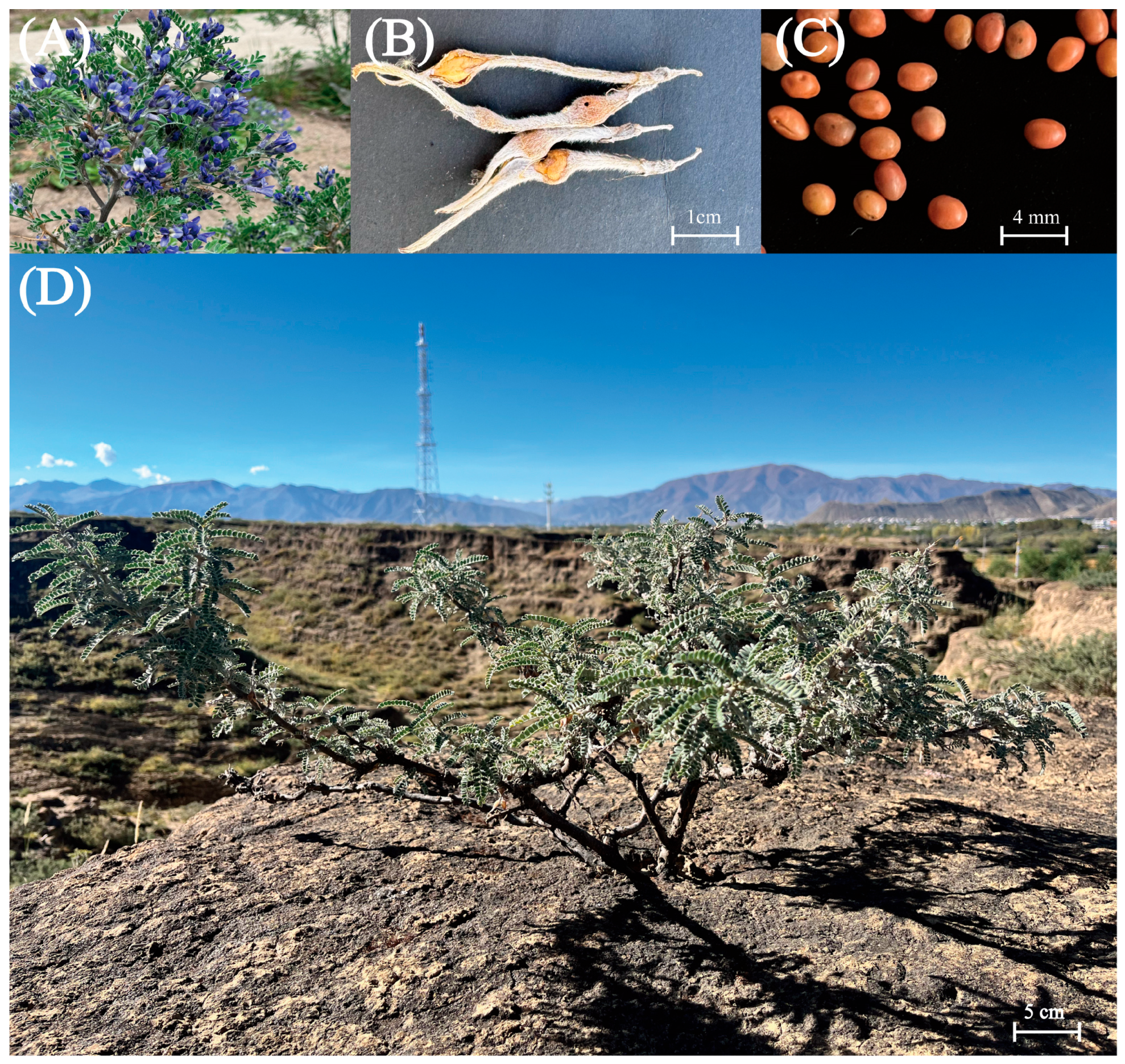
Unlocking the Potential of Sophora moorcroftiana (Fabaceae): The Overlooked Xizang Endemic
Abstract
1. Introduction
2. Ecological and Environmental Value
2.1. Windbreak and Sand Stabilization in Combating Desertification
2.2. Soil and Water Conservation and Fertility Enhancement
2.3. Genetic Regulation of Drought Stress Responses in Sophora moorcroftiana
| Genes | Description | Gene_ID | Function | Refs. |
|---|---|---|---|---|
| SmDREB1 | Drought-responsive element-binding protein 1 | KM527092 | Regulates downstream drought resistance genes | [22,43,44] |
| SmDREB2 | Drought-responsive element-binding protein 2 | KM527093 | Protects cells and helps retention | [22] |
| SmDREB3 | Drought-responsive element-binding protein 3 | KM527094 | Protects cells | [22] |
| SmDREB4 | Drought-responsive element-binding protein 4 | KM527095 | Facilitates water transport | [22] |
| AMY3 | Alpha-amylase | Ssh0029976 | Hydrolyzes starch to release sugars | [45] |
| CWINV1 | Beta-fructofuranosidase | Ssh0033248 | Hydrolyzes sucrose | [45] |
3. Medicinal Value
3.1. Active Compounds and Clinical Applications
3.2. Safety of Consumption
4. Pests and Disease Management
4.1. Major Pests and Diseases
4.1.1. Pod Borer (Etiella zinckenella)
4.1.2. Seed Wasp (Bruchophagus onois)
4.1.3. Current Status of Diseases
- Powdery mildew (Erysiphales): During 2024 fieldwork in Xizang, powdery mildew infections were observed on greenhouse-cultivated S. moorcroftiana, manifesting as white mycelial growth on leaves and stems. This pathogen thrives under high humidity (>70%) and moderate temperatures (15–25 °C), conditions common in controlled environments [72]. Infected plants exhibited reduced photosynthetic capacity and stunted growth, highlighting risks for intensive cultivation systems.
