1. Introduction
As an environment of microorganisms, soil represents an important and inevitable link in the chain of circulation of matter and energy in nature.
Soil bacteria is a vital component of the soil microbial community, with essential roles in the functioning of forest ecosystems. These microorganisms are predominantly found in the uppermost layers of the soil, particularly in the organic (O) and accumulation (A) horizons, where they are exposed to various environmental stressors, including drought and subsequent rewetting periods [
1,
2]. Bacteria contribute to the transformation of organic compounds into forms that can be utilized by plants, thereby enhancing soil fertility and health [
3]. Microbial interactions with plant roots, such as mycorrhizal associations, facilitate nutrient uptake, indicating a complex relationship that underscores the significance of bacteria in promoting plant health and soil structure formation [
3]. Numerous studies have shown that the total number of bacteria can be used as an indicator of the general activity of soil [
4,
5,
6,
7].
Actinomycetes contribute to the decomposition of organic matter and play a vital role in nutrient cycling, particularly through their production of extracellular enzymes that facilitate the breakdown of complex organic substances [
8]. Within forest ecosystems, actinomycetes represent a small but important fraction of the bacterial population, often comprising around 4.5% of the overall microbiota [
8], influential in enhancing soil health and maintaining ecological balance by participating in the degradation of organic materials and the release of essential nutrients, and characterized by its ability to thrive in various environmental conditions and contribute to essential soil processes, particularly in the presence of organic matter [
9]. Their number is closely linked to the composition and diversity of the surrounding vegetation, which serves as a primary regulator of soil characteristics and microbial communities [
3,
8]. The enzymatic activities of actinomycetes, such as dehydrogenase, play a crucial role in soil biochemical processes [
9].
The diversity and activity of fungi in forest soils are affected by both biotic factors, such as plant community composition, and abiotic factors, including soil pH, moisture, and temperature conditions [
1,
10]. Fungi are essential decomposers in forest ecosystems, breaking down complex organic matter and facilitating nutrient availability for plants. The decomposers are saprophytic fungi that transform dead organic matter into the biomass of their cells, carbon dioxide, organic acids, etc. The saprophytic group of fungi is important for the immobilization and storage of nutrients in the soil. The most numerous fungi decomposers in the soil are
Penicillium,
Aspergillus,
Pleurotus,
Mucor,
Alternaria,
Trichoderma,
Fusarium,
Rhizopus, etc. [
11]. Their extensive hyphal networks enable them to explore larger soil volumes compared to bacteria, allowing fungi to access and decompose substrates that are resistant to bacterial degradation [
3,
10]. This capability not only aids in the recycling of nutrients but also contributes to the stabilization of soil organic matter, which is vital for maintaining soil health and fertility [
1].
Seasonal variations affect the activity levels of actinomycetes, with increased dehydrogenase activity observed during the warmer months. Actinomycetes interact synergistically with other soil microorganisms, including fungi and bacteria, enhancing nutrient availability and soil structure. They are capable of degrading pectin, lignin and xenobiotics, substances that are significantly more resistant to microbial degradation [
7].
The interactions among bacteria, actinomycetes, and fungi in forest soils play a crucial role in enhancing dehydrogenase activity, which is an important indicator of microbial activity and soil health [
12]. Studies indicate that these microorganisms not only coexist but also engage in complex interactions that can be either synergistic or antagonistic, significantly affecting soil biochemical processes and ecosystem functions [
10]. Fungi–bacteria interactions are significant drivers of ecosystem functions as they engage in complex relationships that can range from mutualism to antagonism, affecting microbial community dynamics and soil health [
10]. Several soil parameters such as soil pH, moisture, and nutrient concentrations correlate with microbial diversity indices, which in turn affect the dehydrogenase activity in the soil [
8].
The decomposition of organic matter in soil as an oxidation-reduction process is catalyzed by dehydrogenase enzymes and in the soil, they are most often of microbial origin. Dehydrogenases are enzymes that catalyze the reaction of hydrogen separation from various organic compounds and their transfer to oxygen (aerobic dehydrogenases) or to organic compounds (anaerobic dehydrogenases). Certain studies indicate that the general microbiological activity of the soil can be assessed based on dehydrogenase activity [
13]. The activity of the dehydrogenase enzyme depends on the overall physiological activity of microorganisms, with higher dehydrogenase activity indicating a higher-intensity mineralization of organic matter in the soil [
14,
15]. Variations in environmental conditions, particularly air and soil temperature and humidity, are the principal causes of seasonal variations in dehydrogenase activity values [
16].
