Nitrogen Enrichment Alters Plant Root, Soil Microbial Structure, Diversity, and Function in Mountain Forests of North China
Abstract
1. Introduction
2. Materials and Methods
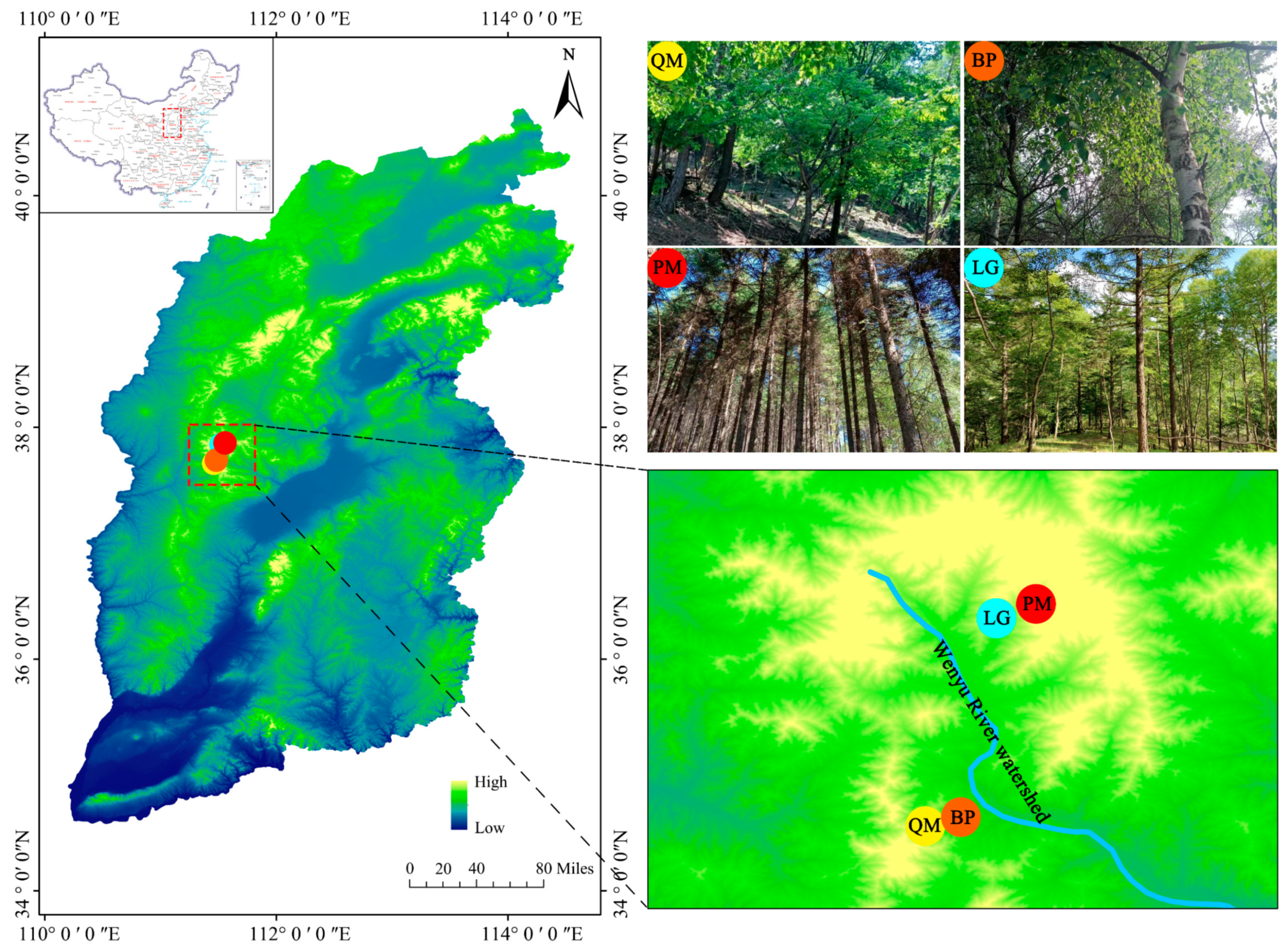
2.1. Study Site
2.2. Experimental Design and Sample Collection
2.3. Root Analysis
2.4. Soil Properties
2.5. Analysis of Soil Microbial Communities
2.5.1. DNA Extraction, Amplification, and Sequencing DNA
2.5.2. Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
3.1. Effects of N Treatments and Tree Species on Soil Properties, Soil Enzyme Activities, and Root Morphology
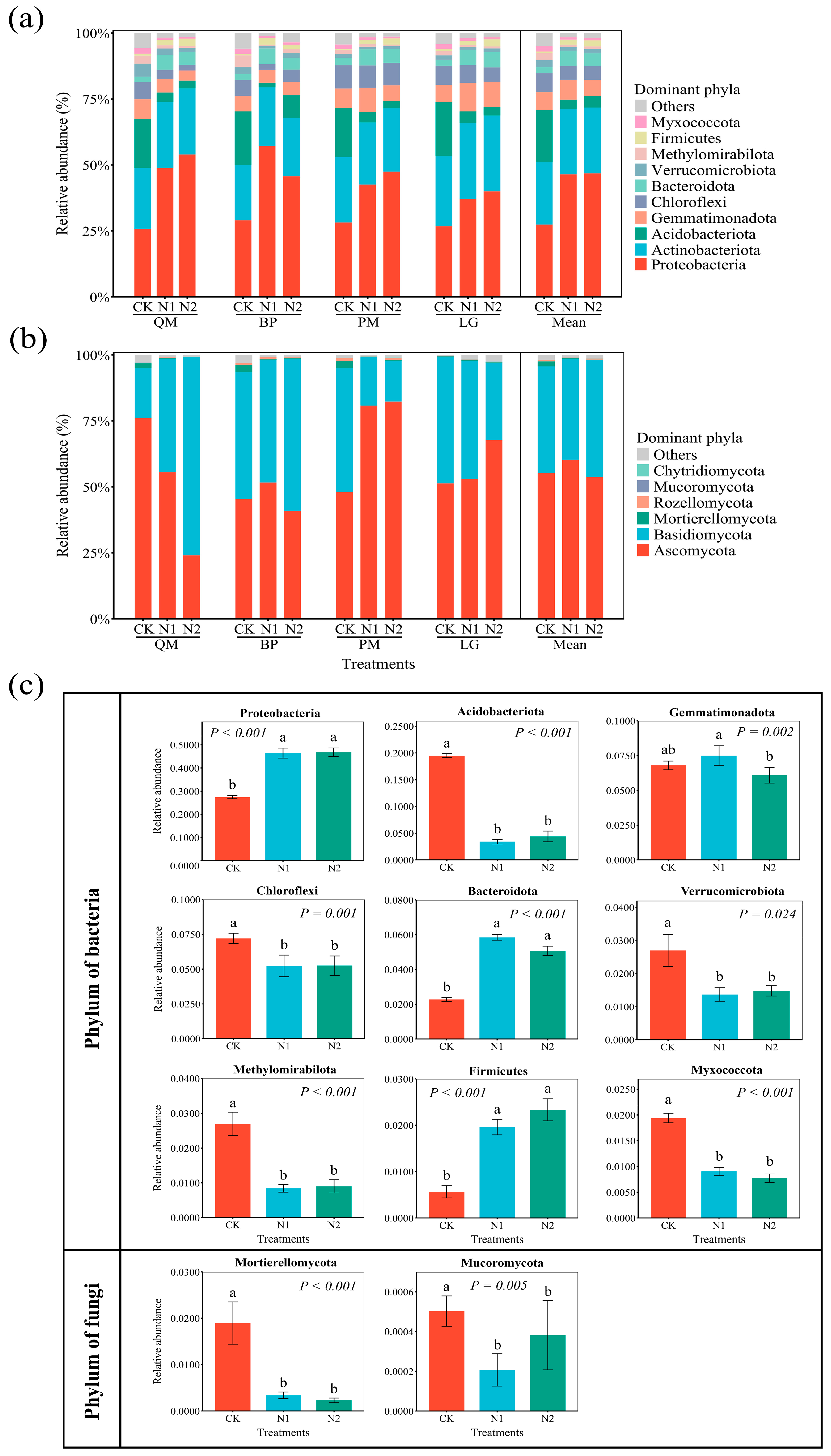
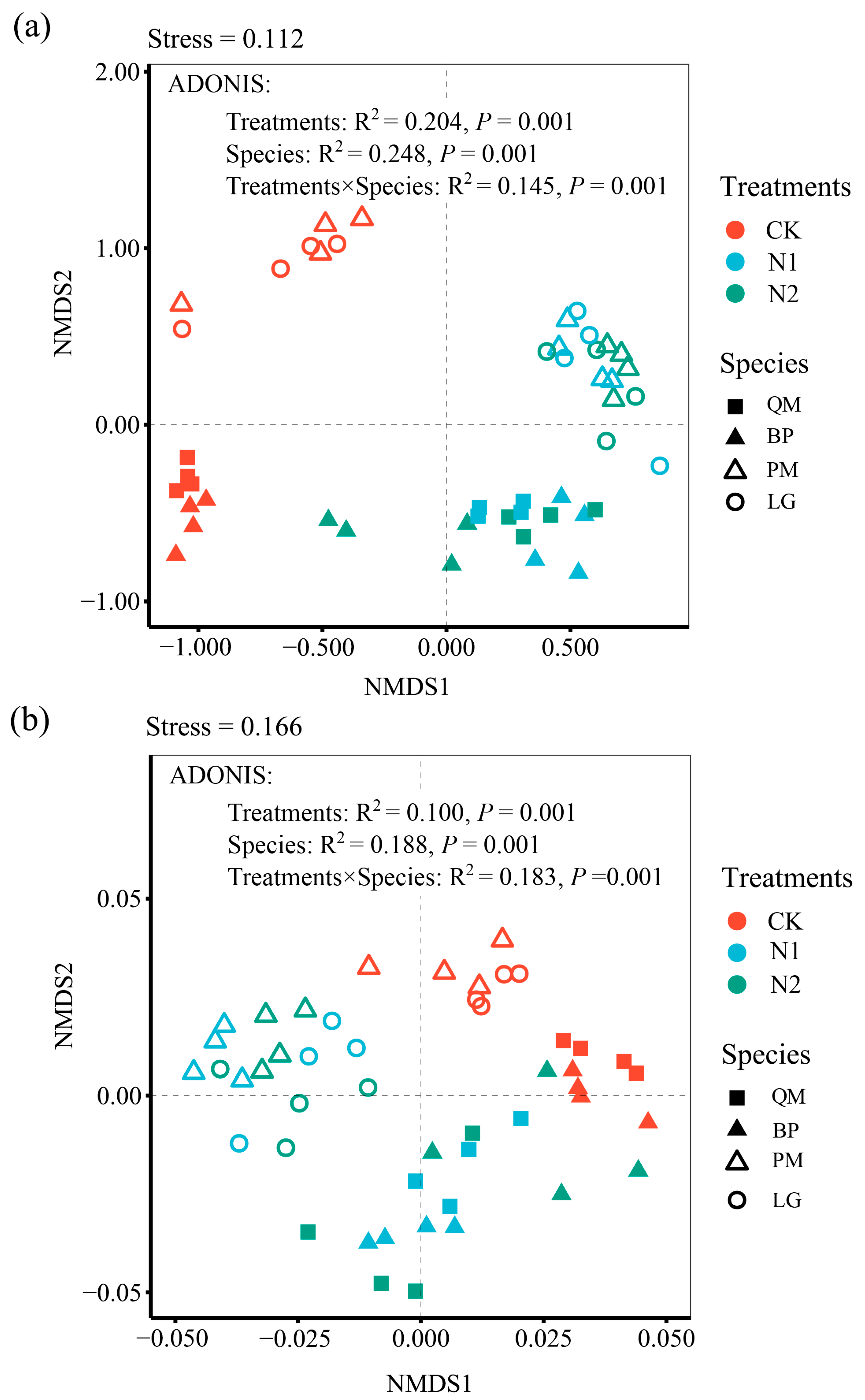
3.2. Impact of N Addition on Microbial Community Structure
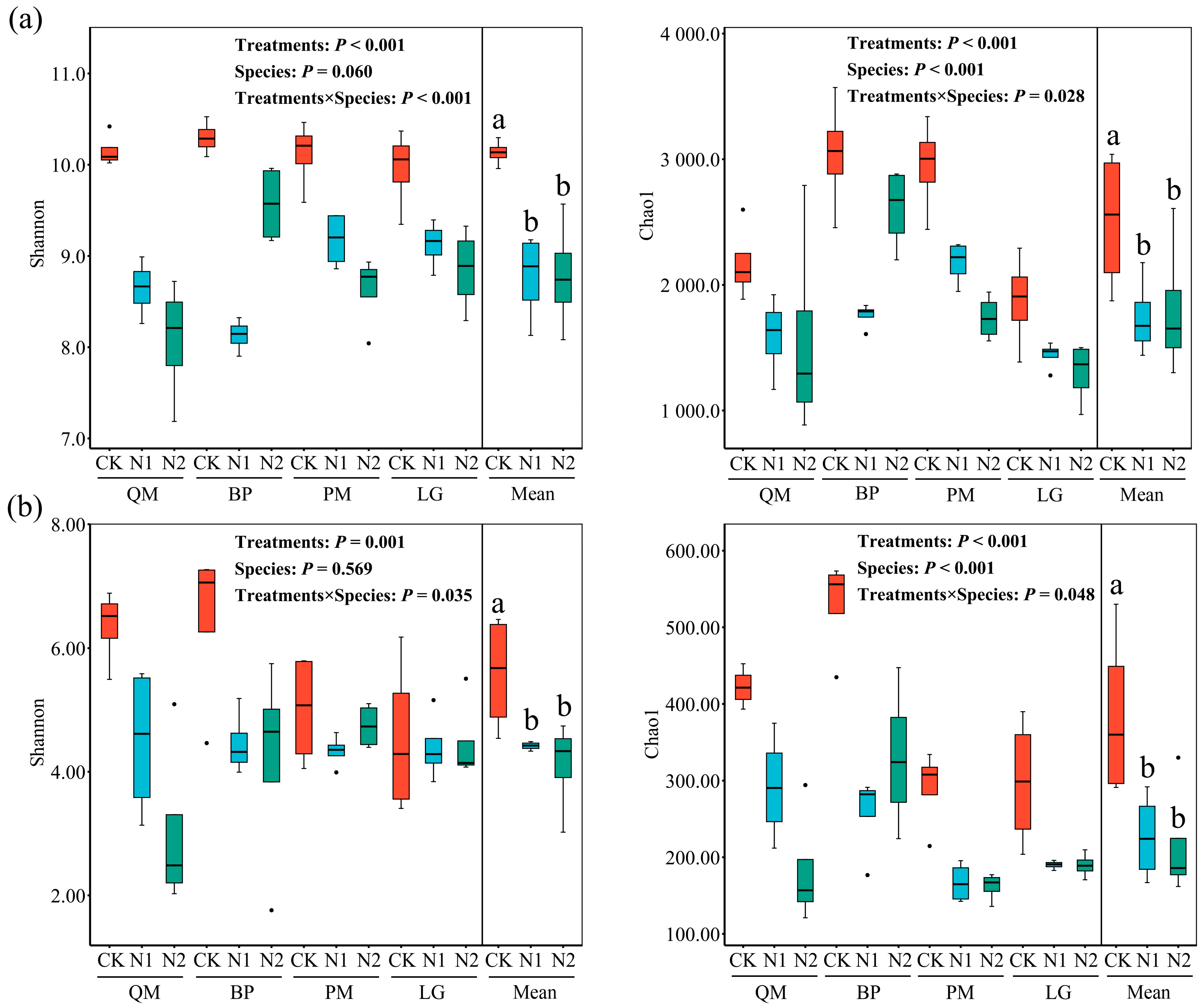
3.3. Effects of N Treatments and Tree Species on Microbial Diversity
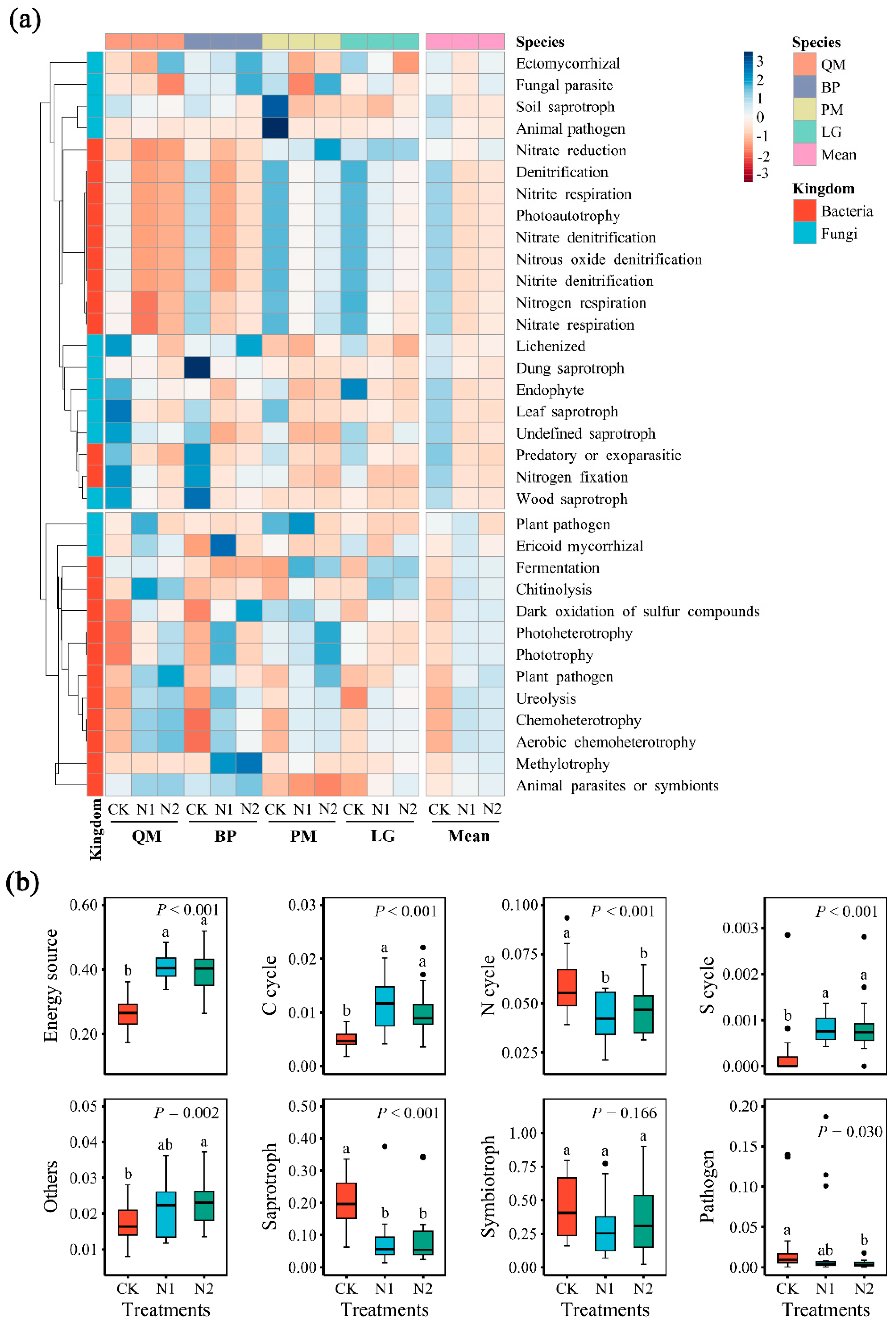
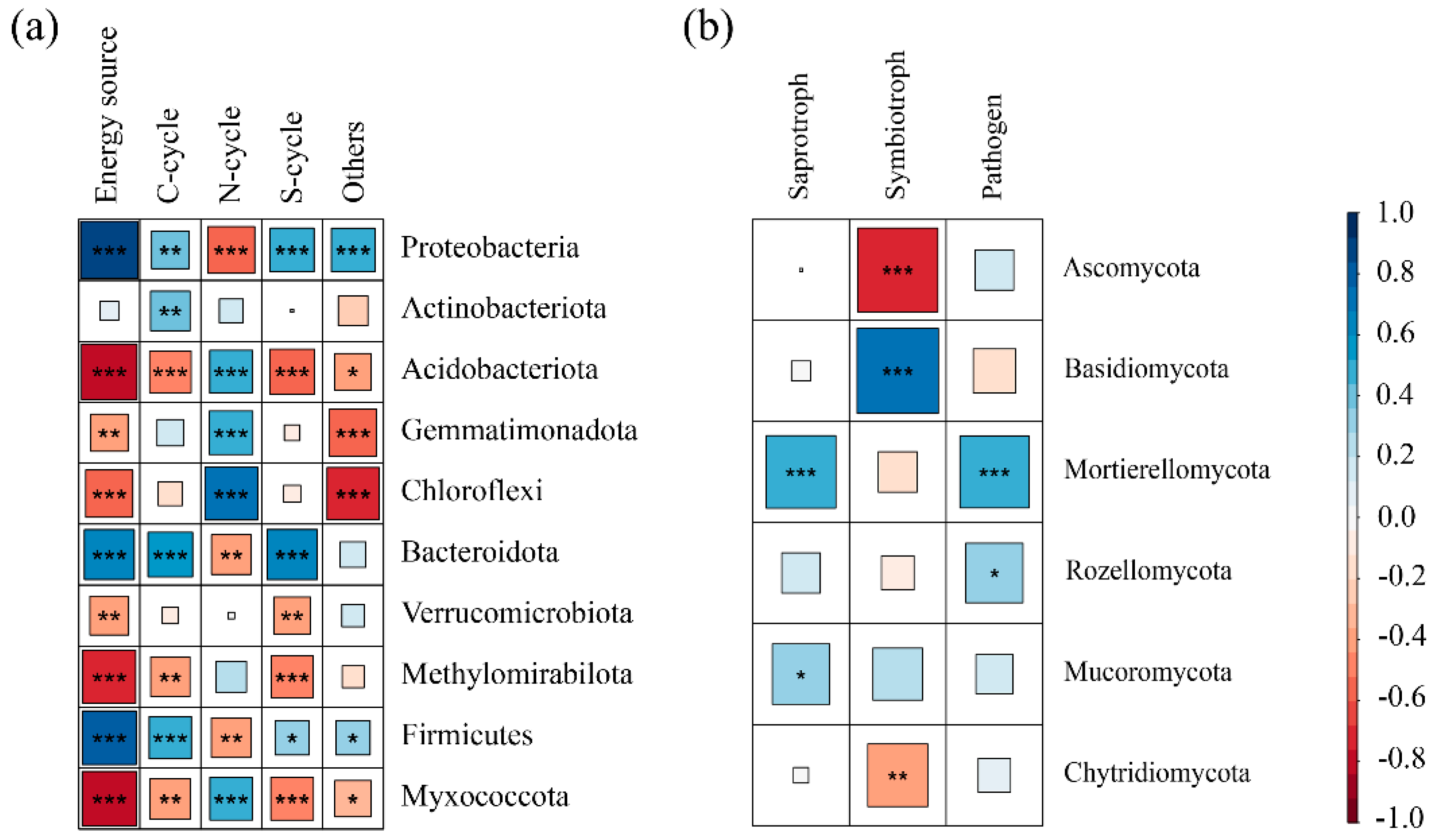
3.4. Changes in the Relative Abundance of Microbial Community Functions and the Relationship Between Function and Dominant Phyla
3.5. Microbial Correlation Networks Under Different N Treatments
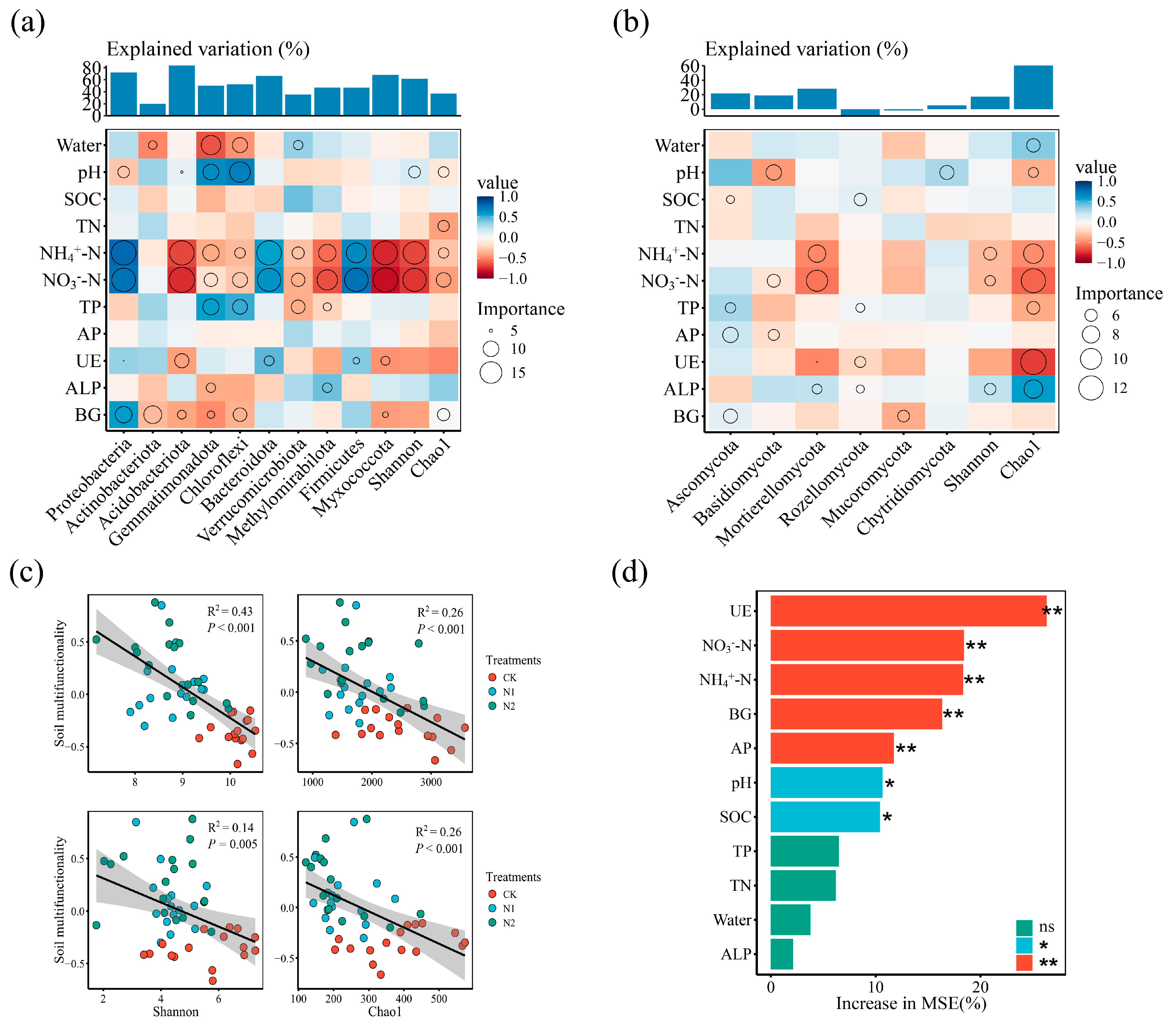
3.6. Soil Factors Affecting Microbial Dominance Phylum, Diversity Indices, and Soil Multifunctionality
4. Discussion
4.1. N Addition Significantly Altered the Morphological Traits of Absorbing Roots
4.2. Impact of N Addition on Soil Microbial Composition and Diversity
4.3. Effects on Soil Microbial Functional Groups
4.4. N Enrichment Reduces the Complexity and Robustness of Microbial Symbiotic Networks
4.5. Microbial Diversity and Soil Multifunctionality Are Significantly Negatively Correlated
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B 2013, 368, 20130116. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Steadman, C.E.; Stevenson, D.; Coyle, M.; Rees, R.M.; Skiba, U.M.; Sutton, M.A.; Cape, J.N.; Dore, A.J.; Vieno, M.; et al. Effects of global change during the 21st century on the nitrogen cycle. Atmos. Chem. Phys. 2015, 15, 13849–13893. [Google Scholar] [CrossRef]
- Melillo, J.M. Disruption of the global nitrogen cycle: A grand challenge for the twenty-first century. Ambio 2021, 50, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Zhou, G.Y.; Zou, X.M.; Bai, E.; Scanlon, T.M.; Hou, E.Q.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef] [PubMed]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar] [CrossRef]
