Response of Leaf Functional Traits and Rhizosphere Microbial Communities of Castanopsis hystrix in Three Subtropical Plantations with Leguminous or Non-Leguminous Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Study Site
2.1.2. Experiment Design and Sampling
2.2. Methods
2.2.1. Measurement of Leaf Functional Traits
2.2.2. High-Throughput Amplicon Sequencing of Soil Microbes
Extraction of Genomic DNA from Soil Microorganisms
PCR Amplification and High-Throughput Sequencing
2.2.3. Data Analysis
3. Results
3.1. Castanopsis Hystrix Leaf Functional Traits
3.2. Rhizosphere Soil Microbial Community of Castanopsis hystrix
3.2.1. Sample Size Testing
3.2.2. Effects of Tree Species Mixing on the Composition of Soil Microbial Communities in the Rhizosphere of C. hystrix
3.2.3. Effects of Tree Species Mixing on the Diversity of Soil Microbial Communities in the Rhizosphere of Castanopsis hystrix
3.2.4. Discriminant Analysis of Species Differences in Rhizosphere Soil Microbial Communities of Castanopsis hystrix Across Three Forest Types
3.3. Association of Leaf Functional Traits with Rhizosphere Soil Microbial Diversity
3.3.1. Relationship Between Leaf Functional Traits and Rhizosphere Bacterial and Fungal Communities
3.3.2. Correlations of Rhizosphere Microbial Alpha (α) Diversity, Relative Abundance of Dominant Flora, and Leaf Functional Traits
4. Discussion
4.1. Response of Leaf Functional Traits of Castanopsis hystrix to the Mixing of Leguminous or Non-Leguminous Tree Species
4.2. Response of Rhizosphere Bacterial and Fungal Communities of Castanopsis hystrix to Mixing of Leguminous or Non-Leguminous Tree Species
4.3. Correlations of Leaf Functional Traits with Rhizosphere Soil Microbial Community Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Z.; Hu, J.; Xiao, Q.; Feng, Q.; He, P.; Li, R. Analysis on characteristics and development countermeasures of plantation resources in China. Cent. South For. Invent. Plan 2020, 39, 5–10. [Google Scholar]
- Administration State Forest E. A Report of Forest Resources in China (2009–2013); China Forestry Press: Beijing, China, 2014. [Google Scholar]
- Zeng, W.; Xiao, F.; Yu, L. Plantation Forest Resource Dynamic and Its Development Strategy Research in China; Academic Symposium on Ecological Forestry; Livelihood-Oriented Forestry and Technological Innovation in Western China: Guiyang, China, 2013. [Google Scholar]
- Liu, S.; Yang, Y.; Wang, H. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10. [Google Scholar]
- Coll, L.; Ameztegui, A.; Collet, C.; Löf, M.; Mason, B.; Pach, M.; Verheyen, K.; Abrudan, I.; Barbati, A.; Barreiro, S.; et al. Knowledge gaps about mixed forests: What do European forest managers want to know and what answers can science provide? Ecol. Manag. 2018, 42, 106–115. [Google Scholar] [CrossRef]
