Concentration Characteristics of Culturable Airborne Microbes in the Urban Forests of Yangzhou
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Airborne Microbial Collection
2.3. Determination of Airborne Microbial Concentration
2.4. Microclimate Environment and Data Collection
2.5. Statistical Analysis
3. Results
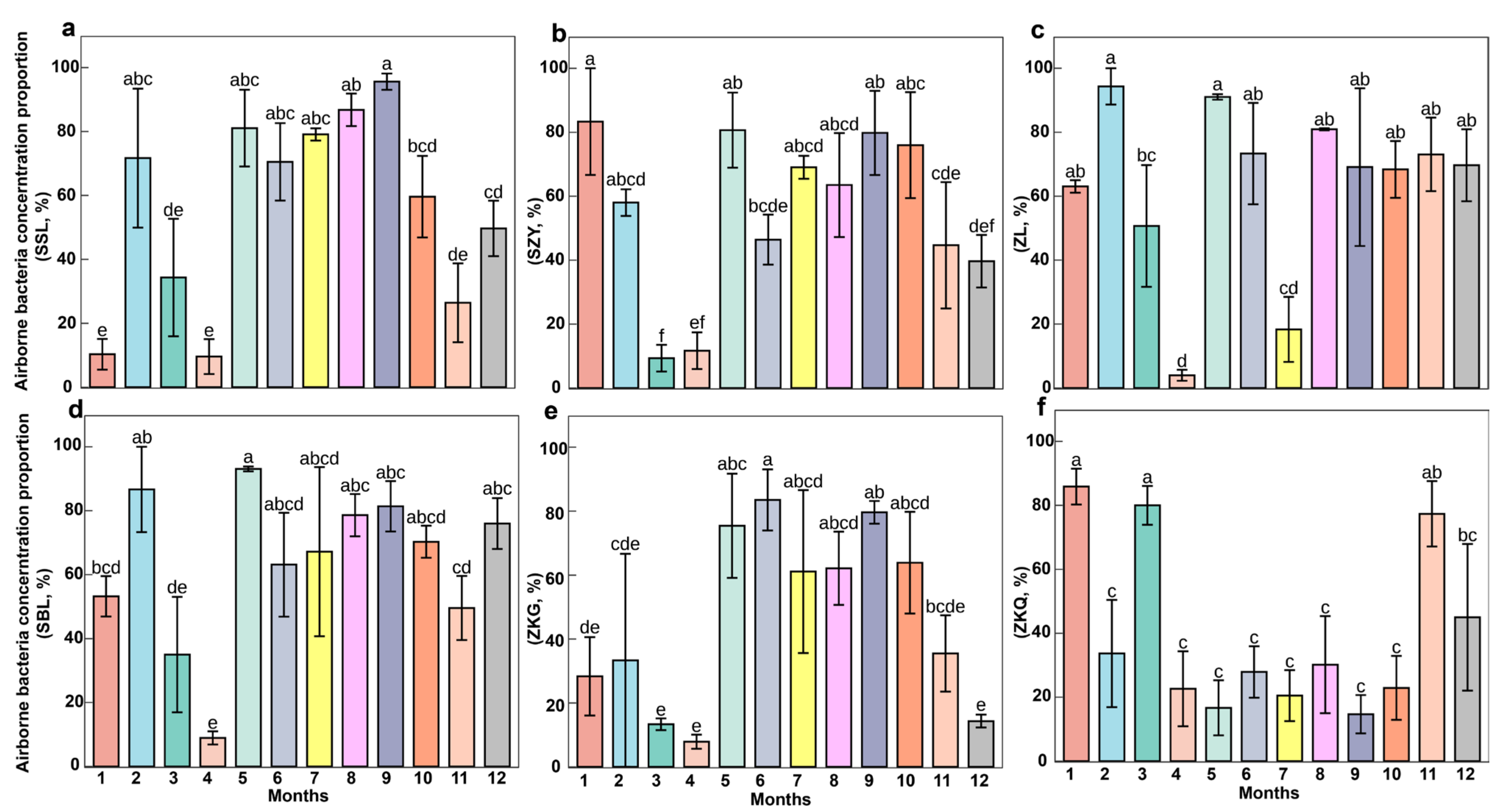
3.1. Proportion of Bacteria in Airborne Microorganisms Across Different Forest Stands
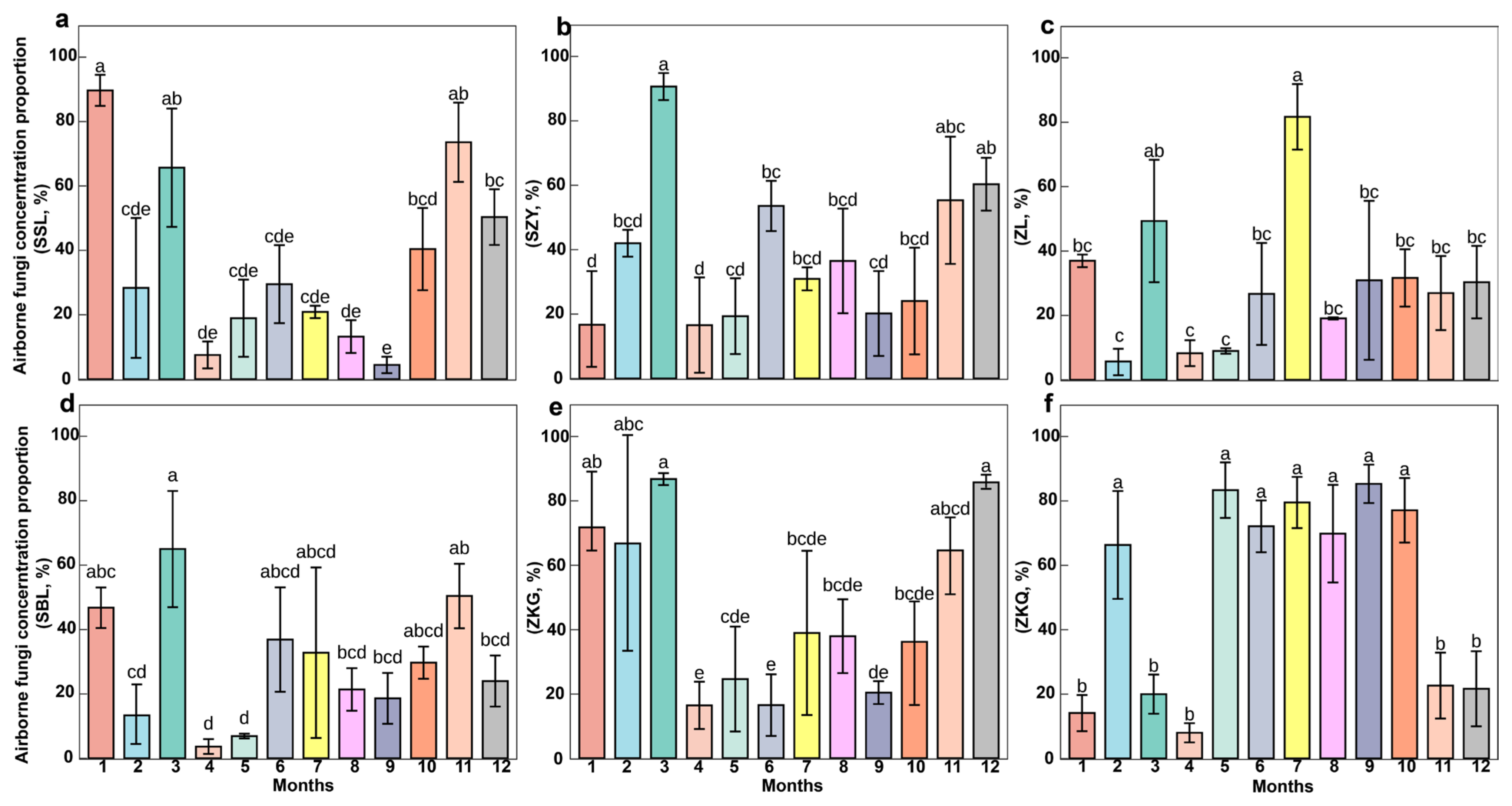
3.2. Proportion of Fungi in Airborne Microorganisms Across Different Forest Stands
3.3. Total Concentration of Airborne Microorganisms Across Different Forest Stands
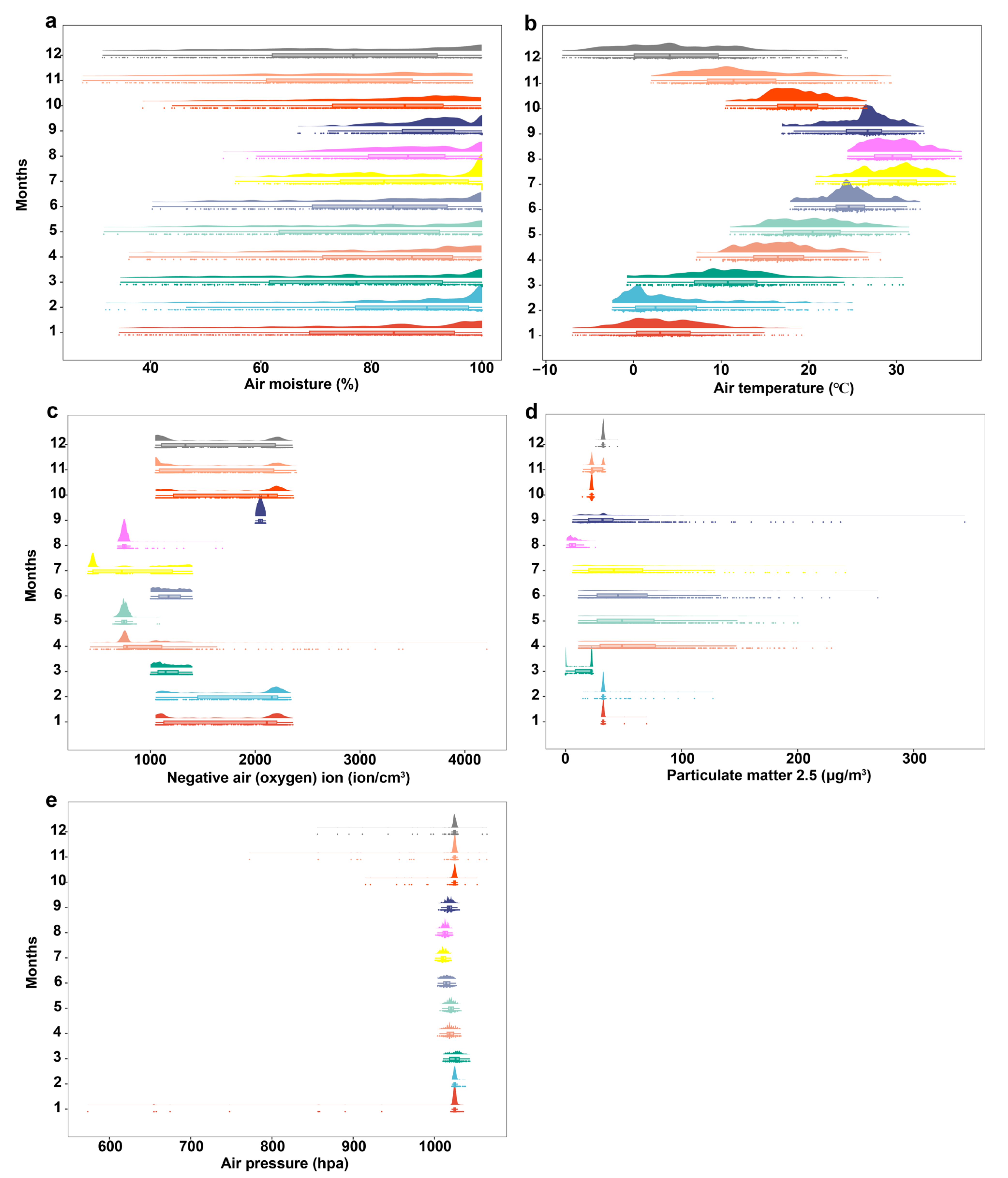
3.4. Changes in Air Factors
3.5. Correlation Analysis
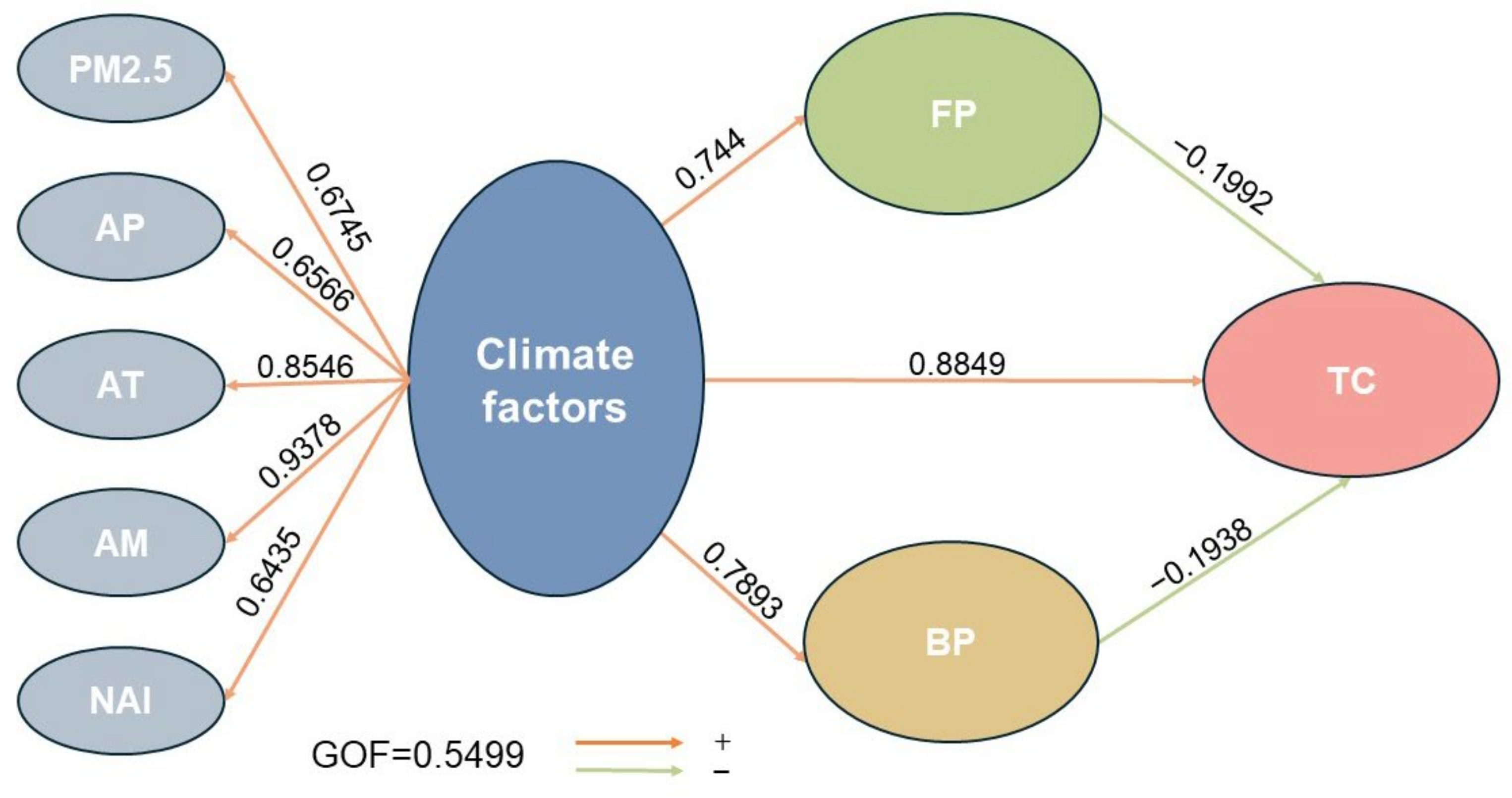
3.6. Relationships Between Air Factors, Bacterial Concentration, Fungal Concentration, and Total Microbial Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pearce, D.W. The Economic Value of Forest Ecosystems. Ecosyst. Health 2002, 7, 284–296. [Google Scholar] [CrossRef]
- Karjalainen, E.; Sarjala, T.; Raitio, H. Promoting human health through forests: Overview and major challenges. Environ. Health Prev. Med. 2009, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weinbrenner, H.; Breithut, J.; Hebermehl, W.; Kaufmann, A.; Klinger, T.; Palm, T.; Wirth, K. “The Forest Has Become Our New Living Room”—The Critical Importance of Urban Forests During the COVID-19 Pandemic. Front. For. Glob. Change 2021, 4, 672909. [Google Scholar] [CrossRef]