Several studies on the properties of the soils of the Vojvodina region [
17] and their microbial activity [
18] have been carried out in the past. However, most of these studies were conducted on agricultural soils [
19,
20,
21]. Dehydrogenase enzyme activity has been previously researched in forest soils of the National Park “Frushka gora”, focusing on soil microbial abundance and its impact on dehydrogenases in 2015, and indicating a directly proportional correlation of soil microorganisms and dehydrogenase activity [
22]. Yet, the dehydrogenase activity in forest soils has been insufficiently studied, and without considering seasonal abiotic variations impact.
The aim of this study is to reveal the mutual interaction of the abiotic (climate) and biotic (bacteria, actinomycetes and fungi) factors in the soil within Quercetum montanum typicum forest community at NP “Frushka gora” and determine their impact on dehydrogenase enzyme activity through data analysis of site-specific values such as air and soil temperature and humidity, number of bacteria, actinomycetes and fungi, and dehydrogenase activity values. It is expected that the abiotic and biotic factors impact the intensity of dehydrogenase activity in forest soils.
3. Results
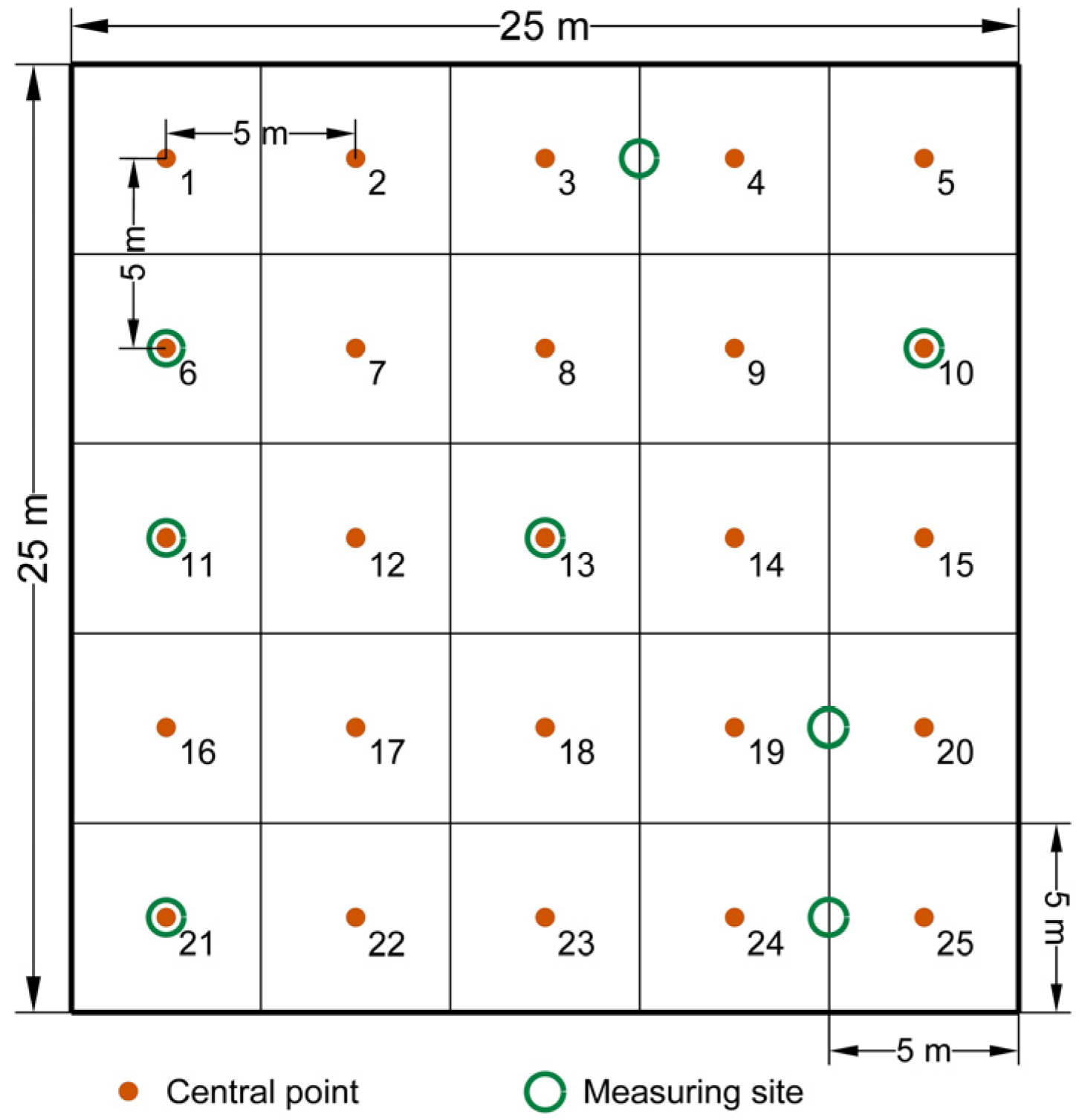
A total of 32 field visits were conducted between 2014 and 2017 to measure site-specific air temperature and humidity at 30 cm and 200 cm, soil humidity and soil sampling.
