Biological Characteristics of the Scale Insect Matsucoccus sinensis (Hemiptera: Coccoidae), a Pest Damaging the Chinese Red Pine Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Life History Observation
2.3. Biological Characteristics Observation
2.4. The Rhythm of Male Cocoon Emergence and Adult Mating
2.5. Determination of the Developmental Period of Eggs Laid by Adult Female M. sinensis
- Effective accumulated temperature = K − (T − C) N
- Development rate = V − 1/V
- Calculated Value of T = T − C + KV
2.6. Data Processing and Analysis Methods
3. Results
3.1. Damage Characteristics
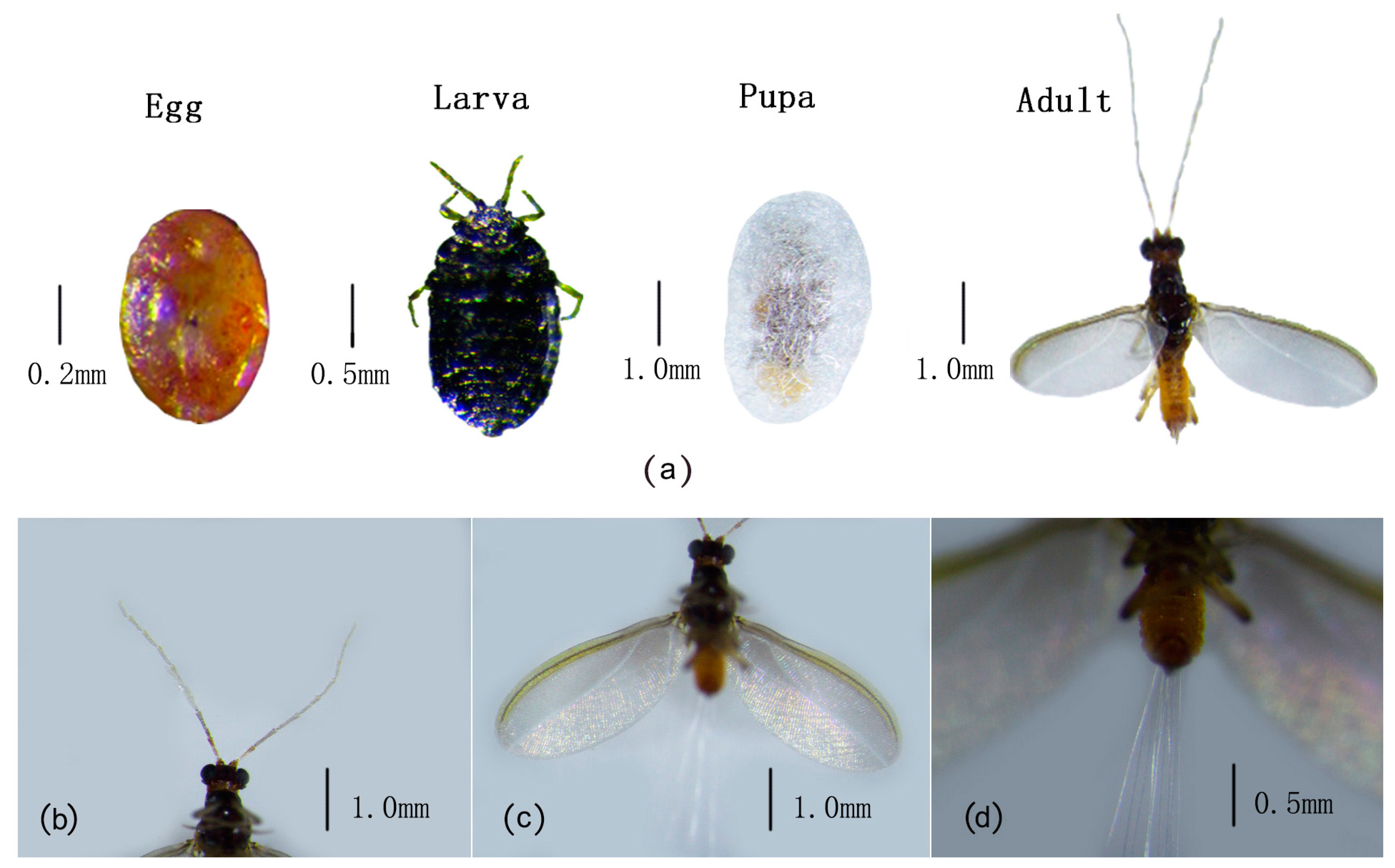
3.2. Morphological Characteristics and Life Cycle of M. sinensis
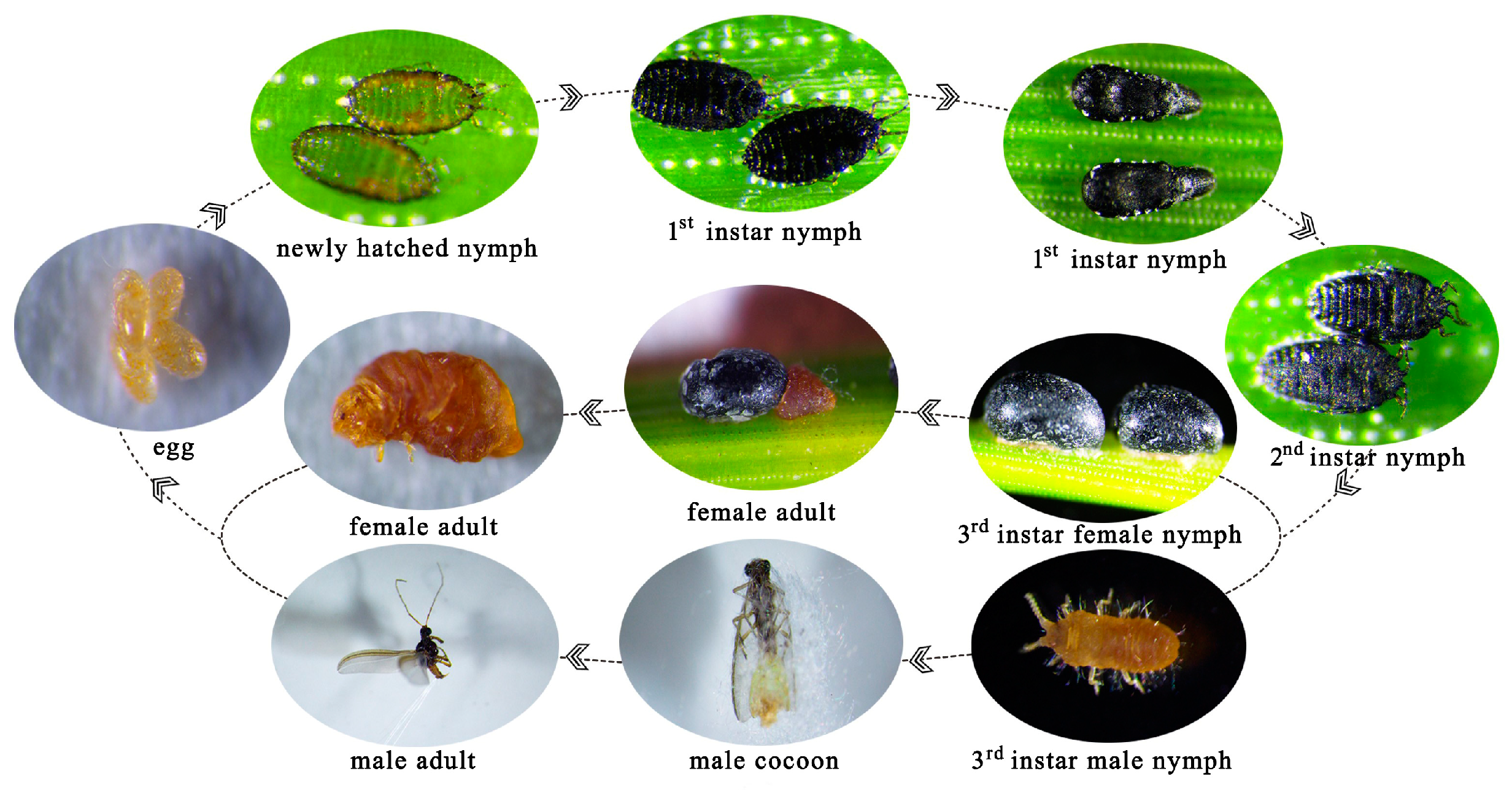
3.3. Life History and Habits
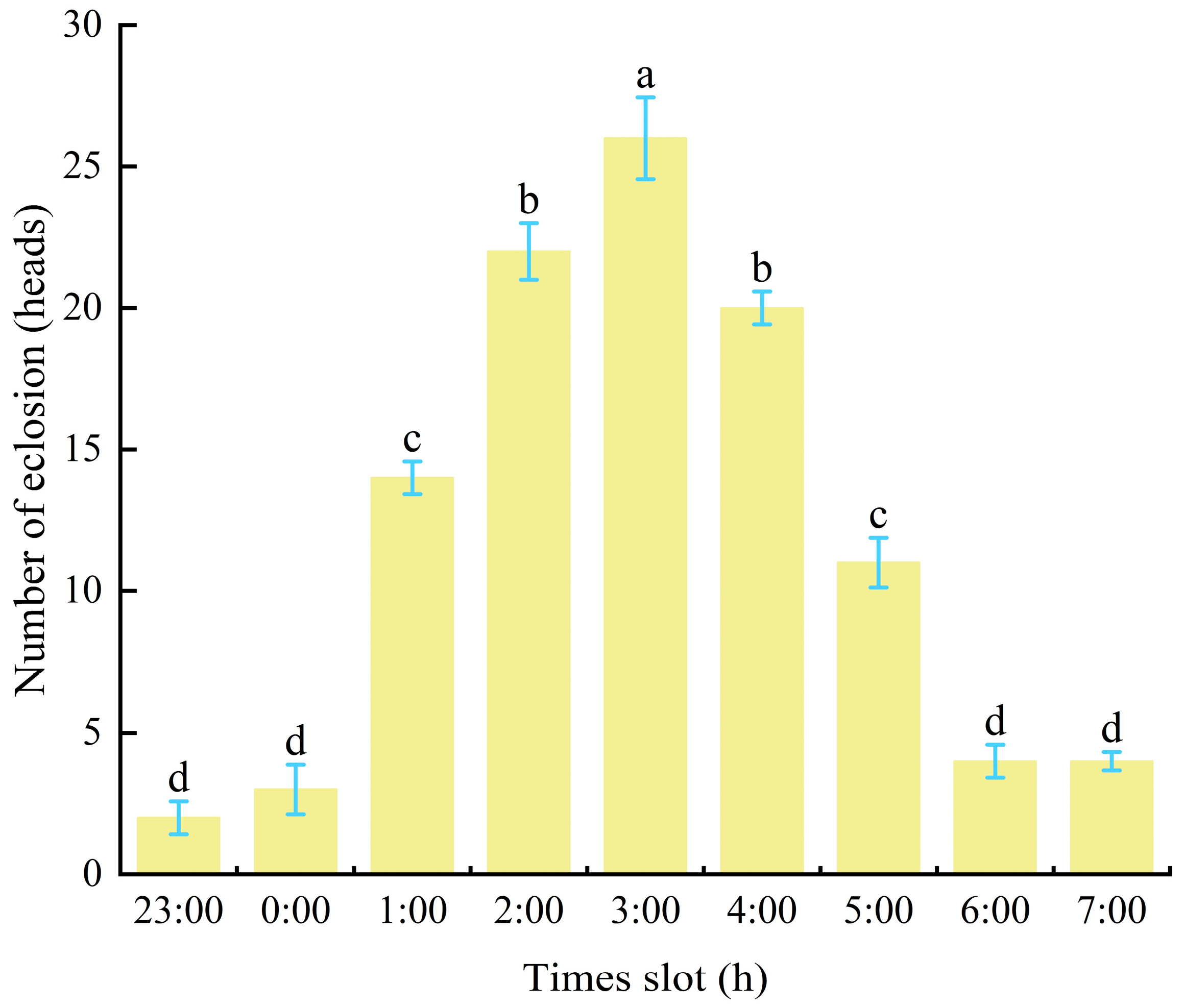
3.4. Male Adult Emergence Rhythm
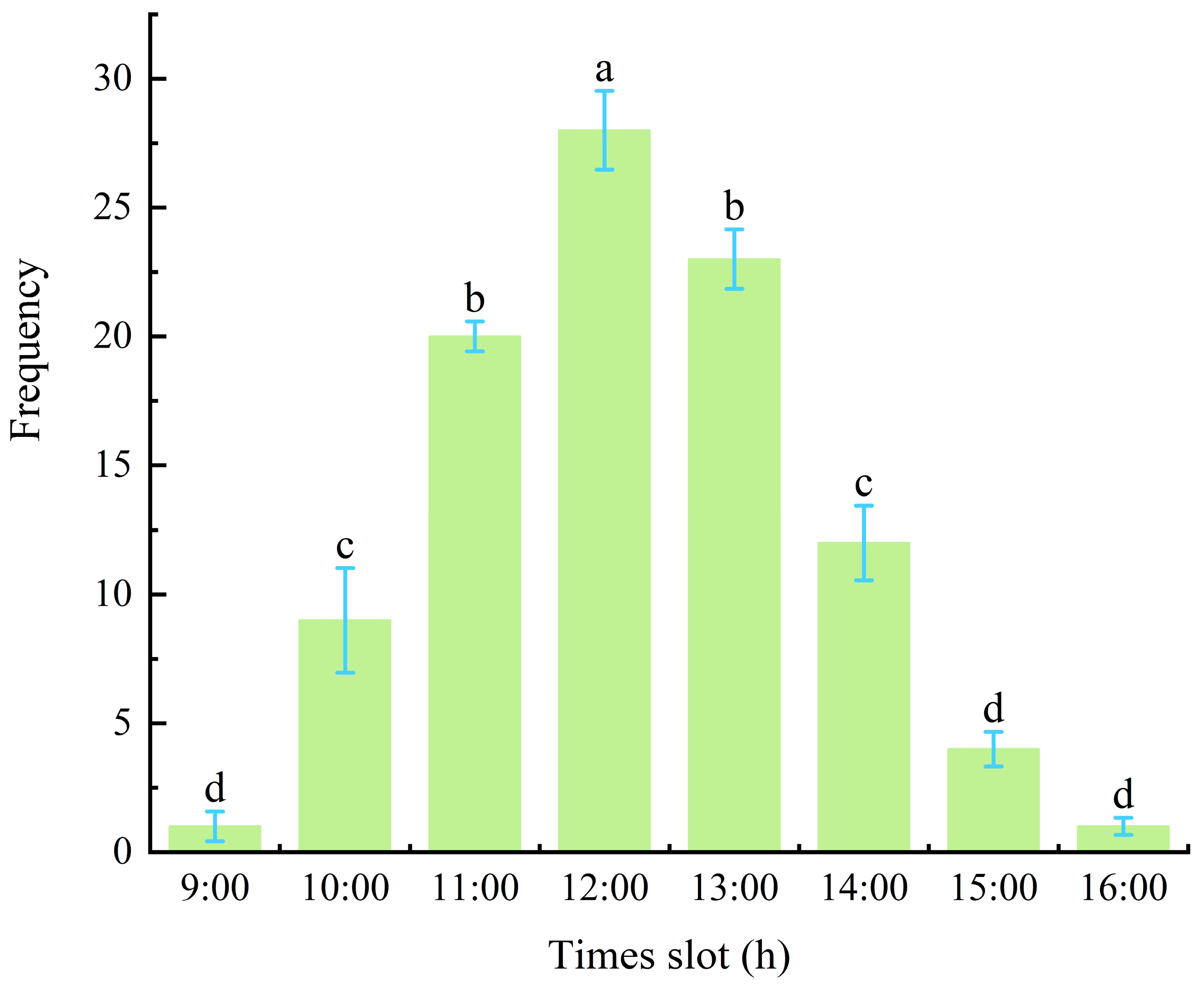
3.5. Duration and Frequency of Adult Mating
3.6. Oviposition Characteristics of Female Adults
3.7. Cocoon and Pupation Sites
3.8. Development Rate and Duration of Eggs at Different Temperatures