- Kim, H.; Shoji, Y.; Mameno, K.; Kubo, T.; Aikoh, T. Changes in visits to green spaces due to the COVID-19 pandemic: Focusing on the proportion of repeat visitors and the distances between green spaces and visitors’ places of residences. Urban For. Urban Green. 2023, 80, 127828. [Google Scholar] [CrossRef]
- Lou, X.; Yang, Q.; Xie, J.; Fang, Z. Concentration characteristics of culturable airborne microbes in family homes in Hangzhou, China. Aerobiologia 2023, 39, 119–131. [Google Scholar] [CrossRef]
- Song, L.; Zhou, J.; Wang, C.; Meng, G.; Li, Y.; Jarin, M.; Wu, Z.; Xie, X. Airborne pathogenic microorganisms and air cleaning technology development: A review. J. Hazard. Mater. 2022, 424, 127429. [Google Scholar] [CrossRef]
- Kumar, P.; Kausar, M.A.; Singh, A.B.; Singh, R. Biological contaminants in the indoor air environment and their impacts on human health. Air Qual. Atmos. Health 2021, 14, 1723–1736. [Google Scholar] [CrossRef]
- Hisano, M.; Searle, E.B.; Chen, H.Y.H. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. 2017, 93, 439–456. [Google Scholar] [CrossRef]
- Li, H.; Wu, Z.-F.; Yang, X.-R.; An, X.-L.; Ren, Y.; Su, J.-Q. Urban greenness and plant species are key factors in shaping air microbiomes and reducing airborne pathogens. Environ. Int. 2021, 153, 106539. [Google Scholar] [CrossRef]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Forzieri, G.; Dakos, V.; McDowell, N.G.; Ramdane, A.; Cescatti, A. Emerging signals of declining forest resilience under climate change. Nature 2022, 608, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; McLetchie, S.; Knight, R.; Fierer, N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 2011, 5, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Lighthart, B. The ecology of bacteria in the alfresco atmosphere. FEMS Microbiol. Ecol. 1997, 23, 263–274. [Google Scholar] [CrossRef]
- Gao, J.-F.; Fan, X.-Y.; Li, H.-Y.; Pan, K.-L. Airborne bacterial communities of PM2. 5 in Beijing-Tianjin-Hebei megalopolis, China as revealed by Illumina MiSeq sequencing: A case study. Aerosol Air Qual. Res. 2017, 17, 788–798. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Ma, A.; Ramachandran, S. Negative air ions and their effects on human health and air quality improvement. Int. J. Mol. Sci. 2018, 19, 2966. [Google Scholar] [CrossRef]
- Kolarž, P.; Ilić, A.Ž.; Janković, M.; Janićijević, A.; Trbovich, A. Estimating aerosol particle removal in indoor air by ion-enhanced deposition. J. Aerosol Sci. 2023, 173, 106199. [Google Scholar] [CrossRef]
- Santl-Temkiv, T.; Amato, P.; Gosewinkel, U.; Thyrhaug, R.; Charton, A.; Chicot, B.; Finster, K.; Bratbak, G.; Londahl, J. High-flow-rate impinger for the study of concentration, viability, metabolic activity, and ice-nucleation activity of airborne bacteria. Environ. Sci. Technol. 2017, 51, 11224–11234. [Google Scholar] [CrossRef]
- DasSarma, P.; DasSarma, S. Survival of microbes in Earth’s stratosphere. Curr. Opin. Microbiol. 2018, 43, 24–30. [Google Scholar] [CrossRef]