The climate data analysis for the 2014–2017 study period (
Figure 2) reveals that mean annual air temperature ranges between 12.34 °C and 12.88 °C, the annual sum of precipitation is higher in 2014 and 2016, the highest mean monthly air temperature of 27.01 °C is calculated for July 2015, the mean monthly air temperatures below 0 °C are calculated in December 2016 and January 2017, and the June–September period is noticeably warmer and drier throughout the study period.
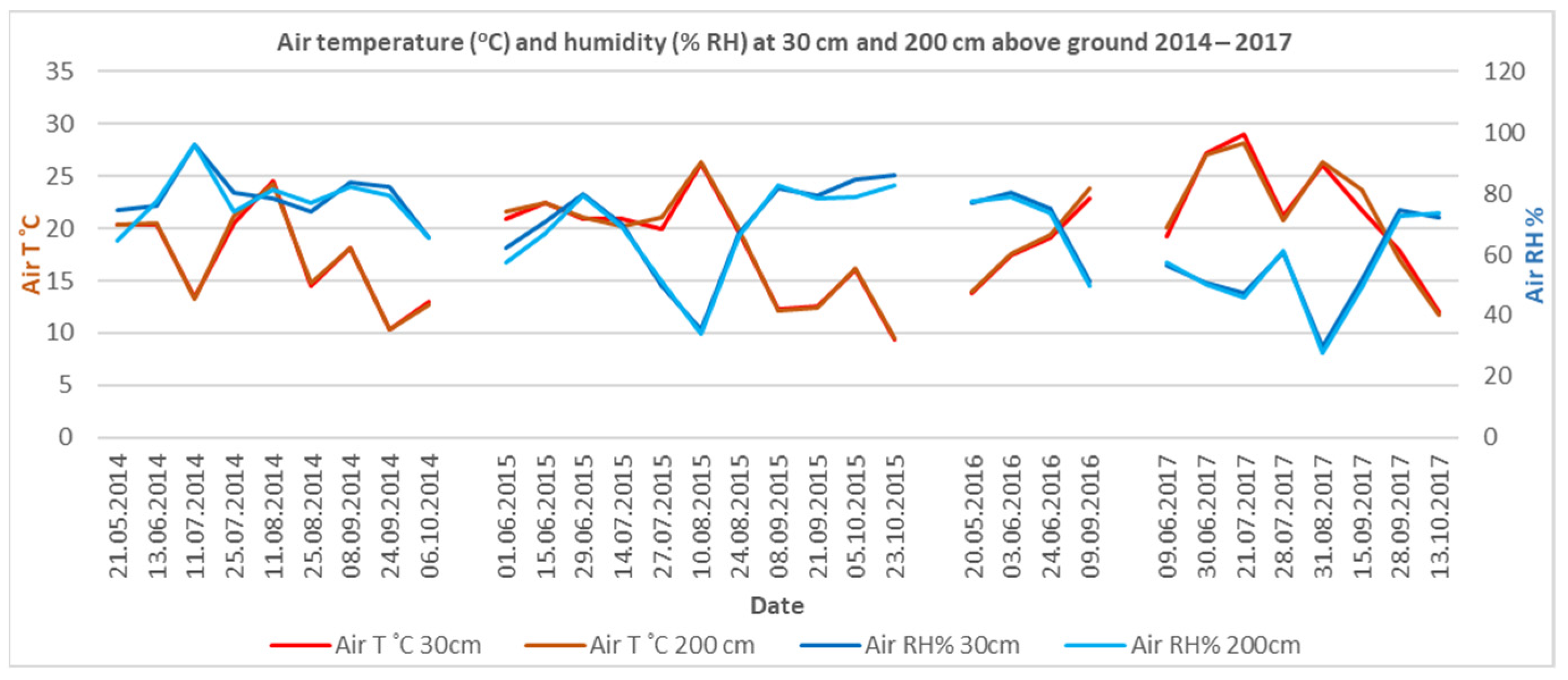