- Potential fungal threats: Comparative studies on related legumes (e.g., Sophora flavescens) indicate susceptibility to root rot (Fusarium spp.) and leaf spot (Alternaria spp.) under prolonged rainfall or poor drainage [73,74]. Although not yet reported in S. moorcroftiana, these pathogens could colonize stressed plants in waterlogged soils or monsoon-affected regions.
- Bacterial risks: In arid regions, Xanthomonas spp. have caused stem cankers in drought-stressed leguminous shrubs [75]. Such pathogens may exploit physiological weaknesses in S. moorcroftiana during extreme drought heatwave events.
4.2. Control Strategies
4.2.1. Control of Etiella zinckenella
- Chemical control: A 5% chlorantraniliprole suspension concentrate is highly effective against E. zinckenella. When applied 3 and 7 days after fertilization, this pesticide achieved 100% efficacy in controlling larvae that damage flowers and 98.26% and 94.91% efficacy in controlling larvae infesting pods, respectively [79]. Additionally, a 25% spinetoram water-dispersible granule was found to be effective, although slightly less potent than chlorantraniliprole.
- Ecological control: Emamectin benzoate and fresh neem oil significantly reduce pod borer populations while having minimal impact on parasitoid species. These products are particularly recommended when pest control must be balanced with ecological conservation [80].
4.2.2. Control of Bruchophagus onois
- Plant quarantine: Strict regulations prohibit the transport of infested seeds to pest-free areas. Rigorous quarantine protocols must be enforced during seed transportation to prevent pest spread.
- Seed treatment: Seed flotation in water can help remove damaged seeds, while fumigation with aluminum phosphide effectively eliminates pests from infested seeds.
- Forest management: Severely infested plantations may require coppicing to remove infected plants, disrupt the habitat of B. onois, and reduce pest sources.
- Chemical control (B. onois): During the adult emergence period of B. onois, two applications of a 50% malathion emulsifiable concentrate diluted 1:1000 are recommended. The first application should occur from late June to early July to target first-generation adults, and the second from late August to early September to control the second-generation adults [81].
4.2.3. Recommendations for Integrated Pest Management (IPM)
- Ecological impact mitigation: Minimize non-target effects on pollinators and natural enemies by using selective pesticides (e.g., emamectin benzoate) and habitat management [82].
- Long-term monitoring: Establish regional pest surveillance networks to track population dynamics and predict outbreaks [83].
- Climate-adaptive disease monitoring: Establish disease risk models incorporating regional climatic variables (e.g., humidity and precipitation) to predict outbreaks of powdery mildew and other humidity-dependent pathogens [84].
- Resistant germplasm screening: Screen wild S. moorcroftiana populations for disease-resistant traits, particularly against powdery mildew and root rot, to inform breeding programs [85].
- Endophyte-based biocontrol: Leverage antimicrobial endophytes isolated from S. moorcroftiana seeds (e.g., Bacillus subtilis strains) to develop biofungicides targeting emerging pathogens [86].
- Given the cryptic nature of E. zinckenella and B. onois, alongside their significant impact on S. moorcroftiana seed production, the strategy of “prevention first, integrated control” is strongly advised.
- Early detection: Integrating molecular techniques with field monitoring can enable the precise prediction of pest occurrence and severity, enhancing early detection and intervention capabilities.
- Rotational use of pesticides: To mitigate the development of resistance, it is crucial to avoid reliance on a single pesticide. Alternating between various highly effective, low-toxicity chemicals can disrupt pest adaptation and prolong the efficacy of pest control measures.
- Eco-friendly strategies: Effective habitat management and the use of natural enemies, such as parasitoid wasps, can be optimized to regulate pest populations, offering a sustainable and environmentally friendly approach to pest control.
- Disease research and control: In-depth studies on the antimicrobial mechanisms of endophytic microorganisms associated with S. moorcroftiana should be conducted to explore their potential in developing novel methods for preventing emerging plant diseases.
