New Insights into the Role of Cytokinin in Regulating Anthocyanin Biosynthesis and Leaf Expansion: An Integrated Transcriptomic, Metabolomic, and Physiological Analysis of Hypericum monogynum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Agrobacterium-Mediated Transformation on Arabidopsis and Eucalyptus
2.3. RNA Extraction and qRT-PCR
2.4. Determination of Chlorophyll and Anthocyanin Content
2.5. Analysis of the Soluble Sugars, Starch, Free Amino Acid, and Total Flavonoid
2.6. Transcriptome Analysis
2.7. Metabolome Analysis
2.8. Statistical Analysis
3. Results
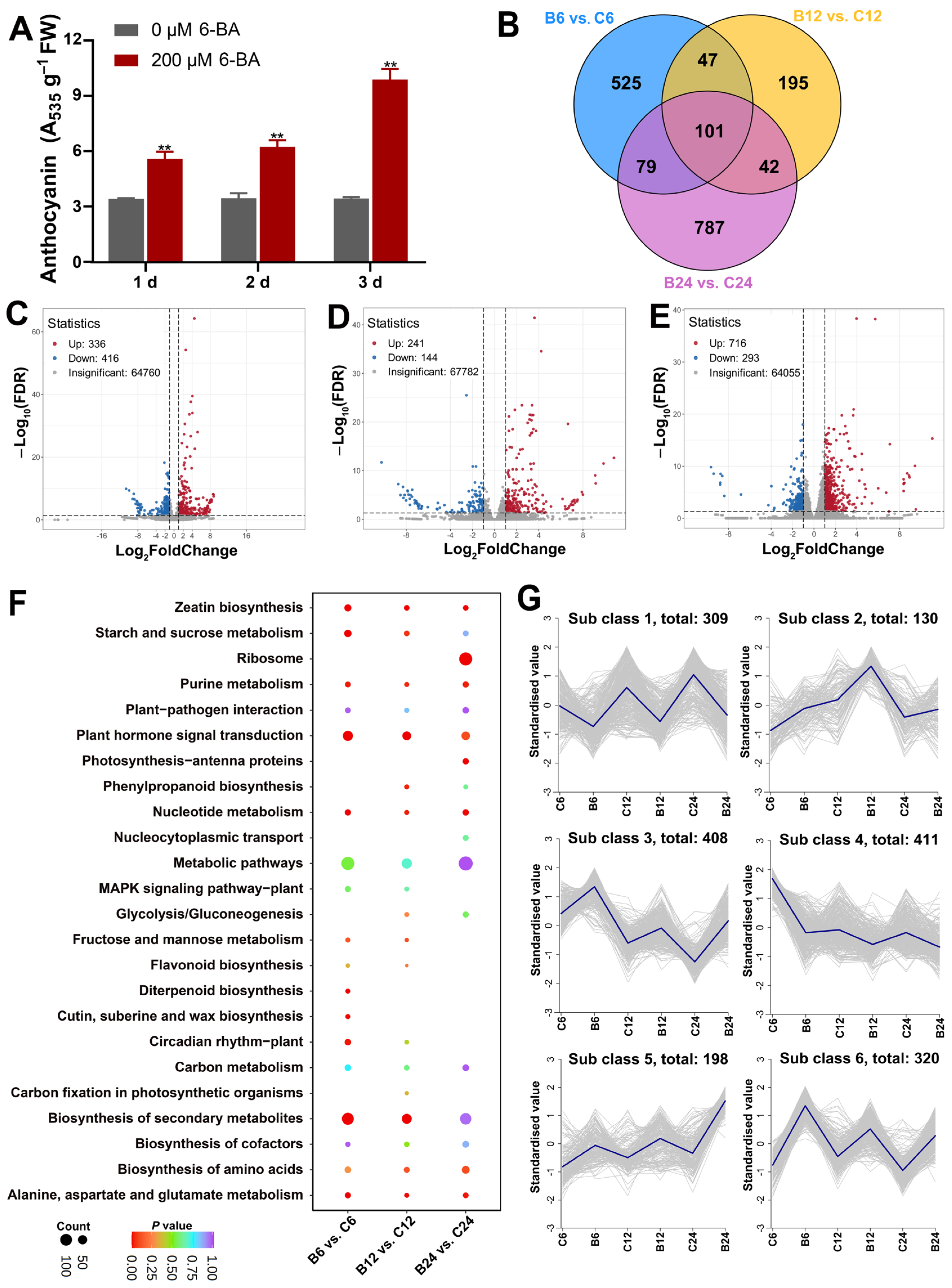
3.1. Anthocyanin Biosynthesis in the Leaves of H. monogynum Can Be Effectively Induced by Cytokinin Treatment
3.2. Transcriptome Analysis of H. monogynum Leaves After Cytokinin Treatment
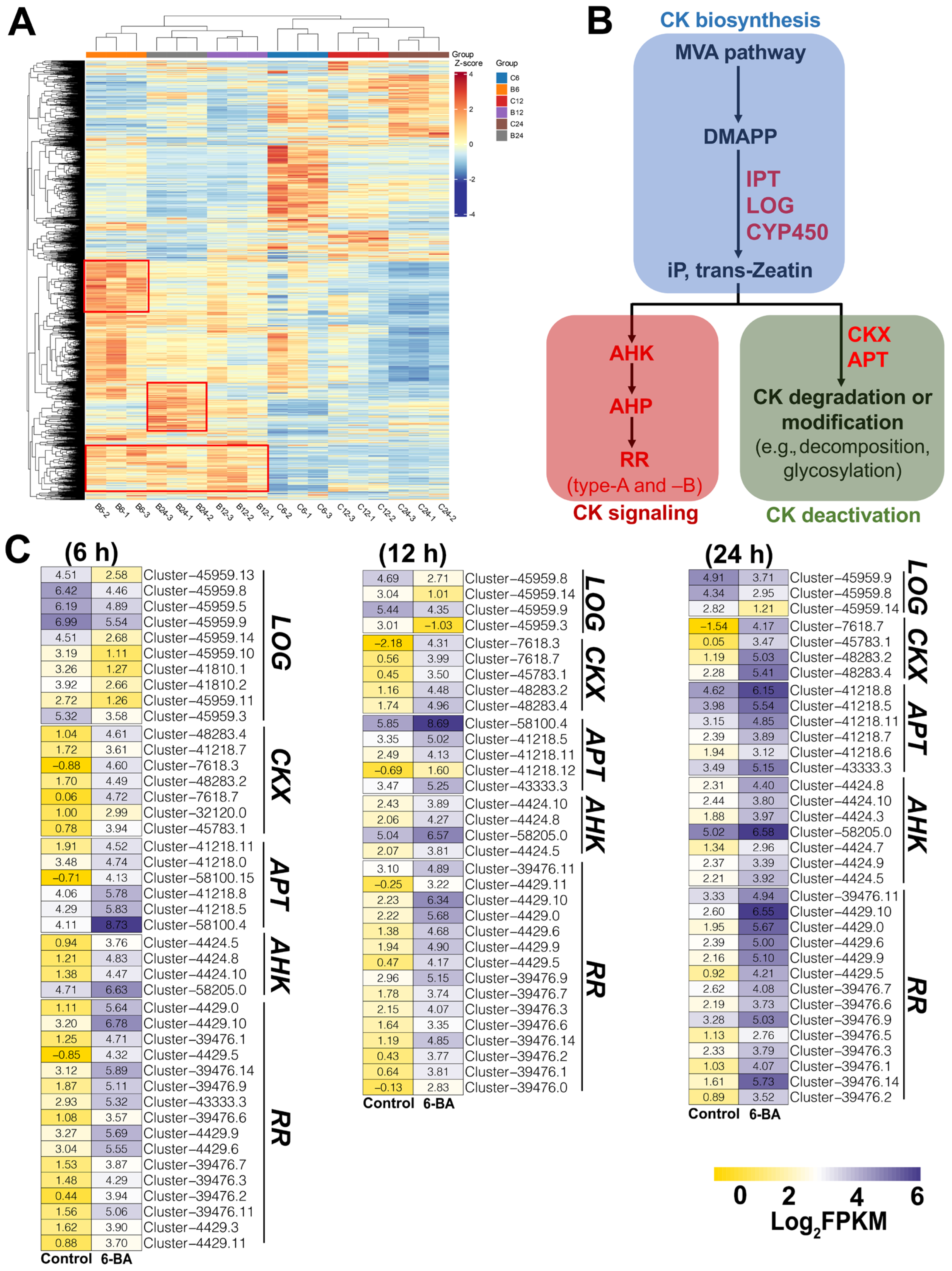
3.3. Identification of Key DEGs Related to CK Biosynthesis, Metabolism, and Signaling
3.4. Identification of Key DEGs Related to Leaf Growth and Expansion
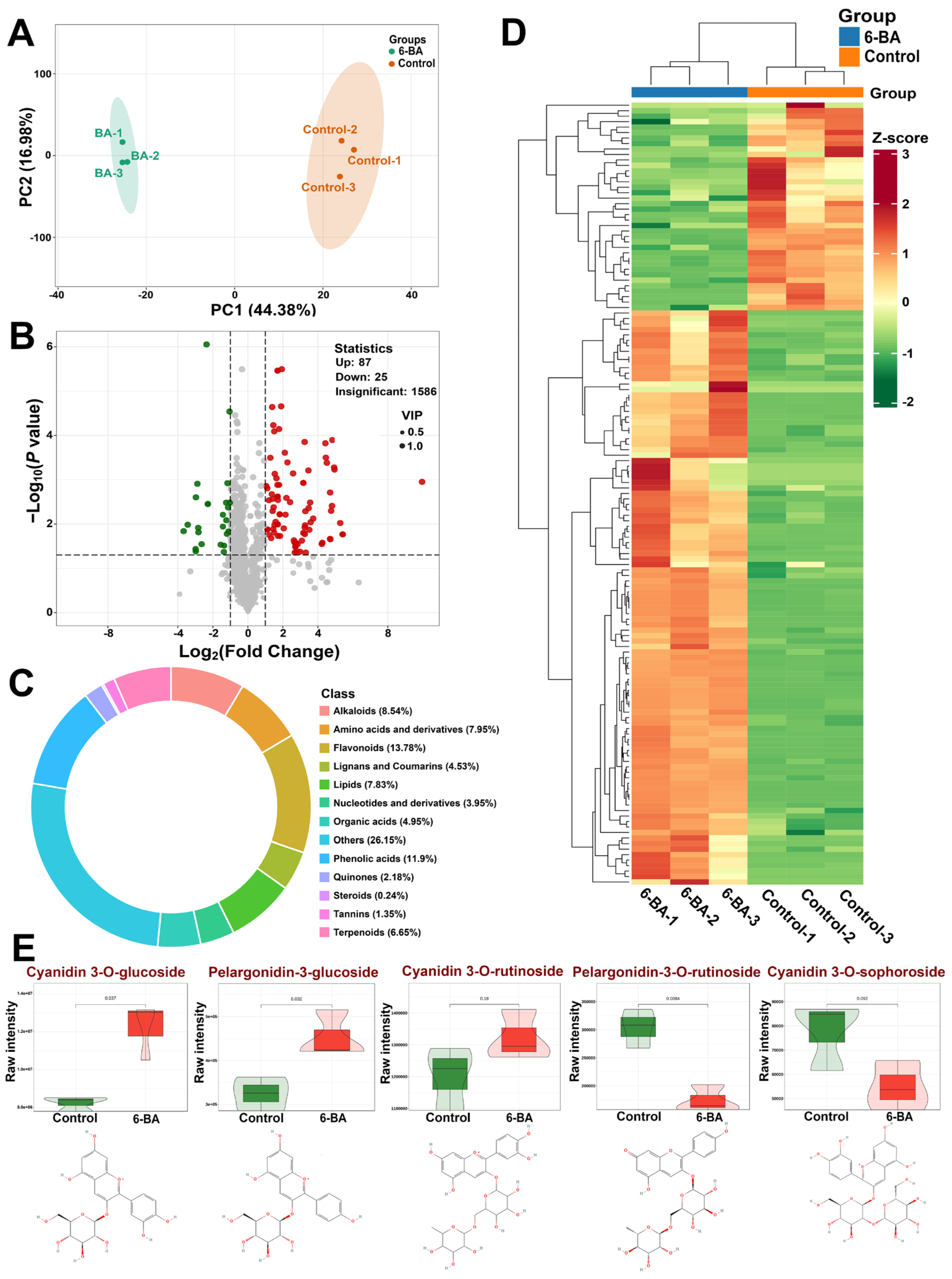
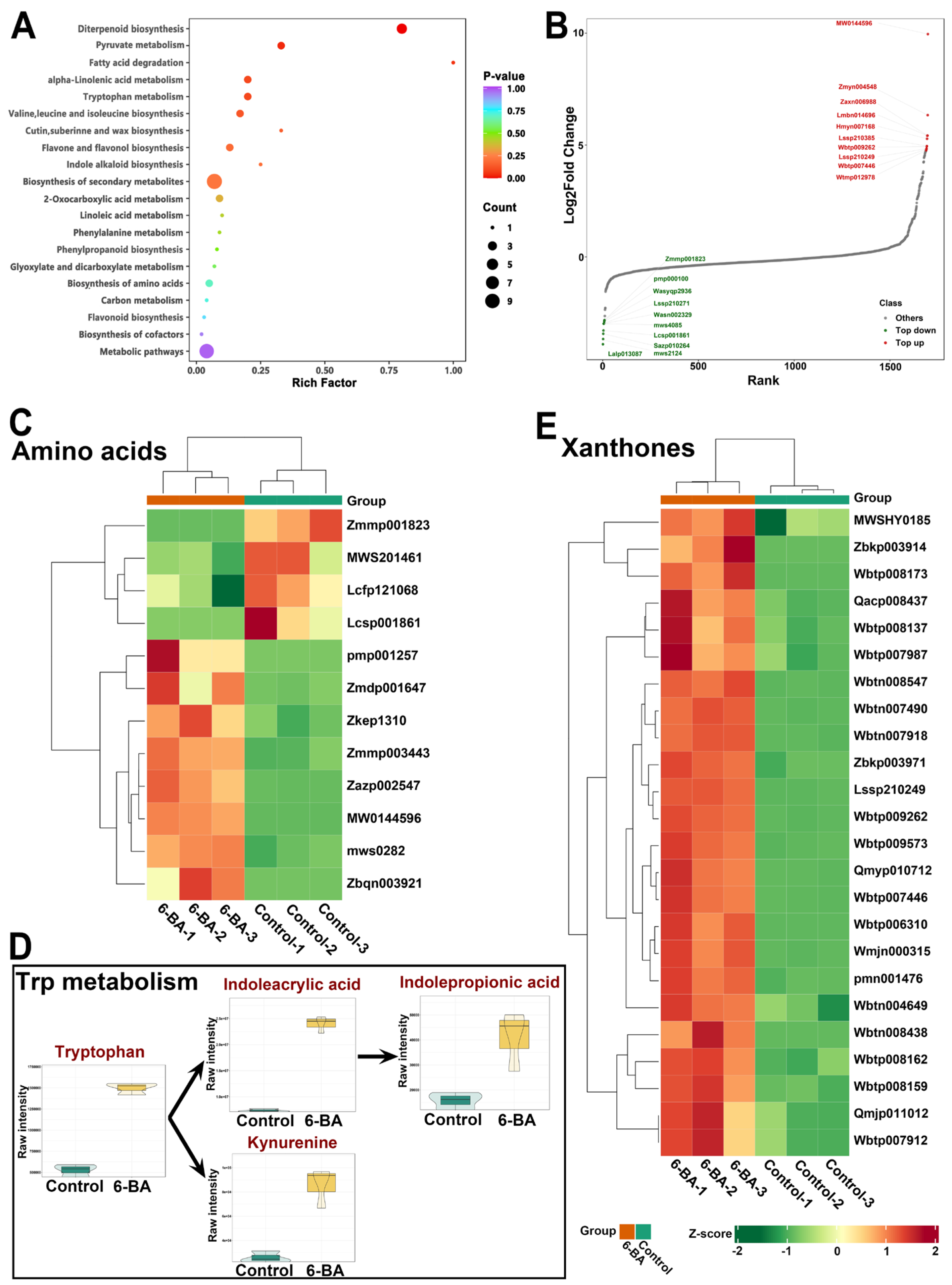
3.5. Widely Targeted Metabolome Analysis of H. monogynum Leaves by Cytokinin Treatment
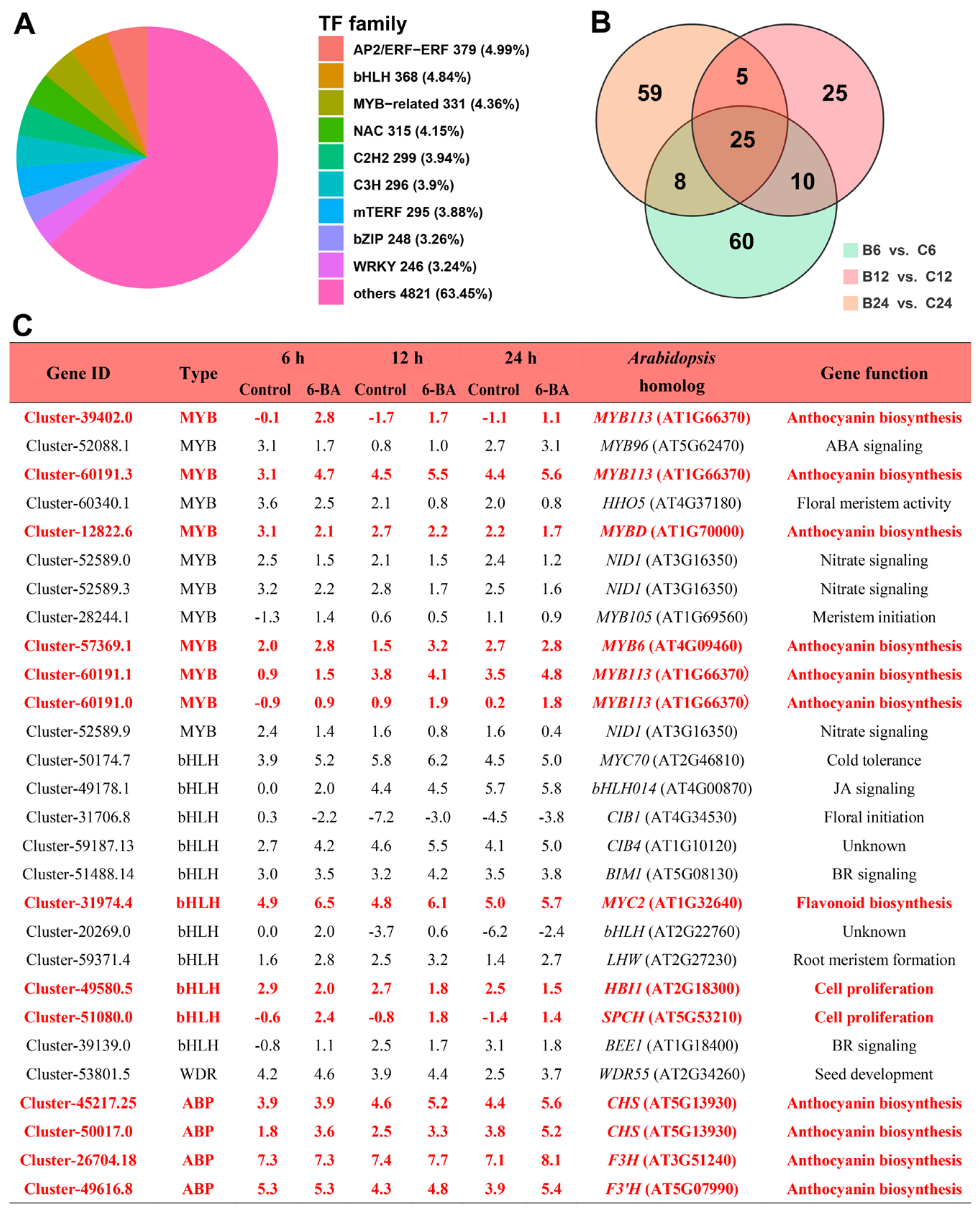
3.6. Identification of Key CK-Responsive Transcription Factors and ABP Genes in H. monogynum