Climate conditions were found to be proportionately correlated with the site-gathered data for soil temperature and humidity at 20 cm below ground and air temperature and humidity at 30 cm and 200 cm above ground. April and May have the highest air humidity (
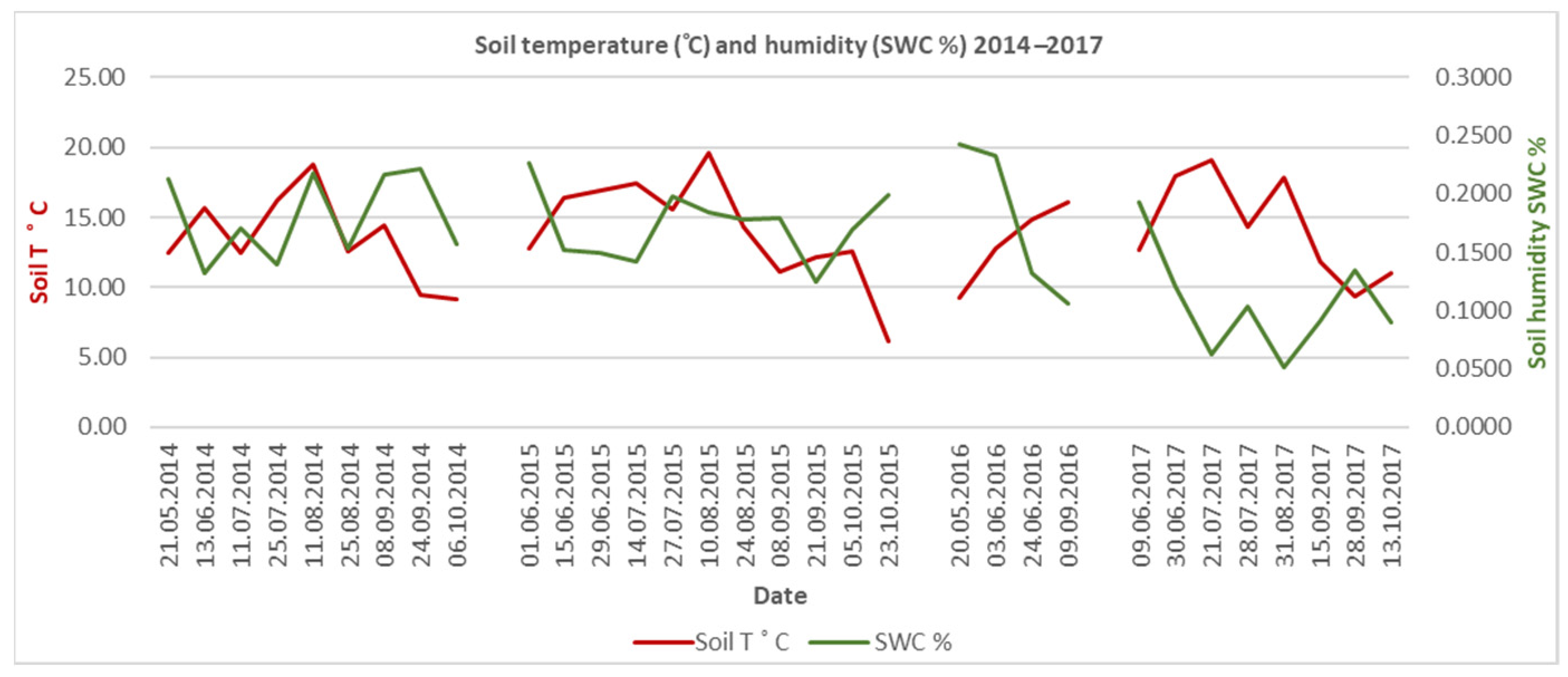
Figure 3), followed by the hot and dry June–September period. The period from October to March has a close total monthly precipitation value and a consistent distribution pattern. Soil humidity and soil temperature are strongly correlated. Large-scale climate and site-specific air temperature and humidity correlate with soil temperature and humidity (
Figure 4).