5. Conservation of Germplasm Resources of S. moorcroftiana
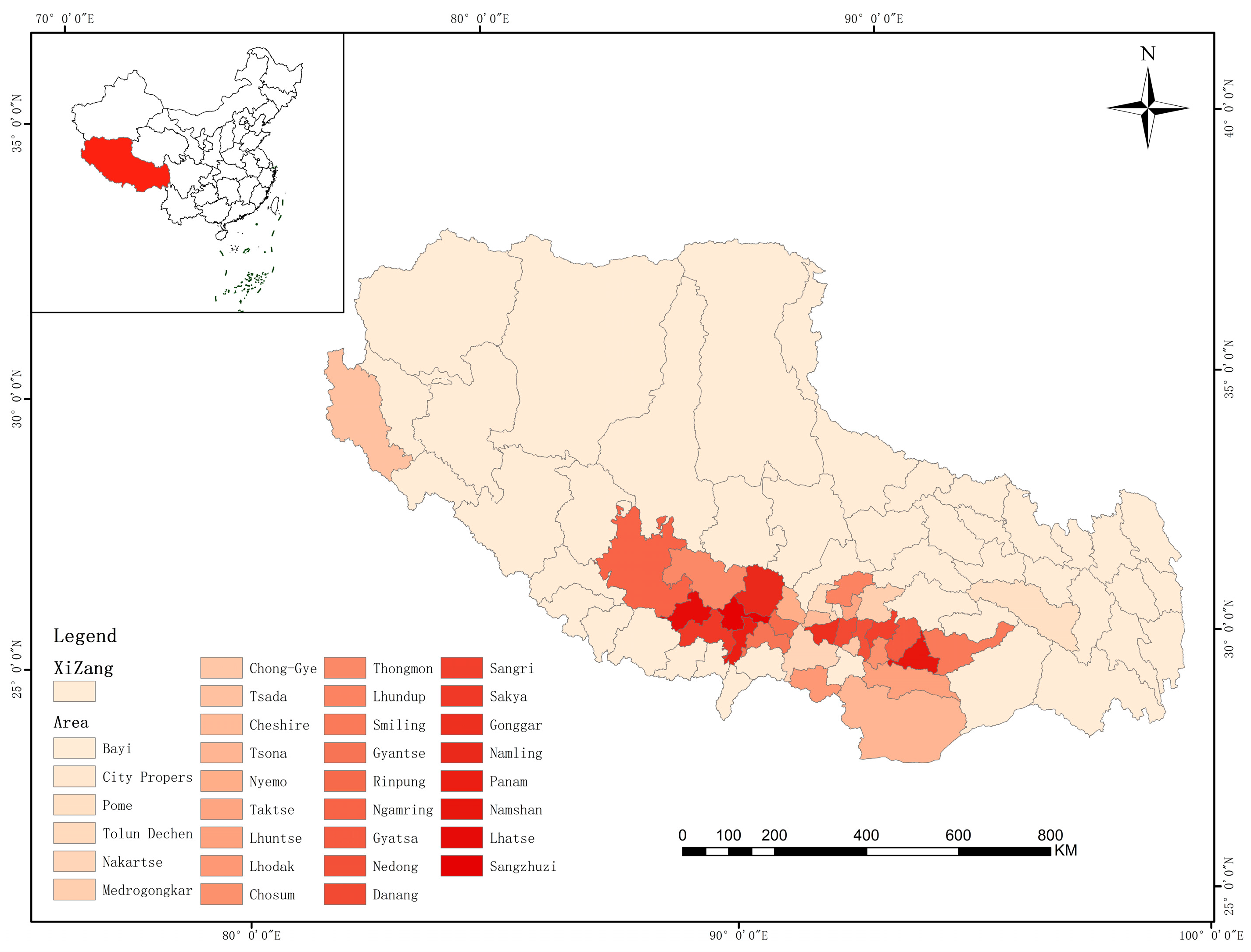
5.1. Current Population Distribution and Degradation Issues
5.2. Genetic Diversity
5.3. Conservation and Restoration Strategies
6. Future Perspectives
6.1. Basic Research Directions
- Deepening gene function studies: Future research should leverage gene-editing technologies such as CRISPR-Cas9 to validate the roles of these genes in drought tolerance and root development. Integrating transcriptomics and metabolomics will enable a comprehensive analysis of gene expression patterns under diverse environmental stresses, revealing molecular mechanisms that facilitate adaptation to plateau-specific adversities. Additionally, whole-genome resequencing of diverse germplasm resources will deepen the understanding of genetic diversity, population structure, and the genetic bases of adaptation, thereby supporting breeding and conservation initiatives.
- Multi-omics integration: Combining genomic, transcriptomic, proteomics, and metabolomics data will allow the construction of a comprehensive molecular network model of drought adaptation.
- Population genetics analysis: Resequencing geographically distinct populations will provide insights into genetic diversity and the adaptive evolution, supporting breeding and conservation initiatives. Specifically, investigating whether the expansion of sucrose metabolism-related genes is associated with evolutionary adaptations to arid environments will provide valuable insights and high-quality germplasm resources for genetic improvement.
6.2. Applied Research Directions
- Breeding drought-resistant varieties: Molecular marker-assisted selection (MAS) and gene-editing technologies can expedite the development of drought-resistant S. moorcroftiana varieties.
- Developing functional microbial agents: Probiotic microbial strains, such as nitrogen-fixing and phosphate-solubilizing bacteria, that promote root development and improve drought tolerance.
- Exploiting functional compounds: Secondary metabolites in sucrose metabolism-related pathways should be further investigated for their roles in drought resistance and antioxidative properties.
6.3. Sustainable Utilization and Management
- Habitat protection strategies: Strengthening environmental monitoring and establishing conservation zones to mitigate human-induced pressures on S. moorcroftiana populations.
- Desertification control and ecological restoration: Harnessing S. moorcroftiana’s robust root systems and stress tolerance can enhance ecosystem stability in plateau and desertified regions.