3.9. Threshold and Effective Accumulated Temperature for Egg Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Q.; Liu, Z.H.; Wang, S.H.; Li, Z.Q. Genetic diversity and phylogenetic relationships among five endemic Pinus taxa (Pinaceae) of China as revealed by SRAP Markers. Biochem. Syst. Ecol. 2015, 62, 115–120. [Google Scholar] [CrossRef]
- Li, G.; Xu, G.; Guo, K.; Du, S. Geographical boundary and climatic analysis of Pinus tabulaeformis in China: Insights on its afforestation. Ecol. Eng. 2016, 86, 75–84. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The effects of drought and re-watering on non-structural carbohydrates of Pinus tabulaeformis seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, Y.F.; Wang, W.H.; Lv, G.Z.; Liu, B.Y. Morphological characteristics and biological properties of Pinus tabulaeformis. Mod. Agric. Sci. Technol. 2012, 16, 203. [Google Scholar]
- Fu, X.J. Occurrence and control of pests and diseases in oil pine forests in nature reserves. Mod. Rural Sci. Technol. 2025, 1, 35–36. [Google Scholar]
- Wang, W.R. Causes and Control Measures for Matsucoccus sinensis. Mod. Rural Sci. Technol. 2023, 4, 37–38. [Google Scholar]
- Zhang, X. Comprehensive analysis of the breeding and cultivation techniques and the main pest and disease control methods of Chinese red pine. Seed World 2024, 12, 192–194. [Google Scholar]