- Ibáñez, A.; Garrido-Chamorro, S.; Barreiro, C. Microorganisms and Climate Change: A Not so Invisible Effect. Microbiol. Res. 2023, 14, 918–947. [Google Scholar] [CrossRef]
- Šantl-Temkiv, T.; Amato, P.; Casamayor, E.O.; Lee, P.K.H.; Pointing, S.B. Microbial ecology of the atmosphere. FEMS Microbiol. Rev. 2022, 46, fuac009. [Google Scholar] [CrossRef] [PubMed]
- Núñez, A.; García, A.M.; Moreno, D.A.; Guantes, R. Seasonal changes dominate long-term variability of the urban air microbiome across space and time. Environ. Int. 2021, 150, 106423. [Google Scholar] [CrossRef] [PubMed]
- Uçar, Z.; Akay, A.E.; Bilici, E. Towards Green Smart Cities: Importance of Urban Forestry and Urban Vegetation. Int. Arch.Photogramm. Remote Sens. Spat. Inf. Sci. 2020, XLIV-4/W3-2020, 399–403. [Google Scholar] [CrossRef]
- McSpadden Gardener, B.B.; Weller, D.M. Changes in Populations of Rhizosphere Bacteria Associated with Take-All Disease of Wheat. Appl. Environ. Microbiol. 2001, 67, 4414–4425. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, M.; Li, X. Temporal and Spatial Evolution, Prediction, and Driving-Factor Analysis of Net Primary Productivity of Vegetation at City Scale: A Case Study from Yangzhou City, China. Sustainability 2023, 15, 14518. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, R.; Li, L.; Yang, C.; Lian, J.; Zhang, J.; Liu, S.; Xing, W.; Yuan, Y. Factors Influencing the Concentration of Negative Air Ions in Urban Forests of the Zhuyu Bay Scenic Area in Yangzhou, China. Atmosphere 2024, 15, 316. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, R.; Yuan, Y.; Xing, W.; Liu, S. Microbiota associated with urban forests. PeerJ 2024, 12, e16987. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Liao, Z.-N.; Xu, H.-J.; Ma, J.; Li, M.; He, C.; Zhang, Q.; Xu, S. Seasonal and vegetational variations of culturable bacteria concentrations in air from urban forest parks: A case study in Hunan, China. Environ. Sci. Pollut. Res. 2022, 29, 28933–28945. [Google Scholar] [CrossRef]
- Godefroid, S.; Koedam, N. Distribution pattern of the flora in a peri-urban forest: An effect of the city–forest ecotone. Landsc. Urban Plan. 2003, 65, 169–185. [Google Scholar] [CrossRef]
- Han, I.D.; Koo, C.-D. The Effect of Forest Healing Program on the Resilience of Elderly People in Urban Forest. J. People Plants Environ. 2018, 21, 293–303. [Google Scholar] [CrossRef]
- Elshafei, A.M. General Overview of Climate Change and Global Warming: Their Effect on Microorganisms. Int. J. Environ. Clim. Change 2022, 12, 1378–1387. [Google Scholar] [CrossRef]
- Valiullin, L.R.; Mukhammadiev, R.S.; Egorov, V.; Mukhammadiev, R.S.; Yarullin, A.I.; Sakhnov, V.V.; Singh, R.K.; Sushkova, S.N.; Kozmenko, S.V.; Minkina, T.M. Microorganism Isolates Stimulate Forest Crop Growth and Protection. Preprints 2023, preprint. [Google Scholar]
- Hutchins, D.A.; Jansson, J.K.; Remais, J.V.; Rich, V.I.; Singh, B.K.; Trivedi, P. Climate change microbiology—Problems and perspectives. Nat. Rev. Microbiol. 2019, 17, 391–396. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R. Managing Forests for Climate Change Mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