- Socioeconomic value promotion: Developing industries around S. moorcroftiana products, such as functional foods and medicinal products, can stimulate economic growth and conservation efforts.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, J.Z.; Gao, X.J.; Bai, Y.Y.; Han, J.W.; Zhaxi, C.R.; Da, Q.; Liu, X. A Brief Introduction of Caragana korshinskii, Haloxylon ammodendron and Sophora moorcroftiana and Research Progress on Their Feeding Value. Anim. Husb. Feed Sci. 2018, 39, 40–43. [Google Scholar]
- Nan, J.B.; Li, Y.H.; Danzeng, N.M.; Min, X.H.; Li, B.Z. Study on Natural Population Structure and Species Diversity of Sophora moorcroftiana in Tibet. J. Plateau Agric. 2021, 5, 592–597. [Google Scholar]
- PuBu, D.Z.; Hou, W.; Bianba, D.J.; Pu, L.; Xu, X.Y. Distribution Characteristics and Stumping-Rejuvenating Technology of Sophora moorcroftiana in Tibet. For. Constr. 2020, 6, 27–35. [Google Scholar]
- Yang, L.; Li, Q.; Guo, Q.Q.; Zhang, Y.F.; Li, H.E. Anthocyanin-related compounds during flower coloration of Sophora moorcroftiana. Eur. J. Hortic. Sci 2022, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- An, Y.P.; Hou, X.F.; Huang, Y.; Li, Y.X. Study on Seed Dormancy and Release Method of Sophora moorcroftiana in Tibet. J. Plateau Agric. 2020, 4, 244–248. [Google Scholar]
- Wu, G.Q.; Chen, X.L.; Zhang, T.; Zhuoma, Y.Z. The Characteristic Nectar Plant Sophora moorcroftiana in Shannan Area of Tibet. Apic. China 2020, 71, 42–43. [Google Scholar]
- Ma, Z.J. Study on Seed Germination and Growth of Sophora moorcroftiana Under Different Treatments. Agric. Sci. Eng. China 2021, 33, 34–37. [Google Scholar]
- Ning, X.B.; Zhang, X.C.; Shi, W. Investigation and Discussion on Resource Status Quo of Sophora moorcroftiana Populations in Tibet. Cent. South For. Inventory Plan. 2022, 41, 50–54. [Google Scholar]
- Editorial Board of China Traditional Chinese Medicine News. Sophora moorcroftiana: The ‘Gold’ of the XiZang Plateau. China Traditional Chinese Medicine News, 1 March 2007. [Google Scholar]
- Ma, X.M.; Li, H.Y.; Yi, S.F.; Wang, B. Study on Anti-Inflammatory and Antibacterial Activity of Alkaloids from Sophora moorcroftiana. Acta Chin. Med. Pharmacol. 2004, 32, 23–25+1. [Google Scholar]
- Du, T. Experimental Study on Anti-Angiogenesis and Anti-Tumor Effects of Total Alkaloids of Sophora moocrorftiana In Vivo and In Vitro. Master’s Thesis, Qinghai University, Qinghai, China, 2023. [Google Scholar]
- Yao, W.J.; Zhang, Y.F.; Dan, Q.; Guo, Q.Q.; Li, H.E. Stomatal Feature of Sophora moorcroftiana Leaf Under Drought and Cold Stress. Guizhou Agric. Sci. 2015, 43, 23–29. [Google Scholar]
- Guo, Q.Q.; Zhang, W.H.; Li, H.E. Comparison of photosynthesis and antioxidative protection in Sophora moorcroftiana and Caragana maximovicziana under water stress. J. Arid Land 2014, 6, 637–645. [Google Scholar] [CrossRef][Green Version]
- Wei, W.; Zhou, J.J.; Baima, G.W.; Wang, Y.T. Research Status and Prospects for the Endemic Species of Sophora moorcroftiana in the Tibet. Chin. Wild Plant Resour. 2024, 43, 70–75. [Google Scholar]
- Zhang, B.J.; Xiong, D.H.; Liu, L.; Tang, Y.F. Wind erodibility indices of aeolian sandy soils impacted by different vegetation restoration: A case study from the Shannan valley of the Yarlung Zangbo River. J. Mt. Sci. 2022, 19, 2830–2845. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Zhang, Z.H.; Li, Q.Y. Growth and Reproduction of Sophora moorcroftiana Responding to Altitude and Sand Burial in the Middle Tibet. Environ. Geol. 2007, 53, 11–17. [Google Scholar] [CrossRef]
- Zhao, W. A Preliminary Study on the Arenaceous Adaptability of Sophora moorcroftiana. Acta Phytoecol. Sin. 1998, 22, 379–384. [Google Scholar]
- Tang, Y.F. Effects of Typical Vegetation Ecological Projects Implementation on Thehydrological Function of Aeolian Sandy Landsoil of the Yarlung Zangbo River Valley. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2022. [Google Scholar]
- Zang, J.C.; Sun, T. The Complete Mitochondrial Genome of Seed Pest from Sophora moorcroftiana. Mitochondrial DNA Part B Resour. 2019, 4, 149–150. [Google Scholar] [CrossRef]
- Liu, Z.M.; Jiang, D.M. The Quicksand and Its Control in Tibet. In Proceedings of the Abstracts of the 2005 Qinghai-Tibet Plateau Environment and Change Seminar, Guilin, China, 10–11 October 2005; p. 1. [Google Scholar]