- Xu, H.D.; Yu, M.K.; Cheng, X.R. Abundant fungal and rare bacterial taxa jointly reveal soil nutrient cycling and multifunctionality in uneven-aged mixed plantations. Ecol. Indic. 2021, 21, 107932. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y.; Paré, D. Differences in fine root productivity between mixed- and single-species stands. Funct. Ecol. 2011, 25, 238–246. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Avacaritei, D.; Coomes, D.A.; Knops, J.; Knops, J. Stabilizing effects of diversity on aboveground wood production in forest ecosystems: Linking patterns and processes. Ecol. Lett. 2014, 17, 1560–1569. [Google Scholar] [CrossRef]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.; Zhu, J.; Hong, P.; Fang, J.; et al. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. Ecol. Manag. 2014, 39, 282–292. [Google Scholar] [CrossRef]
- Pretzsch, H.; Forrester, D.I. Stand dynamics of mixed species stands compared with monocultures. In Mixed-Species Forests; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Yin, Z.F.; Sun, H.G.; Tan, Z.F.; Liu, W. Research progress on productivity of mixed forests. Chin. J. Appl. Ecol. 2023, 34, 3135–3143. [Google Scholar]
- Li, J.F.; Wang, H.; Huang, H.M.; Tong, X.L.; Wang, J.; Liao, Y.L.; Huang, X.M.; Ming, A.G.; You, Y.M. Effects of mixed plantation of Pinus massoniana with different broadleaf species on soil microbial community composition and diversity. Guangxi Sci. 2024, 31, 439–450. [Google Scholar]
- Gei, M.G.; Powers, J.S. Do legumes and non-legumes tree species affect soil properties in unmanaged forests and plantations in Costa Rican dry forests? Soil. Biol. Biochem. 2013, 57, 264–272. [Google Scholar] [CrossRef]
- Ren, Y.W.; Zhong, X.Y.; Yi, H.P.; Chang, Y. Effects of different stand types on leaf functional traits, understory species diversity and soil nutrients. SciEngine 2023, 36, 161–168. [Google Scholar]
- Tedersoo, L.; Laanisto, L.; Rahimlou, S.; Toussaint, A.; Hallikma, T.; Pärtel, M. Global database of plants with root-symbiotic nitrogen fixation: NodDB. J. Veg. Sci. 2018, 29, 560–568. [Google Scholar] [CrossRef]
- Xu, H.; Detto, M.; Fang, S.; Chazdon, R.L.; Li, Y.; Hau, B.C.H.; Fischer, G.A.; Weiblen, G.D.; Hogan, J.A.; Zimmerman, J.K.; et al. Soil nitrogen concentration mediates the relationship between leguminous trees and neighbor diversity in tropical forests. Commun. Biol. 2020, 3, 317. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Y.; Shen, W.J. Effects of introduced native tree species on plant community soil microbial and chemical properties in two subtropical degraded leguminous plantations in south China. Res. Soil. Water Conserv. 2013, 20, 24–31. [Google Scholar]
- Xu, M.; Gao, D.; Fu, S.; Lu, X.; Wu, S.; Han, X.; Yang, G.; Feng, Y. Long-term effects of vegetation and soil on the microbial communities following afforestation of farmland with Robinia pseudoacacia plantations. Geoderma 2020, 367, 114263. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Sun, S.; Zhao, C. Effects of tree species and soil enzyme activities on soil nutrients in dryland plantations. Forests 2021, 12, 1153. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Su, Y.Q.; Kang, Y.X.; Xu, X.M.; Qin, L. Carbon sequestration of young Robinia pseudoacacia plantation in Loess Plateau. Chin. J. Appl. Ecol. 2009, 20, 2911–2916. [Google Scholar]