- Mao, Q.G.; Lu, X.K.; Mo, H.; Gundersen, P.; Mo, J.M. Effects of simulated N deposition on foliar nutrient status, N metabolism and photosynthetic capacity of three dominant understory plant species in a mature tropical forest. Sci. Total Environ. 2018, 610, 555–562. [Google Scholar] [CrossRef]
- Payne, R.J.; Dise, N.B.; Field, C.D.; Dore, A.J.; Caporn, S.J.M.; Stevens, C.J. Nitrogen deposition and plant biodiversity: Past, present, and future. Front. Ecol. Environ. 2017, 15, 431–436. [Google Scholar] [CrossRef]
- Craig, H.; Antwis, R.E.; Cordero, I.; Ashworth, D.; Robinson, C.H.; Osborne, T.Z.; Bardgett, R.D.; Rowntree, J.K.; Simpson, L.T. Nitrogen addition alters composition, diversity, and functioning of microbial communities in mangrove soils: An incubation experiment. Soil Biol. Biochem. 2021, 153, 108076. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Kou, L.; Guo, D.L.; Yang, H.; Gao, W.L.; Li, S.G. Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 2015, 391, 207–218. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Gong, L. The morphological and chemical properties of fine roots respond to nitrogen addition in a temperate Schrenk’s spruce (Picea schrenkiana) forest. Sci. Rep. 2021, 11, 3839. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, S.; Shao, Y.; Zhang, K. Soil bacteria are more sensitive than fungi in response to nitrogen and phosphorus enrichment. Front. Microbiol. 2022, 13, 999385. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; McCormack, M.L.; Eissenstat, D.M. Foraging strategies in trees of different root morphology: The role of root lifespan. Tree Physiol. 2013, 33, 940–948. [Google Scholar] [CrossRef]
- Du, X.Z.; Wei, X. Definition of fine roots on the basis of the root anatomy, diameter, and branch orders of one-year old seedlings. J. For. Res. 2018, 29, 1321–1327. [Google Scholar] [CrossRef]
- Gu, J.C.; Wei, X.; Wang, J.; Dong, X.Y.; Wang, Z.Q. Marked differences in standing biomass, length density, anatomy and physiological activity between white and brown roots in Rupr. plantation. Plant Soil 2015, 392, 267–277. [Google Scholar] [CrossRef]
- Da, R.; Fan, C.; Zhang, C.; Zhao, X.; von Gadow, K. Are absorptive root traits good predictors of ecosystem functioning? A test in a natural temperate forest. New Phytol. 2023, 239, 75–86. [Google Scholar] [CrossRef]