- Calderón-Ezquerro, M.d.C.; Serrano-Silva, N.; Brunner-Mendoza, C. Aerobiological study of bacterial and fungal community composition in the atmosphere of Mexico City throughout an annual cycle. Environ. Pollut. 2021, 278, 1165858. [Google Scholar] [CrossRef]
- Els, N.; Greilinger, M.; Reisecker, M.; Tignat-Perrier, R.; Baumann-Stanzer, K.; Kasper-Giebl, A.; Sattler, B.; Larose, C. Comparison of Bacterial and Fungal Composition and Their Chemical Interaction in Free Tropospheric Air and Snow Over an Entire Winter Season at Mount Sonnblick, Austria. Front. Microbiol. 2020, 11, 980. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef]
- Pyrri, I.; Kapsanaki-Gotsi, E. Functional relations of airborne fungi to meteorological and pollution factors in a Mediterranean urban environment. Fungal Ecol. 2017, 30, 48–54. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, S.; Li, Y.; Zhou, Z.; Cheng, Q.; Wang, Q. Microbial pollution assessment of storage environment for the ancient Buddhist relics in Tibetan Palace Museum. Aerobiologia 2023, 39, 275–283. [Google Scholar] [CrossRef]
- Huang, X.; Song, J. Urban moisture and dry islands: Spatiotemporal variation patterns and mechanisms of urban air humidity changes across the globe. Environ. Res. Lett. 2023, 18, 103003. [Google Scholar] [CrossRef]
- Joung, Y.S.; Ge, Z.; Buie, C.R. Bioaerosol generation by raindrops on soil. Nat. Commun. 2017, 8, 14668. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.S.; Buie, C.R. Aerosol generation by raindrop impact on soil. Nat. Commun. 2015, 6, 7083. [Google Scholar] [CrossRef]