- Wang, Y. Points of cultivation and pest control technology of oil pine. World Trop. Agric. Inf. 2024, 12, 73–75. [Google Scholar]
- Che, Z.X.; Liu, X.D.; Pan, X.; Li, Y.; Jin, M.; Jing, W.M.; Wang, S.H.L.; Wang, R.X.; Zhao, W.J. The Variation characteristics of nutrients contents of main dominant tree species in Gansu Province. Ecol. Environ. Sci. 2015, 24, 237–243. [Google Scholar] [CrossRef]
- Li, D.C.; Fu, Z.L.; Liu, J.Q.; Zhang, W.Y.; Yang, J. Investigation on Matsucoccus sinensis and its natural enemies and their occurrence patterns in the southern region of Gansu Province. J. Gansu Agric. Univ. 2024, 59, 180–187. [Google Scholar] [CrossRef]
- Song, J.J.; Yang, G. Biological characteristics and life cycle of Matsucoccus sinensis in Longnan City. Shaanxi For. Sci. Technol. 2024, 52, 82–86+92. [Google Scholar] [CrossRef]
- Mo, L. Biological characteristics and control technology of Matsucoccus sinensis. Contemp. Hortic. 2023, 46, 57–58+61. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Dong, X.; Zhao, C.; An, L. Pinus tabulaeformis forests have higher carbon sequestration potential than Larix principis-rupprechtii forests in a dryland mountain ecosystem, northwest China. Forests 2022, 13, 739. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, J.; Don, O.; Zhang, Z. The red turpentine beetle, Dendroctonus valens LeConte (Scolytidae): An exotic invasive pest of pine in China. Biodivers. Conserv. 2005, 14, 1735–1760. [Google Scholar] [CrossRef]
- Liu, H.W.; Cheng, X.Q.; Kang, F.F. Changes of understory plant community in Pinus tabuliformis plantation and associated environmental explanations. Chin. J. Ecol. 2014, 33, 290–295. [Google Scholar] [CrossRef]
- Yang, P.L.; Hu, J.L.; Ren, Z.Y. On pine needle scales. Acta Entomol. Sin. 1980, 23, 42–46. [Google Scholar] [CrossRef]
- Wu, S.A. Checklist and faunistic analysis of scale insect pests (Hemi ptera: Coccoidea) in Chinese Mainland. J. Beijing For. Univ. 2009, 31, 55–63. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, W.R. Studies on Biological Characteristics and Control of Chinese Pine Shoot (Pin) Scale. Fujian For. Sci. Technol. 1987, 1, 21–29. [Google Scholar] [CrossRef]
- Cai, T.T.; Rong, C.H.; Tan, C.; Gao, J.; Liu, P.; Zhang, X.; Wang, L. Spatial Distribution Patterns and Sampling Techniques of Matsucoccus sinensis Adult. J. Southwest For. Univ. 2017, 37, 159–164. [Google Scholar] [CrossRef]
- Zhang, G.X. Relationship between occurrence of Matsucoccus sinensis and environmental factors in western Henan province. Plant Quar. 2009, 23, 11–14. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, W.Y.; Li, D.C. Risk analysis of subalpine Pinus tabulaeformis forest damaged by Matsucoccus sinensis in southern Gansu Province. Shaanxi For. Sci. Technol. 2022, 50, 61–65. [Google Scholar] [CrossRef]
- Yang, H.B. Research on integrated control technology of Matsucoccus sinensis. Seed Sci. Technol. 2019, 37, 96–97. [Google Scholar]
- Li, D.C.; Fu, Z.L.; Xu, H.X.; Cao, X.W.; Liu, J.Q. Effects of stand factors of Pinus tabulaeformis stand on damage of Matsucoccus sinensis in Bailongjiang forest area. For. Res. 2021, 34, 180–186. [Google Scholar] [CrossRef]
- Zhang, C.D.; Li, D.C.; Yang, J.; Zhang, C.A.; Ding, Y.P.; Ding, Q.D. Occurrence and harm of Matsucoccus sinensis in A’xia Provincial nature reserve of Gansu Province. Shaanxi For. Sci. Technol. 2022, 50, 40–43. [Google Scholar] [CrossRef]