- Ostonen, I.; Helmisaari, H.S.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.J.; Nöjd, P.; Uri, V.; Merilä, P.; et al. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob. Change Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- Wang, G.L.; Fahey, T.J.; Xue, S.; Liu, F. Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 2013, 171, 583–590. [Google Scholar] [CrossRef]
- Wang, W.J.; Mo, Q.F.; Han, X.G.; Hui, D.F.; Shen, W.J. Fine root dynamics responses to nitrogen addition depend on root order, soil layer, and experimental duration in a subtropical forest. Biol. Fertil. Soils 2019, 55, 723–736. [Google Scholar] [CrossRef]
- Wang, W.N.; Wang, Y.; Hoch, G.; Wang, Z.Q.; Gu, J.C. Linkage of root morphology to anatomy with increasing nitrogen availability in six temperate tree species. Plant Soil 2018, 425, 189–200. [Google Scholar] [CrossRef]
- Li, W.B.; Jin, C.J.; Guan, D.X.; Wang, Q.K.; Wang, A.Z.; Yuan, F.H.; Wu, J.B. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Anthony, M.A.; Pec, G.J.; Trocha, L.K.; Trzebny, A.; Geyer, K.M.; van Diepen, L.T.A.; Frey, S.D. Fungal community structure and function shifts with atmospheric nitrogen deposition. Glob. Change Biol. 2021, 27, 1349–1364. [Google Scholar] [CrossRef]
- Wang, J.Q.; Shi, X.Z.; Zheng, C.Y.; Suter, H.; Huang, Z.Q. Different responses of soil bacterial and fungal communities to nitrogen deposition in a subtropical forest. Sci. Total Environ. 2021, 755, 142449. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, H.; Huang, Y.; Dong, B.; Fu, S.; Zhang, C.; Li, X.; Tan, Y.; Zhang, X.; Zhang, X. Driving mechanisms of soil bacterial α and β diversity under long-term nitrogen addition: Subtractive heterogenization based on the environment selection. Geoderma 2024, 445, 116886. [Google Scholar] [CrossRef]
- Nie, Y.X.; Wang, M.C.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W.J. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 2018, 624, 407–415. [Google Scholar] [CrossRef]
- Yang, H.L.; Cheng, L.; Che, L.M.G.; Su, Y.Z.; Li, Y.L. Nutrients addition decreases soil fungal diversity and alters fungal guilds and co-occurrence networks in a semi-arid grassland in northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef]
- He, J.H.; Jiao, S.; Tan, X.P.; Wei, H.; Ma, X.M.; Nie, Y.X.; Liu, J.X.; Lu, X.K.; Mo, J.M.; Shen, W.J. Adaptation of Soil Fungal Community Structure and Assembly to Long-Versus Short-Term Nitrogen Addition in a Tropical Forest. Front. Microbiol. 2021, 12, 689674. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Z.; Li, A.; Geng, T.; Liu, L.; Liu, W. Long-term nitrogen input reduces soil bacterial network complexity by shifts in life history strategy in temperate grassland. iMeta 2024, 3, e194. [Google Scholar] [CrossRef]