| Factors | Abbreviation | Measurement Range | Model | Brand | Origin |
|---|---|---|---|---|---|
| Air moisture | AM | 0%–99.9% | PH-ATERH-165 | Puhou | Nanjing, China |
| Air temperature | AT | −20 to 80 °C | PH-ATERH-165 | Puhou | Nanjing, China |
| Negative air (oxygen) ion | NAI | 0–500,000 ion/cm3 | AN-200 | Anion | Chongqing, China |
| Particulate matter 2.5 | PM2.5 | 0–999 μg/m3 | PH-PM-999 | Puhou | Nanjing, China |
| Air pressure | AP | 300–1100 hpa | PH-APRE-101 | Puhou | Nanjing, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Qiu, S.; Xu, C.; Li, L.; Xing, W.; Yuan, Y. Concentration Characteristics of Culturable Airborne Microbes in the Urban Forests of Yangzhou. Forests 2025, 16, 378. https://doi.org/10.3390/f16020378
Wan X, Qiu S, Xu C, Li L, Xing W, Yuan Y. Concentration Characteristics of Culturable Airborne Microbes in the Urban Forests of Yangzhou. Forests. 2025; 16(2):378. https://doi.org/10.3390/f16020378
Chicago/Turabian StyleWan, Xin, Sumei Qiu, Cong Xu, Liwen Li, Wei Xing, and Yingdan Yuan. 2025. "Concentration Characteristics of Culturable Airborne Microbes in the Urban Forests of Yangzhou" Forests 16, no. 2: 378. https://doi.org/10.3390/f16020378
APA StyleWan, X., Qiu, S., Xu, C., Li, L., Xing, W., & Yuan, Y. (2025). Concentration Characteristics of Culturable Airborne Microbes in the Urban Forests of Yangzhou. Forests, 16(2), 378. https://doi.org/10.3390/f16020378