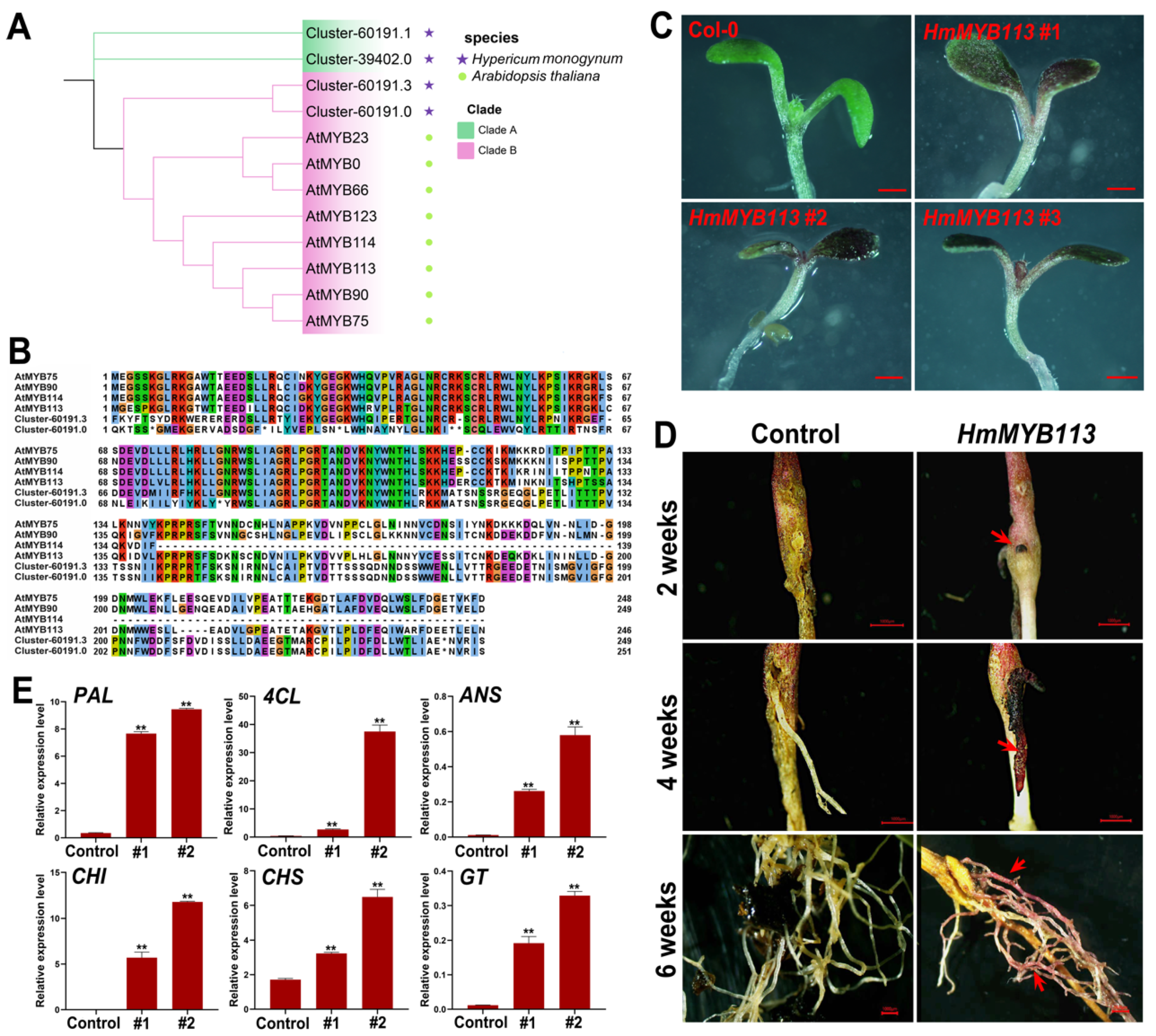
3.7. Functional Characterization of HmMYB113 in the Regulation of Anthocyanin Biosynthesis in the Model Plants Arabidopsis and Eucalyptus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caldeira, G.I.; Gouveia, L.P.; Serrano, R.; Silva, O.D. Hypericum genus as a natural source for biologically active compounds. Plants 2022, 11, 2509. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Grossman, R.B.; Xu, G. Research progress of polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Cui, Z.; Li, Q.; Kong, L.; Luo, J. Functional characterization of a Flavonol 3-O-rhamnosyltransferase and two UDP-rhamnose synthases from Hypericum monogynum. Plant Physiol. Biochem. 2023, 197, 107643. [Google Scholar] [CrossRef] [PubMed]
- Dongfeng, J.; Shuyao, Y.; Qingquan, H.; Qing, L.; Yanqun, H.; Guanglian, L.; Chunhui, H.; Xiaobiao, X. Comprehensive analysis of a red-peel kiwi berry mutant reveals key genes are responsible for anthocyanin biosynthesis in fruit. Sci. Hortic. 2023, 309, 111682. [Google Scholar]
- Glover, B.J.; Martin, C. Anthocyanins. Curr. Biol. 2012, 22, R147–R150. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Kroon, J.; Souer, E.; Graaff, A.D.; Xue, Y.; Koes, R. Cloning and structural analysis of the anthocyanin pigmentation locus Rt of Petunia hybrida: Characterization of insertion sequences in two mutant alleles. Plant J. 2010, 5, 69–80. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Davies, K.M. Modifying Anthocyanin Production in Flowers. In Anthocyanins; Springer: New York, NY, USA, 2008. [Google Scholar]
- Mehdy, M.C.; Lamb, C.J. Chalcone isomerase cDNA cloning and mRNA induction by fungal elicitor, wounding and infection. EMBO J. 1987, 6, 1527–1533. [Google Scholar] [CrossRef]
- Dongmei, G.; Hanyan, W.; Shumin, Z.; Ting, L. The Type III polyketide synthase supergene family in plants: Complex evolutionary history and functional divergence. Plant J. Cell Mol. Biol. 2022, 112, 414–428. [Google Scholar]
- Wenbo, J.; Yaying, X.; Xiaojia, S.; Yongzhen, P. ARF2 positively regulates flavonols and proanthocyanidins biosynthesis in Arabidopsis thaliana. Planta 2022, 256, 44. [Google Scholar]
- Jaakola, L. Phenylpropanoid Metabolism and Biosynthesis of Anthocyanins. In The Molecular Biology and Biochemistry of Fruit Ripening; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Lafountain, A.M.; Yuan, Y.W. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, Y.; Lin, K.; Wu, W.; Duan, L.; Wang, P.; Xiao, X.; Zhang, T.; Chen, X.; Wang, J.; et al. Cytokinin promotes anthocyanin biosynthesis via regulating sugar accumulation and MYB113 expression in Eucalyptus. Tree Physiol. 2024, 44, tpad154. [Google Scholar] [CrossRef]