- Lavorel, S.; Grigulis, K.; Lamarque, P.; Colace, M.P.; Garden, D.; Girel, J.; Pellet, G.; Douzet, R. Using plant functional traits to understand the landscape distribution of multiple ecosystem services. J. Ecol. 2011, 99, 135–147. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, Z.; Gong, S. Comparisons of relationships between leaf and fine root traits in hilly area of the Loess Plateau, Yanhe River basin, Shaanxi Province, China. Acta Ecol. Sin. 2011, 31, 6805–6814. [Google Scholar]
- Zhang, x.; Li, R.; Zhen, Z.; Li, Z.; Gong, L.; Luo, Y.; Wu, X. Leaf functional traits of Tamarix ramosissima in extremely arid region and their relationship with soil physicochemical factors. Acta Ecol. Sin. 2023, 43, 3699–3708. [Google Scholar]
- Wang, J.; Shi, W.; Wu, H.; Hu, B.; Cheng, X.; Han, H. Characteristics of leaf functional traits of typical coniferous plantations in Northern Shanxi and their relationship with soil factors. Acta Bot. Boreali-Occident. Sin. 2023, 43, 835–845. [Google Scholar]
- Luo, J.F.; Luo, Z.X.; Zhao, S.Q.; Wu, S.N.; Luo, X.L.; Sui, X. Responses of leaf functional traits to partial shading of a photovoltaic array and their different ecological strategies. J. West. China For. Sci. 2022, 51, 48–53. [Google Scholar]
- Xing, Z.Y.; Lin, J.Y.; Bo, Y.X.; Lin, J.D.; Zhang, A.P.; Deng, B.W.; Zheng, D.X.; Jin, S.F. Effects of nitrogen addition on functional traits and plasticity of Taxus chinensis var. mairei leaves. Acta Agri Univ. Jiangxiensis 2023, 45, 125–133. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 1st ed.; Academic Press: London, UK, 1996; pp. 1–889. [Google Scholar]
- Fuente Cantó, C.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 2020, 103, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Doornbos, R.F.; Zamioudis, C.; Berendsen, R.L.; Pieterse, C.M.J. Induced systemic resistance and the rhizosphere microbiome. Plant Pathol. J. 2013, 29, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lareen, A.; Burton, F.; Schäfer, P. Plant root-microbe communication in shaping root microbiomes. Plant Mol. Biol. 2016, 90, 575–587. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Paquette, A.; Messier, C.; Kembel, S.W. Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 2017, 546, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, M.; Mommer, L.; de Caluwe, H.; Smit-Tiekstra, A.E.; van der Putten, W.H.; de Kroon, H.; Wurzburger, N. Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding. J. Ecol. 2013, 101, 287–297. [Google Scholar] [CrossRef]
- Zhou, Y.; Clark, M.; Su, J.; Xiao, C. Litter decomposition and soil microbial community composition in three Korean pine (Pinus koraiensis) forests along an altitudinal gradient. Plant Soil 2015, 386, 171–183. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Gou, W.; Wu, G.; Su, P. Relationship between leaf functional traits of mixed desert plants and microbial diversity in rhizosphere. Ecol. Environ. Sci. 2020, 29, 1713–1722. [Google Scholar]