- Sun, J.; Qin, X.J.; Yang, J. The response of vegetation dynamics of the different alpine grassland types to temperature and precipitation on the Tibetan Plateau. Environ. Monit. Assess. 2016, 188, 20. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.E.; Li, Q.; Guo, Q.Q.; Li, J.R. Genetic Diversity Analysis and Potential Distribution Prediction of Sophora moorcroftiana Endemic to Qinghai-Tibet Plateau, China. Forests 2021, 12, 1106. [Google Scholar] [CrossRef]
- Zhang, X.C.; Ning, X.B.; Shi, W.; Liu, H.W. Prediction of Tibet’s Potential Suitable Areas for Sophora moorcroftiana Based on MaxEnt Model. Cent. South For. Inventory Plan. 2023, 42, 41–45, 50. [Google Scholar]
- Zhao, K.T.; Yang, X.L. Study on Degradation Mechanism of Sophora moorcroftiana Shrub in Semiarid Valley Region of Lasa. Master’s Thesis, Agricultural and Animal Husbandry College of Tibet University, Linzhi, China, 2010. [Google Scholar]
- Xia, H.J.; Zhang, T.T.; Li, X.S.; He, T.H.; Wang, X.; Zhang, J.H.; Zhang, K.R. Effects of drought and nutrient deficiencies on the allocation of recently fixed carbon in a plant-soil-microbe system. Tree Physiol. 2023, 43, 1903–1916. [Google Scholar] [CrossRef]
- Zeng, J.Q.; Tian, X.J.; Chi, Z.Z. Benefit of Soil and Water Conservation for Sophora moorcroftiana in Tibet. Prot. For. Sci. Technol. 2015, 8, 4–6+12. [Google Scholar]
- Liao, C.R. Study on Vegetation Recovery Characteristics and Management Contermeasures of Sandy Lands in Alpine Valley of Tibet. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2020. [Google Scholar]
- Liu, W.J.; Yang, H.; Li, Z.S.; Zhang, A.B.; Xin, F.M. Stoichiometric Characteristics of Plants-Fine Roots-Soil of Sophora moorcroftiana in Arid Valley of Middle Reaches of Yarlung Zangbo River. Southwest China J. Agric Sci. 2022, 35, 608–616. [Google Scholar]
- Yuan, Y.D.; Zuo, J.J.; Zhang, H.Y.; Zu, M.T.; Liu, S.L. The Chinese medicinal plants rhizosphere: Metabolites, microorganisms, and interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- Wu, Z.C. Study on Rhizosphere Growth-Promoting Bacteria of Sophora psammophila and Growth-Promoting Effect. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2024. [Google Scholar]
- Dong, J.L.; Xu, Y.H.; Quan, Z.; Yin, Y.L.; Zhao, Y.Y.; Xu, Q.; Tian, K.; Huang, B.; Cai, Z.C.; Ma, Y.; et al. The Obstacles and Countermeasures of Soil Sustainability in Protected Horticulture in China. Acta Pedol. Sin. 2024, 61, 1467–1468. [Google Scholar]
- Haynes, S.J.; Darnajoux, R.; Han, E.; Oleynik, S.; Zimble, E.; Zhang, X. Quantification of biological nitrogen fixation by Mo-independent complementary nitrogenases in environmental samples with low nitrogen fixation activity. Sci. Rep. 2022, 12, 22011. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhao, W.J.; Lu, Y.J.; Liu, X.F.; He, J.Q. Screening of Rhizosphere-Promoting Bacteria in Sophora moorcroftiana and Effects of Drought Stress on Seed Germination. J. Plateau Agric. 2020, 4, 249–258. [Google Scholar]
- Wang, H. Isolation and Identification of Endophytic Bacteria and Their Potential for Biocontrol in the Sandy Acacia. Master’s Thesis, Xizang Agricultural and Animal Husbandry University, Nyingchi, China, 2021. [Google Scholar]
- Yao, W.J.; Fu, Y.R.; Zhang, Y.F.; Li, H.E. Cloning of four DREB genes from Tibetan Sophora moorcroftiana and analysis of their expression during abiotic stress. J. For. Res. 2016, 27, 675–683. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Sakuma, Y.; Kasuga, M.; Ito, Y.; Seki, M.; Goda, H.; Shimada, Y.; Yoshida, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 2004, 38, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Narusaka, M.; Abe, H.; Kasuga, M.; Yamaguchi-Shinozaki, K.; Carninci, P.; Hayashizaki, Y.; Shinozaki, K. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 2001, 13, 61–72. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Schena, M.; Shalon, D.; Heller, R.; Chai, A.; Brown, P.O.; Davis, R.W. Parallel human genome analysis: Microarray-based expression monitoring of 1000 genes. Proc. Natl. Acad. Sci. USA 1996, 93, 10614–10619. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Ohri, P.; Bhardwaj, R. Decoding Sugar Regulation and Homeostasis in Plants: Cracking Functional Roles Under Stresses. J. Plant Growth Regul. 2023, 42, 4797–4817. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Li, H.E.; Yao, W.J.; Fu, Y.R.; Li, S.K.; Guo, Q.Q. De Novo Assembly and Discovery of Genes That Are Involved in Drought Tolerance in Tibetan Sophora moorcroftiana. PLoS ONE 2015, 10, e111054. [Google Scholar] [CrossRef] [PubMed]