- Ding, Y.P.; Zhang, C.D.; Bai, Z.L.; Hu, X.R.; Qi, W.Y.; Li, D.C. Investigation into the mortality of trees in Axia provincial nature reserve of Gansu Province. Shaanxi For. Sci. Technol. 2022, 50, 56–60. [Google Scholar] [CrossRef]
- Booth, J.M.; Gullan, P.J. Synonymy of three pestiferous Matsucoccus scale insects (Hemiptera: Coccoidea: Matsucoccidae) based on morphological and molecular evidence. Proc. Entomol. Soc. Wash. 2006, 108, 749–760. [Google Scholar]
- Foldi, I. The matsucoccidae in the mediterranean basin with a world list of species (Hemiptera: Sternorrhyncha: Coccoidea). Ann. Soc. Entomol. Fr. 2004, 40, 145–168. [Google Scholar] [CrossRef]
- Mendel, Z.; Assael, F.; Dunkelblum, E. Kairomonal attraction of predatory bugs (Heteroptera: Anthocoridae) and brown lacewings (Neuroptera: Hemerobiidae) to sex pheromones of Matsucoccus species (Hemiptera: Matsucoccidae). Biol. Control 2004, 30, 134–140. [Google Scholar] [CrossRef]
- Choi, J.; Cha, D.; Kim, D.S.; Lee, S. Review of Japanese pine bast scale, Matsucoccus matsumurae (Kuwana) (Coccomorpha: Matsucoccidae), occurring on Japanese black pine (Pinus thunbergii Parl.) and Japanese red pine (P. densiflora Siebold & Zucc.) from Korea. Forests 2019, 10, 639. [Google Scholar] [CrossRef]