- Li, Y.; Xu, T.; Ai, Z.; Zhou, Z.; Ma, F. Relationship between plant functional traits and rhizosphere bacterial community structure of two Caragana species. Acta Prataculturae Sincia 2022, 31, 38–49. [Google Scholar]
- Leff, J.W.; Bardgett, R.D.; Wilkinson, A.; Jackson, B.G.; Pritchard, W.J.; De Long, J.R.; Oakley, S.; Mason, K.E.; Ostle, N.J.; Johnson, D.; et al. Predicting the structure of soil communities from plant community taxonomy, phylogeny, and traits. ISME J. 2018, 12, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y. Study on Effects of Intercropping Leguminous Green Manure on Tea Plantation Soil Environment and Tea Leaves Amino Acids Metabolism. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 1 May 2022. [Google Scholar]
- Xu, Y. Guangdong Forestry, 1st ed.; Guangdong Science and Technology Press: Guangzhou, China, 1990; pp. 1–552. [Google Scholar]
- Tang, J.; Zhu, X.; Jia, H.; Zeng, J.; Guo, W.; Huang, D. Growth dynamics and tree form quality of mixed Betula alnoides-Castanopsis hystrix plantation. J. Nanjing For. Univ. 2022, 46, 97–105. [Google Scholar]
- Li, N.; Yang, Y.; Li, Z.; Xu, F.; Chen, X.; Zhu, B.; Zhang, W. Variation analysis of leaf phenotypic in different provenance of Castanopsis hystrix. Subtropical. Plant Sci. 2023, 52, 318–326. [Google Scholar]
- Wang, R.; Ma, J.; Liang, H.; Zhang, Y.; Yang, J.; Chen, F.; Wang, Y.; Yan, W. Changes in soil properties, microbial quantity and enzyme activities in four Castanopsis hystrix forest types in Subtropical China. Plants 2023, 12, 2411. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, H.; Luan, J.; Ma, J.; Ming, A.; Liu, S. Early effects of different tree species mixing on aggregate organic carbon fractions. Chin. J. Ecol. 2024, 43, 3350. [Google Scholar]
- Liang, Y.; Ming, A.; He, Y.; Luo, Y.; Tan, L.; Qin, L. Structure and function of soil bacterial communities in the monoculture and mixed plantation of Pinus massoniana and Castanopsis hystrix in southern subtropical China. Chin. J. Appl. Ecol. 2021, 32, 878–886. [Google Scholar]
- Wang, H.; Liu, S.; Wang, J.; You, Y.; Yang, Y.; Shi, Z.; Huang, X.; Zheng, L.; Li, Z.; Ming, A.; et al. Mixed-species plantation with Pinus massoniana and Castanopsis hystrix accelerates C loss in recalcitrant coniferous litter but slows C loss in labile broadleaf litter in southern China. Ecol. Manag. 2018, 43, 207–213. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, J.; Chen, L.; Wei, G.; Yang, M. Effects of different mixed models on growth and plant diversity in Eucalyptus plantations. Southwest China J. Agric. Sci. 2022, 35, 1185–1192. [Google Scholar]
- Wang, L.; Wen, Y.G.; Zhou, X.G.; Zhu, H.G.; Sun, D.J. Effects of mixing Eucalyptus urophylla × E. grandis with Castanopsis hystrix on understory vegetation and soil properties. Ecol. Environ. Sci. 2022, 31, 1340–1349. [Google Scholar]
- Pang, Z.H.; Dai, Q.H. Afforestation Techniques for Major Tree Species in Guangxi, 1st ed.; Guangxi Science and Technology Press: Nanning, China, 2002; pp. 1–270. [Google Scholar]
