia, G.; Zhao, Y.; Tu, Z.; Deng, W.; Jia, J.; Chen, J. Effects of forest structure on hydrological processes in China. J. Hydrol. 2018, 561, 187–199. [Google Scholar] [CrossRef]
- Gomyo, M.; Kuraji, K. Effect of the litter layer on runoff and evapotranspiration using the paired watershed method. J. Forest Res. 2016, 21, 306–313. [Google Scholar] [CrossRef]
- Ilek, A.; Szostek, M.; Mikołajczyk, A.; Rajtar, M. Does mixing tree species affect water storage capacity of the forest floor? Laboratory test of pine-oak and fir-beech litter layers. Forests 2021, 12, 1674. [Google Scholar] [CrossRef]
- Wu, X.; Liu, L.; Zhang, H.; Sun, L.; Yan, X.; Dong, X.; Yao, Y. Litter water-holding capacity and soil physical properties of main afforestation tree species in sand stone area. J. Soil Water Conserv. 2020, 34, 137–144. (In Chinese) [Google Scholar] [CrossRef]
- Tang, S.; Zhang, J.; Pu, X.; Shi, P.; Meng, C.; Gong, Y. Hydrological effects of forest litters and soil in Guansi River Watershed. J. Northeast Forestry Univ. 2014, 42, 50–53. (In Chinese) [Google Scholar] [CrossRef]
- Acharya, B.S.; Stebler, E.; Zou, C.B. Monitoring litter interception of rainfall using leaf wetness sensor under controlled and field conditions. Hydrol. Process. 2017, 31, 240–249. [Google Scholar] [CrossRef]
- Zagyvai-Kiss, K.A.; Kalicz, P.; Szilágyi, J.; Gribovszki, Z. On the specific water holding capacity of litter for three forest eco-systems in the eastern foothills of the Alps. Agr. Forest Meteorol. 2019, 278, 107656. [Google Scholar] [CrossRef]
- Pereira, L.C.; Balbinot, L.; Lima, M.T.; Bramorski, J.; Tonello, K.C. Aspects of forest restoration and hydrology: The hydrological function of litter. J. For. Res. 2022, 33, 543–552. [Google Scholar] [CrossRef]
- Pang, X.; Bao, W. Effect of substituting plantation species for native shrubs on the water-holding characteristics of the forest floor on the eastern Tibetan Plateau. J Resour. Ecol. 2011, 2, 217–224. [Google Scholar]
- Bai, Y.; Zhou, Y.; Du, J.; Zhang, X.; Di, N. Effects of a broadleaf-oriented transformation of coniferous plantations on the hydrological characteristics of litter layers in subtropical China. Glob. Ecol. Conserv. 2021, 25, e01400. [Google Scholar] [CrossRef]
- Guevara-Escobar, A.; Gonzalez-Sosa, E.; Ramos-Salinas, M.; Hernandez-Delgado, G.D. Experimental analysis of drainage and water storage of litter layers. Hydrol. Earth Syst. Sci. 2007, 11, 1703–1716. [Google Scholar] [CrossRef]