- Mao, W.X.; Yuan, Q.; Fang, Q. Study on biological characteristics and experimental control of Matsucoccus sinensis in Diebu foresty of Bailongjiang. Pract. For. Technol. 2011, 6, 39–41. [Google Scholar] [CrossRef]
- Branco, M.; Franco, J.C.; Dunkelblum, E.A. Common mode of attraction of larvae and adults of insect predators to the sex pheromone of their prey (Hemiptera: Matsucoccidae). Bull. Entomol. Res. 2006, 96, 179–185. [Google Scholar] [CrossRef] [PubMed]





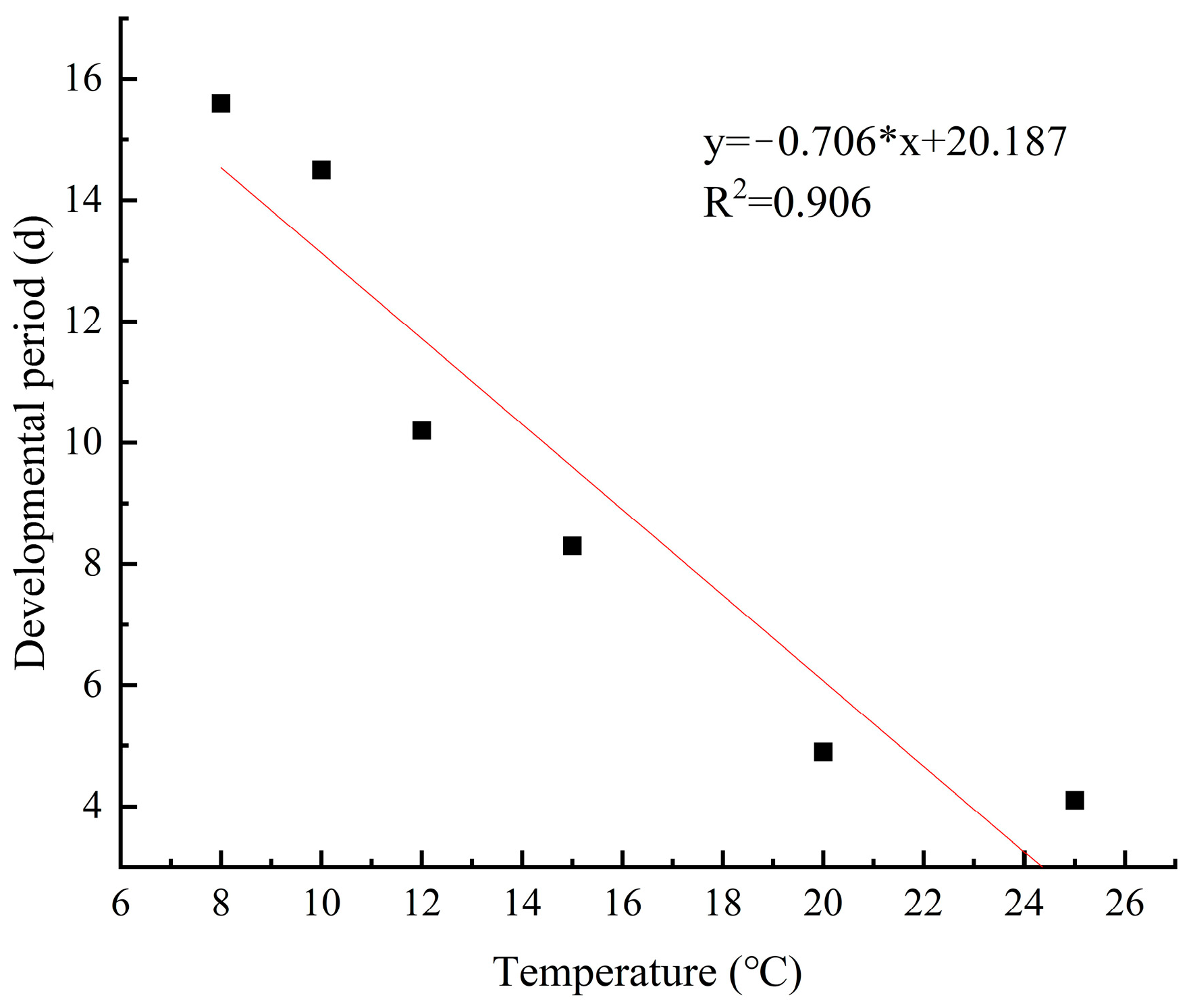
| T (°C) | Development Period (d) Mean ± SE | Development Rate Mean ± SE | Calculated Value of T (°C) Mean ± SE | Standard Error (St) |
|---|---|---|---|---|
| 8 | 15.56 ± 0.230 | 0.06 ± 0.001 | 9.0 ± 0.103 | 0.2 |
| 10 | 14.48 ± 0.286 | 0.07 ± 0.0.001 | 9.4 ± 0.092 | 0.072 |
| 12 | 10.16 ± 0.230 | 0.10 ± 0.002 | 11.9 ± 0.128 | 0.002 |
| 15 | 8.28 ± 0.130 | 0.12 ± 0.002 | 13.9 ± 0.183 | 0.024 |
| 20 | 4.88 ± 0.286 | 0.21 ± 0.012 | 21.1 ± 0.092 | 0.242 |
| 25 | 4.12 ± 0.148 | 0.24 ± 0.009 | 24.5 ± 0.163 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Liu, J.; Yang, J.; Qi, H.; Lin, Y.; Lei, W.; Zhang, W.; Shaarawy, N.; Dewer, Y.; Shang, S.; et