- Li, H.E.; Zhang, Y.F.; Guo, Q.Q.; Yao, W.J. Molecular characterisation of a DREB gene from Sophora moorcroftiana, an endemic species of plateau. Protoplasma 2017, 254, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yang, D.N.; Liu, Y.M.; Yang, S.H.; Zhang, R.; Sun, X.L.; Liu, H.X.; Duan, Y.W.; Yang, Y.Q.; Yang, Y.P. Sophora moorcroftiana genome analysis suggests association between sucrose metabolism and drought adaptation. Plant Physiol. 2023, 191, 844–848. [Google Scholar] [CrossRef]
- Peng, X.J.; Ma, X.Y.; Fan, W.H.; Su, M.; Cheng, L.Q.; Alam, I.; Lee, B.H.; Qi, D.M.; Shen, S.H.; Liu, G.S. Improved drought and salt tolerance of Arabidopsis thaliana by transgenic expression of a novel DREB gene from Leymus chinensis. Plant Cell Rep. 2011, 30, 1493–1502. [Google Scholar]
- Farooq, M.; Romdhane, L.; Rehman, A.; Al-Alawi, A.K.M.; Al-Busaidi, W.M.; Asad, S.A.; Lee, D.J. Integration of Seed Priming and Biochar Application Improves Drought Tolerance in Cowpea. J. Plant Growth Regul. 2021, 40, 1972–1980. [Google Scholar] [CrossRef]
- Wang, Y.T.; Pubu, C.R.; Ma, W.J.; Liu, Y.; Danzeng, L.B. Analysis on Contents and Correlation of Alkaloids in Sophora moorcroftiana of Different Growing Populations in Tibet. Acta Bot. Boreali-Occident. Sin. 2018, 38, 1913–1917. [Google Scholar]
- Kang, S.Y.; Chen, T.T.; Hao, Z.H.; Yang, X.; Wang, M.F.; Zhang, Z.F.; Hao, S.J.; Lang, F.T.; Hao, H.X. Oxymatrine Alleviates Gentamicin-Induced Renal Injury in Rats. Molecules 2022, 27, 6209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.X. Study on the Intervention Effect and Mechanism of Water-Soluble Alkaloid from Sophora moorcroftiana Seeds in a Mice Model of Ulcerative Colitis. Master’s Thesis, Lanzhou University, Lanzhou, China, 2023. [Google Scholar]
- Yang, Z.T.; Yin, R.L.; Cong, Y.F.; Yang, Z.Q.; Zhou, E.R.S.; Wei, Z.K.; Liu, Z.C.; Cao, Y.G.; Zhang, N.S. Oxymatrine Lightened the Inflammatory Response of LPS-Induced Mastitis in Mice Through Affecting NF-κB and MAPKs Signaling Pathways. Inflammation 2014, 37, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.I.; Lee, J.-y.; Jeon, H.W.; Kim, M.H.; Chung, Y.S. In Vivo Genotoxicity Assessment of Matrine and the Water Extract of Sophorae radix Using a Comet Assay. J. Food Hyg. Saf. 2021, 36, 118–123. [Google Scholar] [CrossRef]
- Lu, Z.G.; Li, M.H.; Wang, J.S.; Wei, D.D.; Liu, Q.W.; Kong, L.Y. Developmental Toxicity and Neurotoxicity of Two Matrine-Type Alkaloids, Matrine and Sophocarpine, in Zebrafish (Danio rerio) Embryos/Larvae. Reprod. Toxicol. 2014, 47, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, J.Y.; Li, L. Inhibitory Effects of Sophocarpine on Human Colon Cancer HCT116 Cells via the Mitochondria-Mediated Apoptosis. Lat. Am. J. Pharm. 2019, 38, 1014–1019. [Google Scholar]
- Kong, X.M.; Du, T.; Hu, C.H.; Yang, W.C.; Cheng, Y.Q.; Zhang, F.B. Effects of Total Alkaloids from Xizang Medicine Sophora moocrorftia-na on the Angiogenesis of Vascular Endothelial Cells and the Expression of VEGF-A and VEGF-R2. J. Chin. High Alt. Med. Biol. 2024, 46, 1–10. [Google Scholar]
- Wang, M.L.; Zhou, Q.L.; Wang, B.X. Studies on Metabolism of Oxymatrine by Human Intestinal Bacteria. China J. Chin. Mater. Med. 2001, 26, 272–274. [Google Scholar]
- Zhang, B.; Wang, X.; Li, Y.; Wu, M.; Wang, S.Y.; Li, S. Matrine Is Identified as a Novel Macropinocytosis Inducer by a Network Target Approach. Front. Pharmacol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Z.; Row, K.H. Molecularly imprinted solid-phase extraction of caffeine from green tea. J. Ind. Eng. Chem. 2006, 12, 494–499. [Google Scholar]
- Jiang, M.J.; Wang, L.S.; Liu, X.; Yang, H.; Ren, F.; Gan, L.Z.; Jiang, W.Z. Synthesis of a Temperature-Sensitive Matrine-Imprinted Polymer and Its Potential Application for the Selective Extraction of Matrine from Radix Sophorae tonkinensis. Int. J. Mol. Sci. 2015, 16, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Quan, J.; Zhang, T.; Ito, Y. Preparative separation of alkaloids from the root of Sophora flavescens Ait by pH-zone-refining counter-current chromatography. J. Chromatogr. A 1998, 822, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Huo, J. Research on Extraction and Separation Technology of Matrine from Sophora flavescens Ait. Master’s Thesis, Tianjin University, Tianjing, China, 2008. [Google Scholar]
- Ma, X.B.; Lin, H.L.; Zhang, J.Y.; She, Y.X.; Zhou, X.Z.; Li, X.Z.; Cui, Y.; Wang, J.; Rabah, T.; Shao, Y. Extraction and identification of matrine-type alkaloids from Sophora moorcroftiana using double-templated molecularly imprinted polymers with HPLC-MS/MS. J. Sep. Sci. 2018, 41, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.M.; Lu, J.Y.; Sun, W.; Jin, R.M.; Ohira, T.; Zhang, Z.; Tian, X.S. Oxymatrine and Its Metabolite Matrine Contribute to the Hepatotoxicity Induced by Radix Sophorae tonkinensis in Mice. Exp. Ther. Med. 2019, 17, 2519–2528. [Google Scholar] [CrossRef]