- Das, P.K.; Shin, D.H.; Choi, S.-B.; Yoo, S.-D.; Choi, G.; Park, Y.-I. Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Mol. Cells 2012, 34, 93–101. [Google Scholar] [CrossRef]
- Das, P.K.; Shin, D.H.; Choi, S.-B.; Park, Y.-I. Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol. Cells 2012, 34, 501–507. [Google Scholar] [CrossRef]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl Jasmonate and Salinity Increase Anthocyanin Accumulation in Radish Sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Mengqi, X.; WenSha, D.; Chu, W.; Wenjia, W.; Shanwen, Y.; Changyang, C.; Xin, H.; Nannan, W.; Weiyuan, B.; Xiaoshan, T.; et al. Production of purple Ma bamboo (Dendrocalamus latiflorus Munro) with enhanced drought and cold stress tolerance by engineering anthocyanin biosynthesis. Planta 2021, 254, 50. [Google Scholar]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, Y.; Zhang, T.; Zeng, Z.; Wang, J.; Duan, L.; Chen, X.; Lin, K.; Liang, X.; Han, Z.; et al. Reactive oxygen species act as the key signaling molecules mediating light-induced anthocyanin biosynthesis in Eucalyptus. Plant Physiol. Biochem. 2024, 212, 108715. [Google Scholar] [CrossRef]