- Liu, D.J.; Luo, H.Y.; Cheng, Y.; Li, X.; Liu, X.J.; Yang, S.F.; Chen, F.Q.; Ju, C.; Liu, J.X.; Lie, Z.Y. Growth and nutrient response to phosphorus addition of different modified tree species in Acacia mangium plantation. J. Trop. Subtrop. Bot. 2024, 34, 737–746. [Google Scholar]
- Wang, F. Overview of the forest land soil survey in Longyandong Forest Farm, Guangdong Province. NongJia KeJi 2015, 31, 18–19. [Google Scholar]
- Chen, F.; Guo, Y.; Huang, M.; Feng, W.; Ye, Q.; Liu, J.; Li, X. Community structure characteristics and management strategies of Castanopsis hystrix plantation with different restoration years. J. Anhui Agric. Univ. 2022, 49, 48–55. [Google Scholar]
- LY/T 2812-2017; Methods for the Determination of Functional Traits in Forest Woody Plants. CSP: Beijing, China, 2017.
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil. Biol. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.T.; Zhang, S.S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 51, 107521. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017, 2, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.X.; Li, X. USEARCH 12: Open-source software for sequencing analysis in bioinformatics and microbiome. iMeta 2024, 3, e236. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 41, D633–D642. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.R. Using BLAST for sequence similarity searching. Curr. Protoc. Bioinform. 2013, 40, 115. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lee, S. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 1984, 87, 210–217. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in microbiome data. J. Vis. Exp. 2022, 19, e61715. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.D.; Li, S.; Leng, X.H.; Yao, X.H. Soil bacterial community structure and co-occurrence pattern during vegetation restoration in Karst Rocky desertification area. Front. Microbiol. 2017, 7, 2377. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Jiang, F.; Cadotte, M.W.; Jin, G. Size- and environment-driven seedling survival and growth are mediated by leaf functional traits. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221400. [Google Scholar] [CrossRef]
- Qin, J.; Shangguan, Z.P. Effects of forest types on leaf functional traits and their interrelationships of Pinus massoniana coniferous and broad-leaved mixed forests in the subtropical mountain, Southeastern China. Ecol. Evol. 2019, 9, 6922–6932. [Google Scholar] [CrossRef]
- Li, S. Effects of Mixed Model on Leaf and Root Functional Traits of Phoebe bournei and Cunninghamia lanceolata. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 1 May 2019. [Google Scholar]