- Zhang, Q.; Buyantuev, A.; Fang, X.N.; Han, P.; Li, A.; Li, F.Y.; Liang, C.Z.; Liu, Q.F.; Ma, Q.; Niu, J.M.; et al. Ecology and sustainability of the Inner Mongolian Grassland: Looking back and moving forward. Landsc. Ecol. 2020, 35, 2413–2432. [Google Scholar] [CrossRef]
- Garland, G.; Banerjee, S.; Edlinger, A.; Oliveira, E.M.; Herzog, C.; Wittwer, R.; Philippot, L.; Maestre, F.T.; van der Heijden, M.G.A. A closer look at the functions behind ecosystem multifunctionality: A review. J. Ecol. 2021, 109, 600–613. [Google Scholar] [CrossRef]
- Ma, L.N.; Zhang, C.X.; Xu, X.F.; Wang, C.W.; Liu, G.F.; Liang, C.Z.; Zuo, X.A.; Wang, C.J.; Lv, Y.; Wang, R.Z. Different facets of bacterial and fungal communities drive soil multifunctionality in grasslands spanning a 3500 km transect. Funct. Ecol. 2022, 36, 3120–3133. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Trivedi, P.; Trivedi, C.; Eldridge, D.J.; Reich, P.B.; Jeffries, T.C.; Singh, B.K. Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.C.; Son, A.; Yu, B.; Zeng, X.B.; Chen, M.S.; Yin, H.Q.; Zhang, X.X.; Sun, B.L.; Fan, F.L. Loss of soil microbial diversity may increase insecticide uptake by crop. Agric. Ecosyst. Environ. 2017, 240, 84–91. [Google Scholar] [CrossRef]
- Chen, G.; Wu, C.; Ge, T.; Chen, J.; Deng, Y. Response of Soil Multifunctionality to Reduced Microbial Diversity. Environ. Sci. 2022, 43, 5274–5285. (In Chinese) [Google Scholar]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef]
- Jia, L.Q.; Chen, G.S.; Zhang, L.H.; Chen, T.T.; Jiang, Q.; Chen, Y.H.; Fan, A.L.; Wang, X. Plastic responses of fine root morphological traits of Castanopsis fabri and Castanopsis carlesii to short-term nitrogen addition. J. Appl. Ecol. 2019, 30, 4003–4011. (In Chinese) [Google Scholar]
- Liu, B.T.; Han, F.; Ning, P.; Li, H.B.; Rengel, Z. Root traits and soil nutrient and carbon availability drive soil microbial diversity and composition in a northern temperate forest. Plant Soil 2022, 479, 281–299. [Google Scholar] [CrossRef]
- Li, L. Analysis on Litter and Soil Nutrient Content of Larix Principis-rupprechtii plantation in Xiaowenshan Forest Farm. Shanxi For. Sci. Technol. 2023, 52, 11–13. (In Chinese) [Google Scholar]