al. Biological Characteristics of the Scale Insect Matsucoccus sinensis (Hemiptera: Coccoidae), a Pest Damaging the Chinese Red Pine Forests. Forests 2025, 16, 349. https://doi.org/10.3390/f16020349
Li D, Liu J, Yang J, Qi H, Lin Y, Lei W, Zhang W, Shaarawy N, Dewer Y, Shang S, et al. Biological Characteristics of the Scale Insect Matsucoccus sinensis (Hemiptera: Coccoidae), a Pest Damaging the Chinese Red Pine Forests. Forests. 2025; 16(2):349. https://doi.org/10.3390/f16020349
Chicago/Turabian StyleLi, Danchun, Jinqian Liu, Jing Yang, Hao Qi, Yuan Lin, Wei Lei, Wenyu Zhang, Nehal Shaarawy, Youssef Dewer, Suqin Shang, and et al. 2025. "Biological Characteristics of the Scale Insect Matsucoccus sinensis (Hemiptera: Coccoidae), a Pest Damaging the Chinese Red Pine Forests" Forests 16, no. 2: 349. https://doi.org/10.3390/f16020349
APA StyleLi, D., Liu, J., Yang, J., Qi, H., Lin, Y., Lei, W., Zhang, W., Shaarawy, N., Dewer, Y., Shang, S., & Fu, Z. (2025). Biological Characteristics of the Scale Insect Matsucoccus sinensis (Hemiptera: Coccoidae), a Pest Damaging the Chinese Red Pine Forests. Forests, 16(2), 349. https://doi.org/10.3390/f16020349