- Zhang, H.; Lan, Y.; Jiang, C.; Cui, Y.; He, Y.; Deng, J.; Lin, M.; Ye, S. Leaf traits explain the growth variation and nitrogen response of Eucalyptus urophylla × Eucalyptus grandis and Dalbergia odorifera in mixed culture. Plants 2024, 13, 988. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Evaluation of leaf features in forest trees: Methods, techniques, obtainable information and limits. Ecol. Indic. 2015, 15, 219–230. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, K.Y.; Dai, J.; Chen, D.X.; Lan, X.; Cheng, L.; Chen, X.M.; Li, K.P. Effects of nitrogen-fixing tree species on total N content and enzyme activity of Cunninghamia lanceolata under mixed plantation pattern. J. South. Agric. 2016, 47, 608–613. [Google Scholar]
- Qin, J.; Shang, G.Z.P. Leaf nutrient contents and photosynthetic physiological characteristics of Ulmus pumila Robinia pseudocacia mixed forests. Chin. J. Appl. Ecol. 2010, 21, 2228–2234. [Google Scholar]
- Liu, M.; Chen, S.; An, Q. Photosynthetic and physiological characteristic of three common species in different communities. Acta Bot. Boreal. Occident. Sin. 2015, 35, 998–1004. [Google Scholar]
- Wang, R.; Balkanski, Y.; Boucher, O.; Ciais, P.; Peñuelas, J.; Tao, S. Significant contribution of combustion-related emissions to the atmospheric phosphorus budget. Nat. Geosci. 2015, 8, 48–54. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, W.; Ren, H.; Chen, H.; Wang, J. Impact of atmospheric nitrogen deposition on soil properties and herb-layer diversity in remnant forests along an urban-rural gradient in Guangzhou, southern China. Plant Ecol. 2012, 213, 1187–1202. [Google Scholar] [CrossRef]
- Wen, X.; Wang, R.; Jiang, Y.; Deng, X.; Wei, S.; Liu, X.; Wang, Y. Diversity and composition of rhizosphere fungal community in pure and mixed forests of south China. Pol. J. Ecol. 2021, 69, 1505–2249. [Google Scholar] [CrossRef]
- Nacke, H.; Thürmer, A.; Wollherr, A.; Will, C.; Hodac, L.; Herold, N.; Schöning, I.; Schrumpf, M.; Daniel, R. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, H.C.; Mo, X.Q.; Xiao, N.; You, Y.M.; Huang, X.M.; Wen, Y.G.; Zhu, H.G. Effects of mixture of valuable nitrogen-fixing tree species Dalbergia odorifera and second-generation Eucalyptus urophylla on structure and function of soil microbial community in subtropical China. Guangxi Zhi Wu 2021, 41, 1476–1485. [Google Scholar]
- Li, Y.; Lee, C.G.; Watanabe, T.; Murase, J.; Asakawa, S.; Kimura, M. Identification of microbial communities that assimilate substrate from root cap cells in an aerobic soil using a DNA-SIP approach. Soil Biol. Biochem. 2011, 43, 1928–1935. [Google Scholar] [CrossRef]
- Annala, M.J.; Lehosmaa, K.; Ahonen, S.H.K.; Karttunen, K.; Markkola, A.M.; Puumala, I.; Mykrä, H. Effect of riparian soil moisture on bacterial, fungal and plant communities and microbial decomposition rates in boreal stream-side forests. Ecol. Manag. 2022, 47, 120344. [Google Scholar] [CrossRef]
- Xu, H.C.; You, L.H.; You, H.M.; Yu, J.L.; Cheng, F.S.; Ye, G.F.; Huang, Y.L.; Huang, A.Z.; Li, J.M. Effects of different soil management patterns on soil fungal community composition in a Castanea henryi orchard. J. Fruit. Sci. 2021, 38, 1942–1955. [Google Scholar]
- Song, Z. Effect of Tree Species Richness on Soil Microbial Diversity in a Planted Mixed Forest in South Subtropical Region, Guangxi, China. Master’s Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 1 May 2020. [Google Scholar]