- Comas, L.H.; Eissenstat, D.M. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct. Ecol. 2004, 18, 388–397. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine north american trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.L.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Guan, S.Y. Soil Enzymes and Its Methodology; China Agriculture Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Witte, C.-P.; Medina-Escobar, N. In-Gel Detection of Urease with Nitroblue Tetrazolium and Quantification of the Enzyme from Different Crop Plants Using the Indophenol Reaction. Anal. Biochem. 2001, 290, 102–107. [Google Scholar] [CrossRef]
- Xiong, J. A Method for the Determination of Soil β-Glucosidase Activity; Panzhihua University: Panzhihua, China, 2022. (In Chinese) [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, S.L.; Wu, H.Y.; Liao, L.R.; Wang, X.T.; Zhang, L.; Liu, G.B.; Wang, G.L.; Fang, L.C.; Song, Z.L. Simplified microbial network reduced microbial structure stability and soil functionality in alpine grassland along a natural aridity gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Wu, J.; Wu, C.; Zhang, Q.; Zhang, H.; Wang, S.; Wang, F.; Jin, S.; Kuzyakov, Y.; Chen, J.; Ge, T. Microbial diversity loss and wheat genotype-triggered rhizosphere bacterial and protistan diversity constrain soil multifunctionality: Evidence from greenhouse experiment. Plant Soil 2024, 504, 475–491. [Google Scholar] [CrossRef]
- Zobel, R.W.; Kinraide, T.B.; Baligar, V.C. Fine root diameters can change in response to changes in nutrient concentrations. Plant Soil 2007, 297, 243–254. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst.-Int. J. Deal. All. Asp. Plant Biol. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Poorter, H.; Ryser, P. The limits to leaf and root plasticity: What is so special about specific root length? New Phytol. 2015, 206, 1188–1190. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Fitter, A. Constraints on the Form and Function of Root Systems. In Root Ecology; de Kroon, H., Visser, E.J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wang, G. Belowground Nutrient-Acquisition Strategies of Different Ectomycorrhizal Tree Species in the Mountainous Regions of North China; Shanxi Agricultural University: Jinzhong, China, 2021. [Google Scholar]