- Levia, D.F.; Bollinger, W.C.; Hrabik, R.A. Evaporation of intercepted precipitation from fruit litter of Liquidambar styraciflua L. (sweetgum) in a clearing as a function of meteorological conditions. Int. J. Biometeorol. 2005, 49, 325–331. [Google Scholar] [CrossRef]
- Farahnak, M.; Mitsuyasu, K.; Otsuki, K.; Shimizu, K.; Kume, A. Factors determining soil water repellency in two coniferous plantations on a hillslope. Forests 2019, 10, 730. [Google Scholar] [CrossRef]
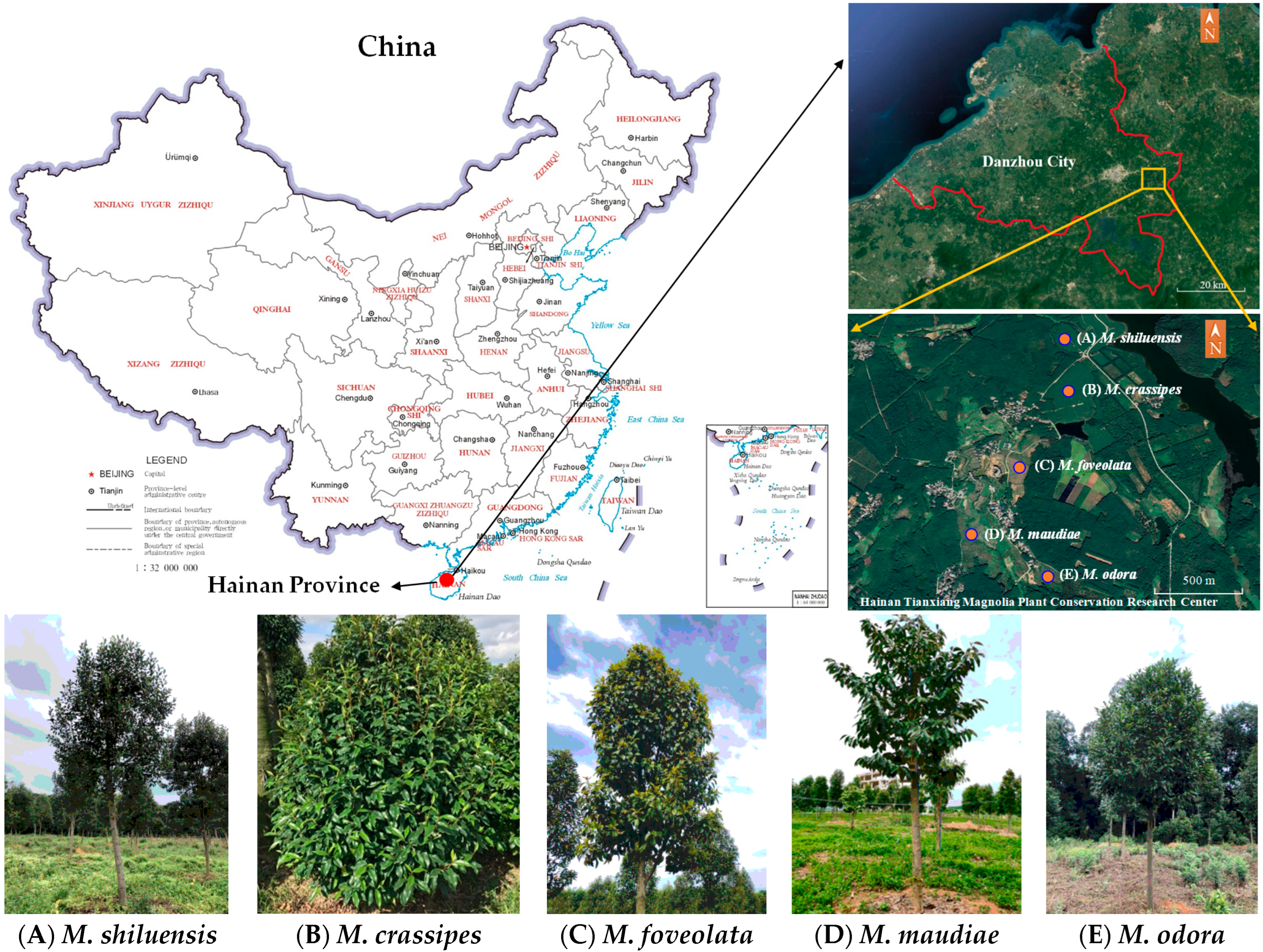


| Tree Species | Stand Age (Years) | Average Tree Height (m) | Average Diameter at Breast Height (cm) | Tree Density (Trees·ha−1) | Canopy Density | Slope Gradient (°) |
|---|---|---|---|---|---|---|
| M. shiluensis | 7 | 4.85 ± 0.42 | 13.09 ± 1.01 | 400 | 0.24 | 3~5 |
| M. crassipes | 7 | 2.39 ± 0.31 | 11.87 ± 1.12 ★ | 400 | 0.22 | 3~5 |
| M. foveolata | 7 | 5.45 ± 0.33 | 12.37 ± 1.52 | 400 | 0.24 | 3~5 |
| M. maudiae | 7 | 5.91 ± 0.53 | 9.63 ± 0.92 | 400 | 0.22 | 3~5 |
| M. odora | 7 | 5.01 ± 0.48 | 11.94 ± 1.21 | 400 | 0.34 | 3~5 |
| Tree Species | Soil Depth (cm) | Bulk Density (g·cm−3) | Non-Capillary Porosity (%) | Capillary Porosity (%) | Total Porosity (%) | Soil Water-Holding Capacity (t·ha−1) |
|---|---|---|---|---|---|---|
| 0 to 10 | 1.33 ± 0.10 a | 7.38 ± 5.15 a | 37.77 ± 3.71 a | 45.15 ± 1.61 a | 73.77 ± 51.52 a | |
| M. shiluensis | 10 to 20 | 1.33 ± 0.10 a | 7.11 ± 6.67 a | 31.82 ± 7.02 a | 38.93 ± 0.40 a | 71.13 ± 66.67 a |
| 20 to 30 | 1.38 ± 0.05 a | 5.95 ± 3.01 a | 37.16 ± 6.04 a | 43.11 ± 4.73 a | 59.47 ± 30.09 b | |
| 0 to 10 | 1.16 ± 0.18 a | 8.46 ± 1.46 a | 38.27 ± 1.80 a | 46.73 ± 2.81 a | 84.60 ± 14.57 a | |
| M. crassipes | 10 to 20 | 1.19 ± 0.10 a | 5.35 ± 1.93 a | 42.36 ± 2.18 a | 47.70 ± 3.86 a | 53.47 ± 19.30 b |
| 20 to 30 | 1.30 ± 0.02 a | 2.72 ± 1.67 b | 42.11 ± 0.96 a | 44.83 ± 2.03 a | 27.20 ± 16.74 c | |
| 0 to 10 | 1.26 ± 0.13 a | 7.81 ± 5.57 a | 39.91 ± 4.98 a | 47.72 ± 1.61 a | 78.07 ± 55.68 a | |
| M. foveolata | 10 to 20 | 1.36 ± 0.27 a | 5.66 ± 3.23 a | 36.80 ± 6.55 a | 42.47 ± 8.09 a | 56.63 ± 32.27 b |
| 20 to 30 | 1.29 ± 0.17 a | 7.63 ± 3.53 a | 37.68 ± 4.64 a | 45.31 ± 8.05 a | 76.30 ± 35.28 a | |
| 0 to 10 | 1.51 ± 0.13 a | 2.82 ± 1.79 a | 39.37 ± 4.33 a | 42.19 ± 5.96 a | 28.17 ± 17.87 a | |
| M. maudiae | 10 to 20 | 1.59 ± 0.12 a | 2.90 ± 1.45 a | 37.17 ± 1.24 a | 40.07 ± 2.10 a | 29.03 ± 14.49 a |