- Li, H.; Su, J.; Yang, X.; Zhu, Y. Distinct rhizosphere effect on active and total bacterial communities in paddy soils. Sci. Total Environ. 2019, 50, 422–430. [Google Scholar] [CrossRef]
- Pereira, A.P.D.A.; Santana, M.C.; Zagatto, M.R.G.; Brandani, C.B.; Wang, J.; Verma, J.P.; Singh, B.K.; Cardoso, E.J.B.N. Nitrogen-fixing trees in mixed forest systems regulate the ecology of fungal community and phosphorus cycling. Sci. Total Environ. 2021, 50, 143711. [Google Scholar] [CrossRef]
- Kabuyah, R.N.T.M.; van Dongen, B.E.; Bewsher, A.D.; Robinson, C.H. Decomposition of lignin in wheat straw in a sand-dune grassland. Soil Biol. Biochem. 2012, 44, 128–131. [Google Scholar] [CrossRef]
- Wei, J.X.; Wang, C.; He, B.; You, Y.M.; Huang, X.M. Research progress on soil microorganisms in Eucalypt forests. J. Zhejiang AF Univ. 2022, 39, 1144–1154. [Google Scholar]
- Qiu, H.; Ge, T.; Liu, J.; Chen, X.; Hu, Y.; Wu, J.; Su, Y.; Kuzyakov, Y. Effects of biotic and abiotic factors on soil organic matter mineralization: Experiments and structural modeling analysis. Eur. J. Soil. Biol. 2018, 26, 27–34. [Google Scholar] [CrossRef]
- Delgado Baquerizo, M.; Fry, E.L.; Eldridge, D.J.; Vries, F.T.; Manning, P.; Hamonts, K.; Kattge, J.; Boenisch, G.; Singh, B.K.; Bardgett, R.D. Plant attributes explain the distribution of soil microbial communities in two contrasting regions of the globe. New Phytol. 2018, 219, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, A.; Wan, W.; Luo, X.; Zheng, L.; He, G.; Huang, D.; Chen, W.; Huang, Q. High salinity inhibits soil bacterial community mediating nitrogen cycling. Appl. Environ. Microbiol. 2021, 87, e136621. [Google Scholar] [CrossRef]
- Trejos-Espeleta, J.C.; Marin-Jaramillo, J.P.; Schmidt, S.K.; Sommers, P.; Bradley, J.A.; Orsi, W.D. Principal role of fungi in soil carbon stabilization during early pedogenesis in the high Arctic. Proc. Natl. Acad. Sci. USA 2024, 121, e1892278175. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Koricheva, J. Functional diversity enhances, but exploitative traits reduce tree mixture effects on microbial biomass. Funct. Ecol. 2020, 34, 276–286. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008, 54, 876–886. [Google Scholar] [CrossRef]
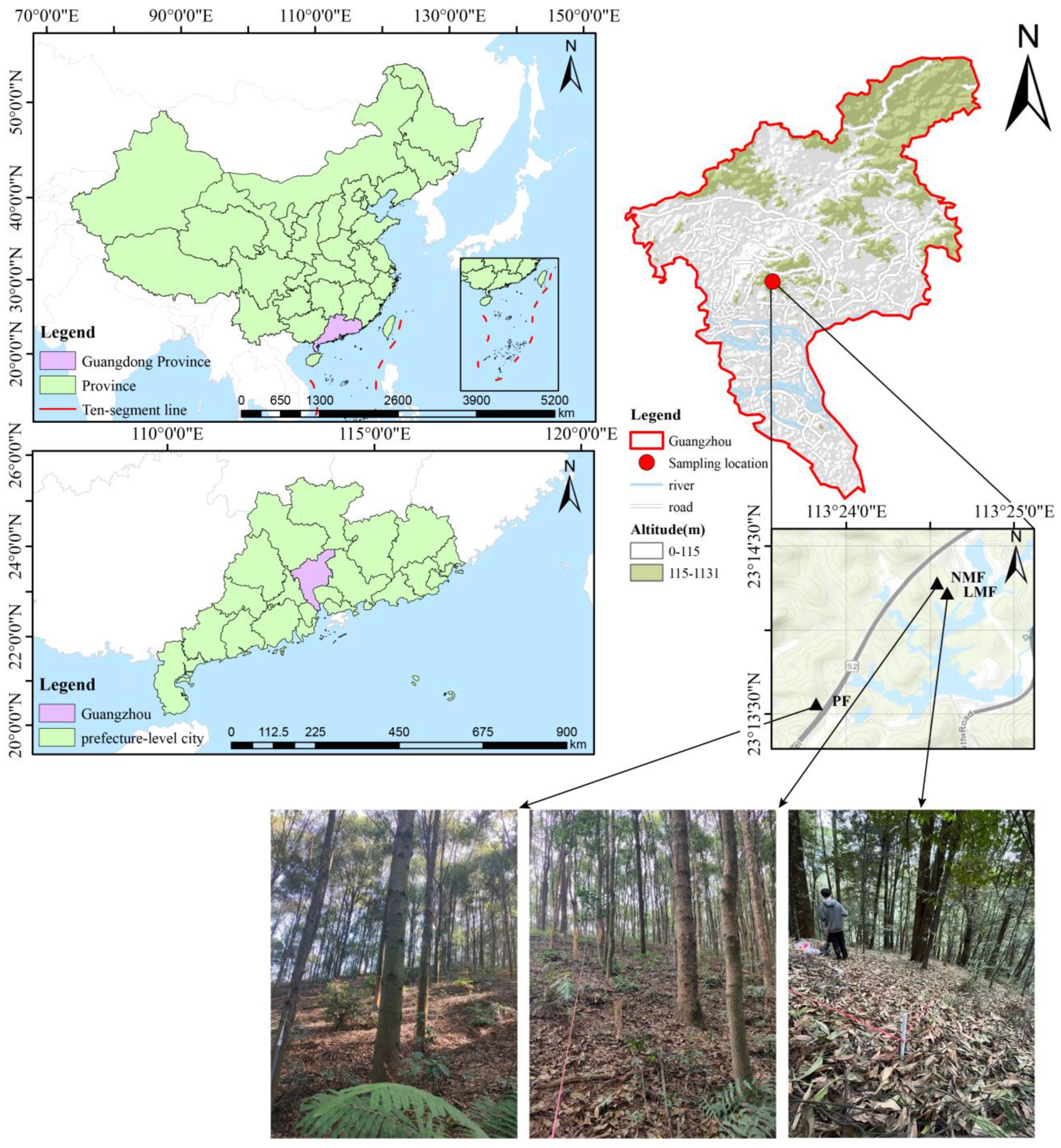


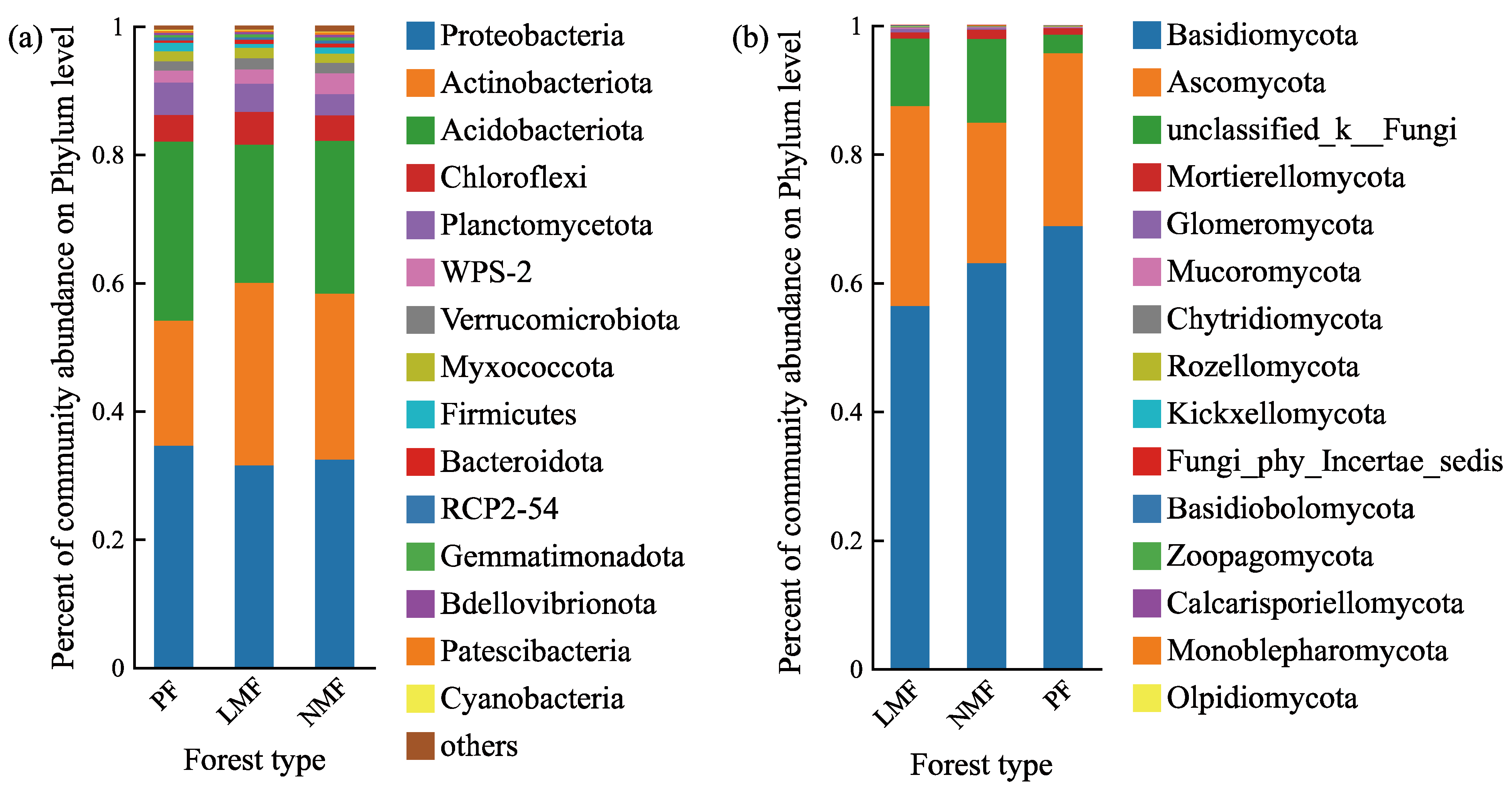

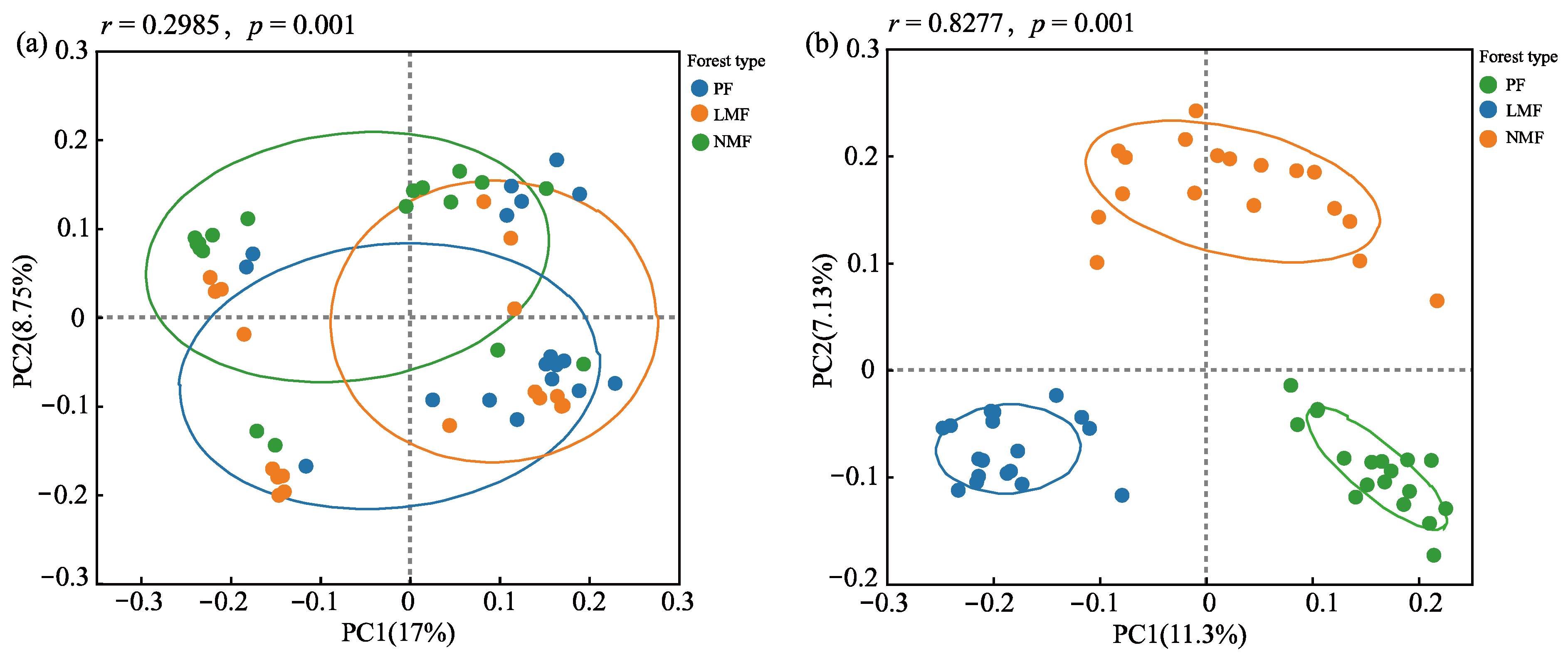
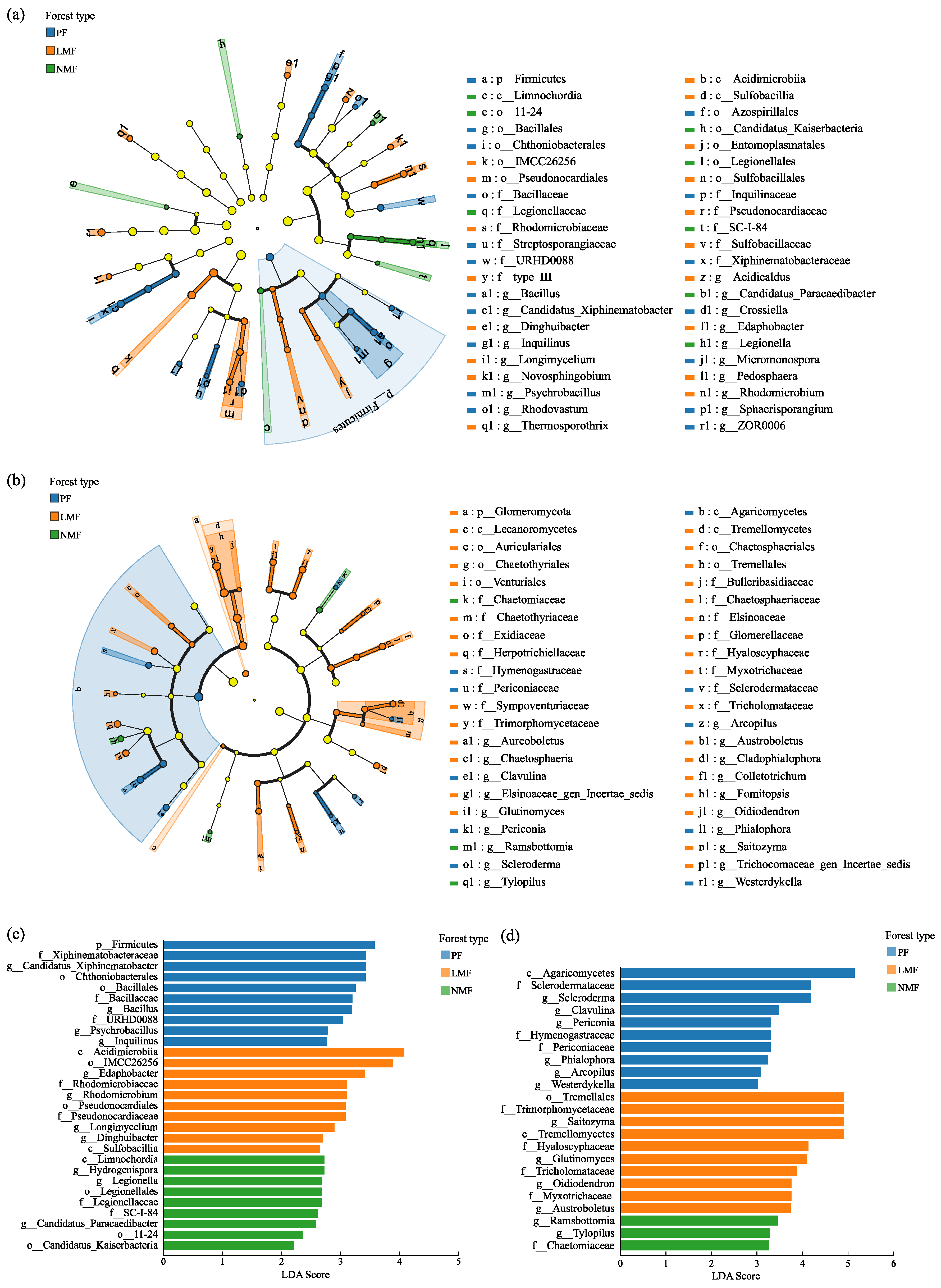


| Forest Type | Pure Forest (PF) | Leguminous Mixed Forest (LMF) | Non-Leguminous Mixed Forest (NMF) | ||
|---|---|---|---|---|---|
| Tree Species | C. hystrix | C. hystrix + A. mangium | C. hystrix + S. superba | ||
| Altitude (m) | 106 | 162 | 157 | ||
| Average age (yr) | 21 ± 3 | 21 ± 3 | 21 ± 3 | ||
| Density (plant ha−1) | 509 | 281 + 278 | 380 + 379 | ||
| Mixing ratio | 1 | 1:1 | 1:1 | ||
| Diameter at breast height (cm) | 20.4 ± 6.6 | 19.5 ± 8.5 | 27.8 ± 9.0 | 23.9 ± 8.0 | 19.3 ± 6.4 |
| Average tree height (m) | 20.4 ± 3.0 | 16.0 ± 5.1 | 19.9 ± 4.0 | 19.8 ± 3.4 | 18.9 ± 3.1 |