- Wang, C.G.; Han, S.J.; Zhou, Y.M.; Yan, C.F.; Cheng, X.B.; Zheng, X.B.; Li, M.H. Responses of Fine Roots and Soil N Availability to Short-Term Nitrogen Fertilization in a Broad-Leaved Korean Pine Mixed Forest in Northeastern China. PLoS ONE 2012, 7, e31042. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Chen, D.M.; Guo, H.; Wei, J.X.; Bai, Y.F.; Shen, Q.R.; Hu, S.J. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Song, W.F.; Wen, S.L.; Wang, B.R.; Zhu, C.Q.; Shen, R.F. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 2017, 113, 240–249. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Arunrat, N.; Sereenonchai, S.; Sansupa, C.; Kongsurakan, P.; Hatano, R. Effect of Rice Straw and Stubble Burning on Soil Physicochemical Properties and Bacterial Communities in Central Thailand. Biology 2023, 12, 501. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; Ju, X.; Zhu, Z.; Zhang, Y.; Chen, H.; Jin, K. Soil acidification drives the negative effects of nitrogen enrichment on soil microbial biomass at the global scale. Plant Soil 2024, 503, 517–528. [Google Scholar] [CrossRef]
- Gonçalves, O.S.; Fernandes, A.S.; Tupy, S.M.; Ferreira, T.G.; Almeida, L.N.; Creevey, C.J.; Santana, M.F. Insights into plant interactions and the biogeochemical role of the globally widespread Acidobacteriota phylum. Soil Biol. Biochem. 2024, 192, 109369. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, M.; Yang, Z.; Cong, M.; Zhu, X.; Jia, H. Soil Microbial Community Response to Nitrogen Application on a Swamp Meadow in the Arid Region of Central Asia. Front. Microbiol. 2022, 12, 797306. [Google Scholar] [CrossRef]
- Ma, A.Z.; Zhuang, X.L.; Wu, J.M.; Cui, M.M.; Lv, D.; Liu, C.Z.; Zhuang, G.Q. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.P.; Lonhienne, T.G.A.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8675. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Wang, J.; Liao, L.R.; Ye, Z.C.; Liu, H.F.; Zhang, C.; Zhang, L.; Liu, G.B.; Wang, G.L. Different bacterial co-occurrence patterns and community assembly between rhizosphere and bulk soils under N addition in the plant-soil system. Plant Soil 2022, 471, 697–713. [Google Scholar] [CrossRef]
- Fu, Y.L. Response of Interroot Microorganisms to Nitrogen Addition in a Northern Forest; Heilongjiang University: Harbin, China, 2021. (In Chinese) [Google Scholar]