| 20 to 30 | 1.54 ± 0.04 a | 2.15 ± 0.62 a | 35.69 ± 2.47 a | 37.84 ± 3.03 a | 21.50 ± 6.18 a | |
| 0 to 10 | 1.44 ± 0.29 a | 4.86 ± 2.70 a | 35.81 ± 3.69 a | 40.67 ± 6.38 a | 48.60 ± 26.97 a | |
| M. odora | 10 to 20 | 1.45 ± 0.31 a | 5.32 ± 3.97 a | 37.19 ± 4.79 a | 42.51 ± 8.73 a | 53.17 ± 39.75 a |
| 20 to 30 | 1.40 ± 0.27 a | 5.67 ± 5.09 a | 39.90 ± 1.71 a | 45.57 ± 6.03 a | 56.73 ± 50.91 a |
| Grade I Index | Weight | Serial No. | Grade II Index | Weight |
|---|---|---|---|---|
| X1 | Litter thickness | 0.1321 | ||
| X2 | Litter mass | 0.1427 | ||
| Litter layer | 0.6152 | X3 | Gm | 0.0816 |
| X4 | Heff | 0.1221 | ||
| X5 | Hmax | 0.1367 | ||
| X6 | Bulk density | 0.0698 | ||
| X7 | Capillary porosity | 0.0807 | ||
| Soil layer | 0.3848 | X8 | Total porosity | 0.0747 |
| X9 | Soil water-holding capacity | 0.0613 | ||
| X10 | Soil infiltration | 0.0983 |
| Tree Species | Water Conservation Capacity | Comprehensive Evaluation Value | Rank | |
|---|---|---|---|---|
| Litter Layer | Soil Layer | |||
| M. shiluensis | 0.2391 | 0.0541 | 0.2932 | 2 |
| M. crassipes | 0.2394 | 0.1028 | 0.3422 | 1 |
| M. foveolata | 0.0515 | 0.1275 | 0.1790 | 3 |
| M. maudiae | 0.0178 | 0.0365 | 0.0543 | 5 |
| M. odora | 0.0637 | 0.0818 | 0.1455 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Han, Y.; Mao, R.; Wang, K.; Yu, Y.; Fan, Y.; Xia, M.; Zhao, Y.; Wu, L.; Tu, Z. Water Conservation Capacity of Soil and Litter Layers of Five Magnoliaceae Plants in Hainan Island, China. Forests 2025, 16, 514. https://doi.org/10.3390/f16030514
Huang Y, Han Y, Mao R, Wang K, Yu Y, Fan Y, Xia M, Zhao Y, Wu L, Tu Z. Water Conservation Capacity of Soil and Litter Layers of Five Magnoliaceae Plants in Hainan Island, China. Forests. 2025; 16(3):514. https://doi.org/10.3390/f16030514
Chicago/Turabian StyleHuang, Yanping, Yujie Han, Ruowen Mao, Kang Wang, Yan Yu, Yanhui Fan, Murong Xia, Yihan Zhao, Liangying Wu, and Zhihua Tu. 2025. "Water Conservation Capacity of Soil and Litter Layers of Five Magnoliaceae Plants in Hainan Island, China" Forests 16, no. 3: 514. https://doi.org/10.3390/f16030514
APA StyleHuang, Y., Han, Y., Mao, R., Wang, K., Yu, Y., Fan, Y., Xia, M., Zhao, Y., Wu, L., & Tu, Z. (2025). Water Conservation Capacity of Soil and Litter Layers of Five Magnoliaceae Plants in Hainan Island, China. Forests, 16(3), 514. https://doi.org/10.3390/f16030514