| Soil pH | 4.1 ± 0.1 | 4.3 ± 0.1 | 4.3 ± 0.1 | ||
| Soil organic carbon (g kg−1) | 13.5 ± 2.0 | 19.2 ± 4.3 | 22.1 ± 6.6 | ||
| Soil total nitrogen (g kg−1) | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.2 ± 0.3 | ||
| Soil total phosphorus (g kg−1) | 0.13 ± 0.0 | 0.13 ± 0.0 | 0.2 ± 0.0 | ||
| Soil available nitrogen (mg kg−1) | 8.0 ± 3.8 | 13.5 ± 4.92 | 8.5 ± 4.3 | ||
| Forest Type | PF | LMF | NMF | ||||
|---|---|---|---|---|---|---|---|
| Bacteria | Chao1 index | 1612 | −224 | 1504 | −157 | 1657 | −238 |
| Shannon index | 5.4 | −0.2 | 5.38 | −0.14 | 5.37 | −0.23 | |
| Simpson index | 0.01 | −0.01 | 0.012 | −0.003 | 0.013 | −0.003 | |
| Fungi | Chao1 index | 509 | −146 | 735 | 120 ** | 678 | 149 ** |
| Shannon index | 2.12 | −0.58 | 3.31 | 0.55 ** | 2.65 | 0.91 * | |
| Simpson index | 0.32 | −0.16 | 0.14 | 0.06 ** | 0.26 | 0.2 * | |
| Functional Trait | Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|
| RDA1 | RDA2 | R2 | p Value | RDA1 | RDA2 | R2 | p Value | |
| Leaf area (LA) | 0.822 | 0.569 | 0.039 | 0.377 | −0.297 | −0.955 | 0.039 | 0.356 |
| Leaf dry matter content (LDMC) | −0.521 | 0.854 | 0.308 | 0.000 ** | 0.709 | −0.706 | 0.042 | 0.329 |
| Specific leaf area (SLA) | 0.988 | 0.154 | 0.147 | 0.019 * | −0.821 | −0.571 | 0.055 | 0.228 |
| Relative chlorophyll content (RCC) | −0.778 | 0.628 | 0.068 | 0.169 | 0.668 | −0.744 | 0.017 | 0.657 |
| Leaf organic carbon content (LOC) | 0.655 | 0.756 | 0.084 | 0.107 | −0.926 | 0.378 | 0.023 | 0.564 |
| Leaf total nitrogen content (LTN) | 0.850 | −0.527 | 0.237 | 0.001 ** | −0.556 | −0.831 | 0.156 | 0.012 * |
| Leaf total phosphorus content (LTP) | 0.021 | −1.000 | 0.337 | 0.000 ** | −0.906 | −0.424 | 0.085 | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, S.; Xie, G.; Shao, Y.; Shi, S.; Lin, J.; Mao, Q.; Li, Y. Response of Leaf Functional Traits and Rhizosphere Microbial Communities of Castanopsis hystrix in Three Subtropical Plantations with Leguminous or Non-Leguminous Trees. Forests 2025, 16, 367. https://doi.org/10.3390/f16020367
Wu Y, Zhang S, Xie G, Shao Y, Shi S, Lin J, Mao Q, Li Y. Response of Leaf Functional Traits and Rhizosphere Microbial Communities of Castanopsis hystrix in Three Subtropical Plantations with Leguminous or Non-Leguminous Trees. Forests. 2025; 16(2):367. https://doi.org/10.3390/f16020367
Chicago/Turabian StyleWu, Yufen, Shihong Zhang, Genglin Xie, Yanqing Shao, Shi Shi, Jieyu Lin, Qinggong Mao, and Yuling Li. 2025. "Response of Leaf Functional Traits and Rhizosphere Microbial Communities of Castanopsis hystrix in Three Subtropical Plantations with Leguminous or Non-Leguminous Trees" Forests 16, no. 2: 367. https://doi.org/10.3390/f16020367
APA StyleWu, Y., Zhang, S., Xie, G., Shao, Y., Shi, S., Lin, J., Mao, Q., & Li, Y. (2025). Response of Leaf Functional Traits and Rhizosphere Microbial Communities of Castanopsis hystrix in Three Subtropical Plantations with Leguminous or Non-Leguminous Trees. Forests, 16(2), 367. https://doi.org/10.3390/f16020367