- Liu, W.B.; Ling, N.; Guo, J.J.; Ruan, Y.; Zhu, C.; Shen, Q.R.; Guo, S.W. Legacy effects of 8-year nitrogen inputs on bacterial assemblage in wheat rhizosphere. Biol. Fertil. Soils 2020, 56, 583–596. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Yang, T.; Chu, H. Threshold effects of soil pH on microbial co-occurrence structure in acidic and alkaline arable lands. Sci. Total Environ. 2021, 800, 149592. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Oladele, O.; Song, X.T.; Ju, X.T.; Jia, Z.J.; Hu, H.W.; Liu, X.J.; Bei, S.K.; Ge, A.H.; Zhang, L.M.; et al. Opportunities and approaches for manipulating soil-plant nicrobiomes for effective crop nitrogen use in agroecosystems. Agric. Sci. Eng. 2022, 9, 333–343. (In Chinese) [Google Scholar]
- Ma, Z.Z.; Qiao, S.S.; Cao, M.W.; Zhou, Y.N.; Liu, J.X.; Jia, T.; Li, C.; Chai, B.F. Environmental selection and dispersal limitation drive the assemblage of bacterial community in temperate forest soils. Chin. J. Appl. Ecol. 2018, 29, 1179–1189. (In Chinese) [Google Scholar]
- Ward, N.L.; Challacombe, J.F.; Janssen, P.H.; Henrissat, B.; Coutinho, P.M.; Wu, M.; Xie, G.; Haft, D.H.; Sait, M.; Badger, J.; et al. Three Genomes from the Phylum Acidobacteria Provide Insight into the Lifestyles of These Microorganisms in Soils. Appl. Environ. Microbiol. 2009, 75, 2046–2056. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Koljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Li, D.; Ni, H.W.; Jiao, S.; Lu, Y.H.; Zhou, J.Z.; Sun, B.; Liang, Y.T. Coexistence patterns of soil methanogens are closely tied to methane generation and community assembly in rice paddies. Microbiome 2021, 9, 20. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Chen, S.Y.; Chen, J.W.; Xue, K.; Chen, S.L.; Wang, X.M.; Chen, T.; Kang, S.C.; Rui, J.P.; Thies, J.E.; et al. Reduced microbial stability in the active layer is associated with carbon loss under alpine permafrost degradation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025321118. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Z.; Yang, S.; Chen, L.; Xin, J.; Xu, L.; Zhang, X.; Zhai, B.; Wang, Z.; Zheng, W.; et al. Nitrogen inhibitors improve soil ecosystem multifunctionality by enhancing soil quality and alleviating microbial nitrogen limitation. Sci. Total Environ. 2023, 880, 163238. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Yao, H.; Zhang, B.; Li, Y.; Jin, S.; Cao, H. Reducing Application of Nitrogen Fertilizer Increases Soil Bacterial Diversity and Drives Co-Occurrence Networks. Microorganisms 2024, 12, 1434. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Qiu, T.; He, H.; Liu, J.; Duan, C.; Cui, Y.; Huang, M.; Wu, C.; Fang, L. High nitrogen fertilizer input enhanced the microbial network complexity in the paddy soil. Soil Ecol. Lett. 2023, 6, 230205. [Google Scholar] [CrossRef]