- Wang, R.Y.; Wang, M.; Wang, S.; Yang, K.; Zhou, P.; Xie, X.H.; Cheng, Q.; Ye, J.X.; Sun, G.B.; Sun, X.B. An integrated characterization of contractile, electrophysiological, and structural cardiotoxicity of Sophora tonkinensis Gapnep. in human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2019, 10, 20. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.L.; Chen, G.; Wu, W.J.; Zhou, L.; Shi, Y.R.; Zeng, Q.; Li, Y.Q.; Sun, Y.W.; Deng, X.M.; et al. Targeting miR-21 with Sophocarpine Inhibits Tumor Progression and Reverses Epithelial-Mesenchymal Transition in Head and Neck Cancer. Mol. Ther. 2017, 25, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Fang, J.P.; Liu, Y.C.; Wang, B. The Spatial Distribution Model of Etiella zinckenella Larvae in Sophora moorcroftiana of Tibet. J. Henan Agric. Sci. 2010, 9, 83–87. [Google Scholar]
- Zang, J.C.; Xin, F.M. Impact of Seed Pests on Seed Quality and Germination of Sophora moorcrofitiana. Acta Agric. Boreali-Occident. Sin. 2012, 21, 198–201. [Google Scholar]
- Das, A.; Chowdhury, S.; Layek, J.; Ramkrushna, G.I.; Panwar, A.S.; Ngachan, S.V.; Buragohain, J. Agro-physical assessment of different pea (Pisum sativum) cultivars in lowland rice (Oryza sativa) fallow under no-till system for enhancing cropping intensity and productivity in mid hills of northeast India. Indian J. Agric. Sci. 2017, 87, 1612–1618. [Google Scholar] [CrossRef]
- Tuda, M.; Chou, L.Y.; Niyomdham, C.; Buranapanichpan, S.; Tateishi, Y. Ecological factors associated with pest status in Callosobruchus (Coleoptera: Bruchidae): High host specificity of non-pests to Cajaninae (Fabaceae). J. Stored Prod. Res. 2005, 41, 31–45. [Google Scholar] [CrossRef]
- Li, R.; Chen, L.J.; Wu, Y.P.; Zhang, R.; Baskin, C.C.; Baskin, J.M.; Hu, X.W. Effects of Cultivar and Maternal Environment on Seed Quality in Vicia sativa. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ma, R.; Wei, J.W.; Song, L.W.; Dewer, Y.; Wang, S.S.; Liu, L.; Zhou, J.J. ApCarE4 and ApPOD3 participate in the adaptation of pea aphids to different alfalfa varieties. Sci. Rep. 2024, 14, 25444. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, L.X.; Li, H.L.; Wei, W.; Liang, G.Q.; Tang, Y.M.; Zhao, Y.Y.; Wei, O.J.H.; Yang, Q.B. Effects of Protected Cultivation on Agronomic, Yield, and Quality Traits of Yard-Long Bean (Vigna unguiculata ssp. unguiculata cv.-gr. sesquipedalis). Horticulturae 2024, 10, 1167. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.P.; Martínez-Rocha, A.L.; Rosales-Castro, M.; Lopez-Rodriguez, A.; Meneses-Morales, I.; Del Campo-Quinteros, E.; Herrera-Ocelotl, K.K.; Gandara-Moreno, G.; Velázquez-Huizar, S.J.; Ibarra-Sánchez, L.; et al. Antifungal Activity of Mexican Oregano (Lippia graveolens Kunth) Extracts from Industrial Waste Residues on Fusarium spp. in Bean Seeds (Phaseolus vulgaris). Agriculture 2024, 14, 1975. [Google Scholar] [CrossRef]
- Almogdad, M.; Jonaviciene, A.; Semaskiene, R. Bruchus rufimanus Boh. Effect on Broad Bean Seed Quality and the Infection Level of Seed-Borne Fungal Pathogens. Plants 2023, 12, 1825. [Google Scholar] [CrossRef] [PubMed]
- Fett, W.F.; Sequeira, L. A New Bacterial Agglutinin from Soybean: II. Evidence Against a Role in Determining Pathogen Specificity. Plant Physiol. 1980, 66, 853–858. [Google Scholar] [CrossRef]
- Wang, W.J. Study on the Characteristics of Seed Vitality and Biological Ecology of Seed Pests of Sophora moorcroftiana in Tibet. Master’s Thesis, Ningxia University, Ningxia, China, 2010. [Google Scholar]
- Yuan, S.L.; Zhang, N.; Zhou, G.N. Study on the Lymantria dispar Linnacus Insect Resistance of the Transgectic Triploid Hybrids of Populus tomentosa Carrying Two-Resistant Genes. North. Hortic. 2012, 2, 143–145. [Google Scholar]
- Chen, Y.Q.; Sun, T.; Zang, J.C.; Hong, D.W. Relationship Between Diversity of Surface Arthropods at Different Altitudes and Seed Damage of Sophora moorcroftiana Shrubs. Southwest China J. Agric. Sci. 2021, 34, 1540–1547. [Google Scholar]
- You, S.Q.; Guo, Q.F.; Ye, J.Q. Field efficacy test of five pesticides against cowpea pod borer. Shanghai Veg. 2024, 5, 37–39. [Google Scholar]
- Imam, M.M.M.; Soyema, K. Evaluation of insecticides against bean pod borer and its larval parasitoid, Tachinid fly. Arch. Phytopathol. Plant Prot. 2023, 56, 547–559. [Google Scholar]