- Zeng, Z.; Liao, Y.; Wang, J.; Liang, X.; Duan, L.; Huang, Y.; Han, Z.; Lin, K.; Hu, H.; Ye, K.; et al. Combined transcriptomic, metabolomic and physiological analysis reveals the key role of nitrogen, but not phosphate and potassium in regulating anthocyanin biosynthesis induced by nutrient deficiency in Eucalyptus. Int. J. Biol. Macromol. 2024, 283, 137564. [Google Scholar] [CrossRef]
- Tonelli, P.C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar]
- Wu, Y.; Wen, J.; Xia, Y.; Zhang, L.; Du, H. Evolution and functional diversification of R2R3-MYB transcription factors in plants. Hortic. Res. 2022, 9, uhac058. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, Z.; Lin, K.; Jiang, W.; Wang, J.; Duan, L.; Liang, X.; Huang, Y.; Han, Z.; Hu, H.; et al. Gibberellin promotes xylem expansion and cell lignification by regulating sugar accumulation and the expression of JcMYB43 and JcMYB63 in the woody plant Jatropha curcas. Int. J. Biol. Macromol. 2025, 294, 139434. [Google Scholar] [CrossRef]
- Pratyusha, D.S.; Sarada, D.V.L. MYB transcription factors-master regulators of phenylpropanoid biosynthesis and diverse developmental and stress responses. Plant Cell Rep. 2022, 41, 2245–2260. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Li, Q.; Li, T.; Zhu, L.; Ma, Q.; Wang, J.; Lan, W.; Ren, J. Systematic analysis of MYB gene family in Acer rubrum and functional characterization of ArMYB89 in regulating anthocyanin biosynthesis. J. Exp. Bot. 2021, 72, 6319–6335. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/MYB transcriptional complex in Arabidopsis seedlings. Plant J. 2010, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wen, L.; Chen, Z.; Zhang, Z.; Pang, X.; Deng, Z.; Liu, T.; Guo, Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chem. 2021, 357, 129791. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Remali, J.; Sahidin, I.; Aizat, W.M. Xanthone biosynthetic pathway in plants: A review. Front. Plant Sci. 2022, 13, 809497. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Zhang, C.; Xia, J.; Li, Y.; Liu, X.; Sun, L.; Tan, S. Indole-3-propionic acid regulates lateral root development by targeting auxin signaling in Arabidopsis. iScience 2024, 27, 110363. [Google Scholar] [CrossRef]
- Zhao, Y.D. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Hussain, S.; Nanda, S.; Zhang, J.; Rehmani, M.I.A.; Suleman, M.; Li, G.; Hou, H. Auxin and cytokinin interplay during leaf morphogenesis and phyllotaxy. Plants 2021, 10, 1732. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.Y.; Zhu, L.; Afzal, S.F.; Zhou, J.B.; Ma, Q.Y.; Li, Q.Z.; Chen, J.H.; Ren, J. Chromosomal-level genome and multi-omics dataset provides new insights into leaf pigmentation in Acer palmatum. Int. J. Biol. Macromol. 