| Parameters | Treatments | Species | Treatments × Species | |||
|---|---|---|---|---|---|---|
| F/H | p | F/H | p | F/H | p | |
| Soil parameters | ||||||
| Water (%) | 0.467 | 0.792 | 39.300 | <0.001 | 6.834 | 0.337 |
| pH | 1.591 | 0.451 | 35.093 | <0.001 | 3.726 | 0.714 |
| SOC (g·kg−1) | 0.465 | 0.793 | 26.779 | <0.001 | 4.815 | 0.568 |
| TN (g·kg−1) | 2.002 | 0.367 | 17.030 | 0.001 | 7.198 | 0.303 |
| NH4+-N (mg·kg−1) | 35.635 | <0.001 | 3.004 | 0.391 | 2.863 | 0.826 |
| NO3−-N (mg·kg−1) | 36.286 | <0.001 | 1.401 | 0.705 | 2.875 | 0.824 |
| TP (g·kg−1) | 0.230 | 0.891 | 37.929 | <0.001 | 4.422 | 0.620 |
| AP (mg·kg−1) | 0.275 | 0.761 | 58.912 | <0.001 | 3.841 | 0.005 |
| SM | 60.304 | <0.001 | 15.743 | <0.001 | 4.194 | 0.003 |
| Soil enzyme activities | ||||||
| BG (µg·g−1·h−1) | 5.857 | 0.053 | 34.908 | <0.001 | 7.654 | 0.265 |
| UE (U·g−1) | 17.384 | <0.001 | 13.483 | 0.004 | 2.079 | 0.912 |
| ALP (U·g−1) | 7.323 | 0.002 | 18.358 | <0.001 | 0.687 | 0.661 |
| Root traits | ||||||
| RD (mm) | 6.777 | 0.034 | 36.887 | <0.001 | 0.589 | 0.997 |
| SRL (m·g−1) | 6.701 | 0.035 | 38.600 | <0.001 | 0.543 | 0.997 |
| SRA (cm2·g−1) | 6.143 | 0.046 | 36.038 | <0.001 | 0.771 | 0.994 |
| RTD (g·cm−3) | 6.255 | 0.044 | 37.096 | <0.001 | 0.317 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Luo, Q.; Chen, Y.; Xuan, Y.; Wu, L.; Qiu, W.; Wu, X.; Chen, Y.; Guo, J. Nitrogen Enrichment Alters Plant Root, Soil Microbial Structure, Diversity, and Function in Mountain Forests of North China. Forests 2025, 16, 459. https://doi.org/10.3390/f16030459
Han X, Luo Q, Chen Y, Xuan Y, Wu L, Qiu W, Wu X, Chen Y, Guo J. Nitrogen Enrichment Alters Plant Root, Soil Microbial Structure, Diversity, and Function in Mountain Forests of North China. Forests. 2025; 16(3):459. https://doi.org/10.3390/f16030459
Chicago/Turabian StyleHan, Xiaoli, Qian Luo, Yuhan Chen, Yajie Xuan, Lei Wu, Wenhui Qiu, Xiaogang Wu, Yinglong Chen, and Jinping Guo. 2025. "Nitrogen Enrichment Alters Plant Root, Soil Microbial Structure, Diversity, and Function in Mountain Forests of North China" Forests 16, no. 3: 459. https://doi.org/10.3390/f16030459
APA StyleHan, X., Luo, Q., Chen, Y., Xuan, Y., Wu, L., Qiu, W., Wu, X., Chen, Y., & Guo, J. (2025). Nitrogen Enrichment Alters Plant Root, Soil Microbial Structure, Diversity, and Function in Mountain Forests of North China. Forests, 16(3), 459. https://doi.org/10.3390/f16030459