- Zang, J.C.; Xin, F.M.; Wang, Z.H. Study on the Damage and Control of Bruchophagus onois (Mayr) to Sophora moorcroftiana Seed. J. Anhui Agric. Sci. 2008, 36, 14179–14180. [Google Scholar]
- da Rocha, L.C.; Cardoso, J.C.F.; de Oliveira, A.C.; Araújo, T.N.; Castro-Melo, A.; Augusto, S.C. How much biodiversity do yellow passionfruit (Passiflora edulis) croplands harbour? Insights from trap-nesting bees, wasps and their natural enemies. J. Nat. Conserv. 2025, 84, 126818. [Google Scholar] [CrossRef]
- Tufail, M.S.; Krebs, G.L.; Southwell, A.; Piltz, J.W.; Wynn, P.C.; Cook, D.F. Significance of honeybee pollination in increasing seed yield of Trifolium alexandrinum (Fabales: Fabaceae) and its impact on economic sustainability of smallholder farmers. J. Econ. Entomol. 2024, 117, 2495–2504. [Google Scholar] [CrossRef]
- Shajitha, P.; Nisha, R.; Sivasamy, M.; Jayaprakash, P.; Vikas, V.K.; Gajalakshmi, K.; Mallick, N.; Babu, P.; Yadav, R.; Vijaishree, S.; et al. Integrating solid stem and multiple disease resistance for developing climate-resilient wheat (Triticum aestivum L.). Cereal Res. Commun. 2024. [Google Scholar] [CrossRef]
- Silva, C.M.D.; Dias, R.D.S.; dos Santos, J.S.; Souza, F.D.; de Melo, N.F. Induction of Polyploidy in Watermelon Genotype with Powdery Mildew Resistance (Podosphaera xanthii). Rev. Caatinga 2022, 35, 505–513. [Google Scholar] [CrossRef]
- Ghimire, B.; Orellana, R.; Chowdhury, S.R.; Vermeer, C.B.; Patel, P.; Raymer, P.; Milla-Lewis, S.; Buck, J.W.; Martinez-Espinoza, A.D.; Bahri, B.A. Assessing Biofungicides and Host Resistance Against Rhizoctonia Large Patch in Zoysiagrass. Pathogens 2024, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.B. Analysis on Distribution Pattern of Sophora moorcroftiana Population in Tibet. Cent. South For. Inventory Plan. 2012, 31, 54–56. [Google Scholar]
- Cidan, Z.M. Analysis of Biological Characteristics and Comprehensive Utilization of Sophora moorcroftiana in Tibet. Farmers Consult. 2021, 21, 177–178. [Google Scholar]
- Liu, Z.M.; Zhao, A.M.; Kang, X.Y.; Zhou, S.L.; López-Pujol, J. Genetic diversity, population structure, and conservation of Sophora moorcroftiana (Fabaceae), a shrub endemic to the Tibetan Plateau. Plant Biol. 2006, 8, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yi, F.; Yang, G.J.; Wang, Y.T.; Pubu, C.; He, R.H.; Xiao, Y.; Wang, J.C.; Lu, N.; Wang, J.H.; et al. Geographic population genetic structure and diversity of Sophora moorcroftiana based on genotyping-by-sequencing (GBS). Peer J. 2020, 8, e9609. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.Y.; Zhu, H.X. Green Hospital Based on Grey System Theory Research on Construction Risk Assessment. J. Liaoning Univ. Technol. 2024, 26, 42–46. [Google Scholar]
- Yang, X.L.; Gong, Z.H.; Ma, H.P. Evaluation on the Degradation of Shrub Community of Sophora moorcroftiana in Semi-Arid Valley of Lhasa. J. Northwest For. Univ. 2012, 27, 11–14. [Google Scholar]
- Li, W.L.; Zhang, Y.Y.; Li, Z.Z.; Du, G.Z.; Huang, L. The Relationship Between Plant Niche Fitness and Productivity and Diversity in Alpine Grassland and Its Response to Grazing. J. Lanzhou Univ. Nat. Sci. 2007, 43, 53–57. [Google Scholar]
- Guo, Q.Q.; Fang, J.P.; Bian, D.; Pu, Q.; Wang, S.L.; Zhong, G.H. Effect of Different Disturbances on Structural Characteristics of Sophora moorcroftiana Communities. Acta Bot. Boreali-Occident. Sin. 2009, 29, 1670–1677. [Google Scholar]
- Xin, F.M.; Liu, J.M.; Chang, C.; Wang, Y.T.; Jia, L.M. Evaluating the Influence of Climate Change on Sophora moorcroftiana (Benth.) Baker Habitat Distribution on the Tibetan Plateau Using Maximum Entropy Model. Forests 2021, 12, 1230. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, D.; Yu, S.; Yu, S.; Cao, F.; Wang, G.; Dai, T. Unlocking the Potential of Sophora moorcroftiana (Fabaceae): The Overlooked Xizang Endemic. Forests 2025, 16, 410. https://doi.org/10.3390/f16030410
Mei D, Yu S, Yu S, Cao F, Wang G, Dai T. Unlocking the Potential of Sophora moorcroftiana (Fabaceae): The Overlooked Xizang Endemic. Forests. 2025; 16(3):410. https://doi.org/10.3390/f16030410
Chicago/Turabian StyleMei, Duozhuoga, Sinong Yu, Shuangyuan Yu, Fuliang Cao, Guibin Wang, and Tingting Dai. 2025. "Unlocking the Potential of Sophora moorcroftiana (Fabaceae): The Overlooked Xizang Endemic" Forests 16, no. 3: 410. https://doi.org/10.3390/f16030410
APA StyleMei, D., Yu, S., Yu, S., Cao, F., Wang, G., & Dai, T. (2025). Unlocking the Potential of Sophora moorcroftiana (Fabaceae): The Overlooked Xizang Endemic. Forests, 16(3), 410. https://doi.org/10.3390/f16030410