2023, 227, 93–104. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Xuan, Y.; Tang, F.; Wang, J.; Shi, D.; Fu, S.; Ren, J. Transcriptome analysis based on a combination of sequencing platforms provides insights into leaf pigmentation in Acer rubrum. BMC Plant Biol. 2019, 19, 240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, X.; Gao, J.; Xuan, Y.; Ren, J. Integrating transcriptomic and metabolomic analysis of hormone pathways in Acer rubrum during developmental leaf senescence. BMC Plant Biol. 2020, 20, 410. [Google Scholar] [CrossRef]
- Zheng, T.; Tan, W.; Yang, H.; Zhang, L.E.; Li, T.; Liu, B.; Zhang, D.; Lin, H. Regulation of anthocyanin accumulation via MYB75/HAT1/TPL-mediated transcriptional repression. PLoS Genet. 2019, 15, e1007993. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hülskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Wang, N.; Xu, H.; Qu, C.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Chen, X. MdMYBL2 helps regulate cytokinin-induced anthocyanin biosynthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana) callus. Funct. Plant Biol. 2019, 46, 187–196. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Zeng, Z.; Tang, Y.; Liao, Y.; Lin, K.; Hu, H.; Xu, Z.-F.; Ni, J. New Insights into the Role of Cytokinin in Regulating Anthocyanin Biosynthesis and Leaf Expansion: An Integrated Transcriptomic, Metabolomic, and Physiological Analysis of Hypericum monogynum. Forests 2025, 16, 465. https://doi.org/10.3390/f16030465
Duan L, Zeng Z, Tang Y, Liao Y, Lin K, Hu H, Xu Z-F, Ni J. New Insights into the Role of Cytokinin in Regulating Anthocyanin Biosynthesis and Leaf Expansion: An Integrated Transcriptomic, Metabolomic, and Physiological Analysis of Hypericum monogynum. Forests. 2025; 16(3):465. https://doi.org/10.3390/f16030465
Chicago/Turabian StyleDuan, Lanjuan, Zhiyu Zeng, Yaodan Tang, Yuwu Liao, Kai Lin, Hao Hu, Zeng-Fu Xu, and Jun Ni. 2025. "New Insights into the Role of Cytokinin in Regulating Anthocyanin Biosynthesis and Leaf Expansion: An Integrated Transcriptomic, Metabolomic, and Physiological Analysis of Hypericum monogynum" Forests 16, no. 3: 465. https://doi.org/10.3390/f16030465
APA StyleDuan, L., Zeng, Z., Tang, Y., Liao, Y., Lin, K., Hu, H., Xu, Z.-F., & Ni, J. (2025). New Insights into the Role of Cytokinin in Regulating Anthocyanin Biosynthesis and Leaf Expansion: An Integrated Transcriptomic, Metabolomic, and Physiological Analysis of Hypericum monogynum. Forests, 16(3), 465. https://doi.org/10.3390/f16030465