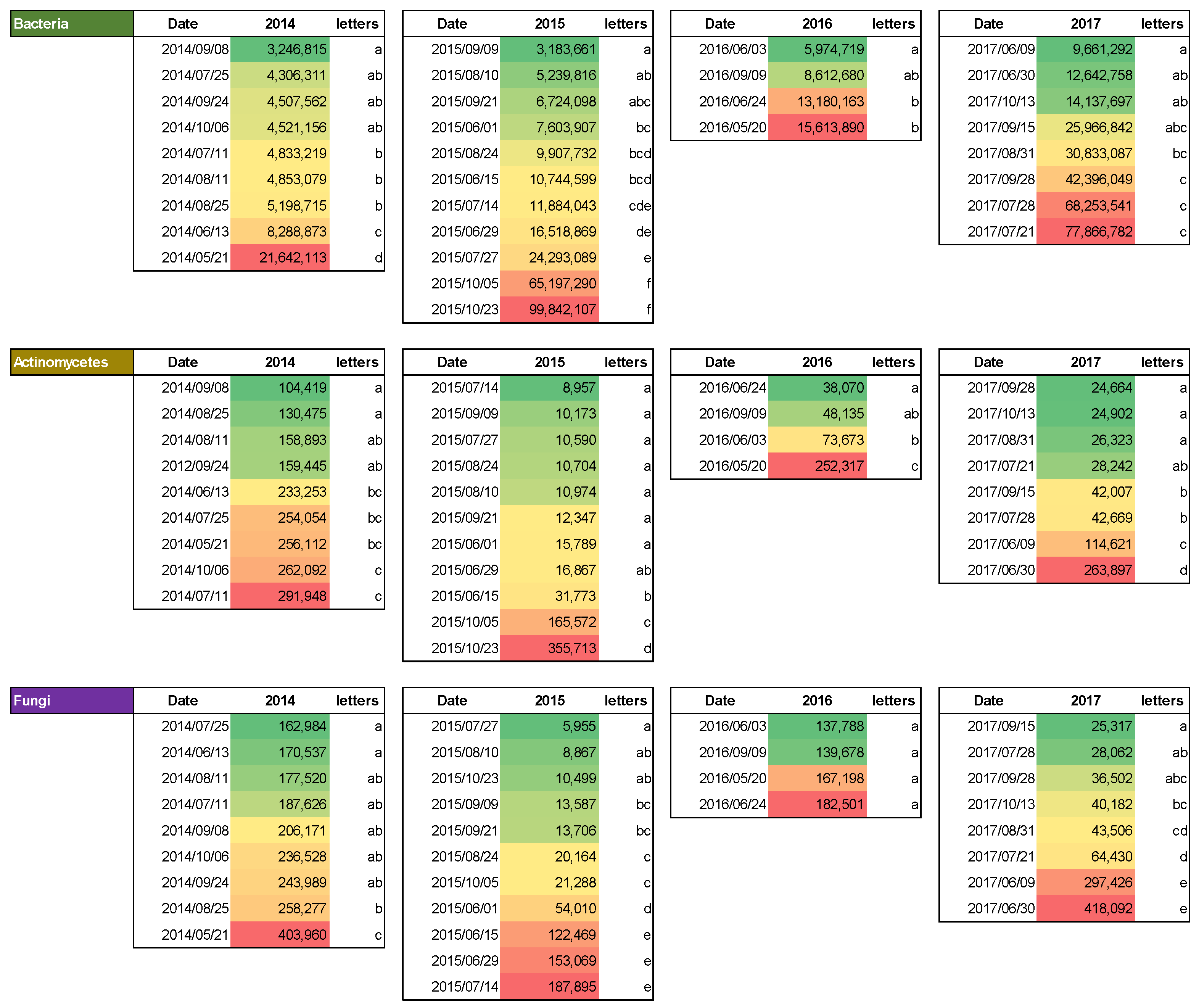
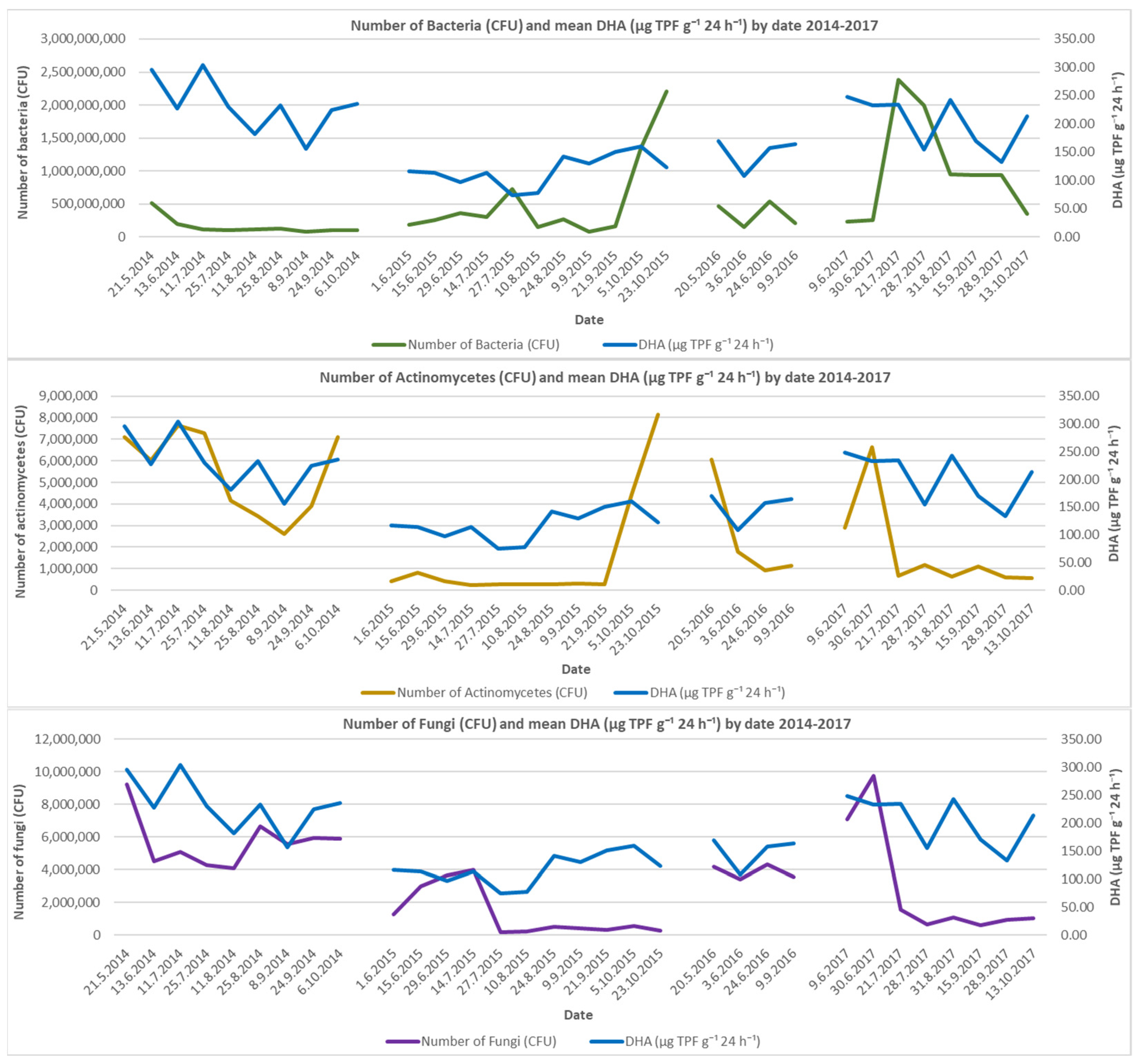
There were variations in the total number of bacteria during the study period: 3.24 × 106 to 21.64 × 106 CFU (8 September 2014, and 21 May 2014, respectively) in 2014; 3.18 × 106 to 99.84 × 106 CFU (9 September 2015 and 23 October 2015) in 2015; 5.97 × 106 to 15.61 × 106 CFU (3 June 2016 and 20 May 2016) in 2016; and 9.66 × 106 to 77.86 × 106 CFU (9 June 2017 and 21 July 2017) in 2017.
The total number of actinomycetes varied from 104.41 × 103 to 291.94 × 103 CFU (8 September 2014, and 11 July 2014, respectively) in 2014; from 8.95 × 103 to 355.71 × 103 CFU (14 July 2015, and 23 October 2015) in 2015; from 38.07 × 103 to 252.31 × 103 CFU (24 June 2016, and 20 May 2016) in 2016; and from 24.66 × 103 to 263.89 × 103 CFU (28 September 2017, and 30 June 2017) in 2017.
The total number of fungi varied from 162.98 × 103 to 403.96 × 103 CFU (25 July 2014, and 21 May 2014, respectively) in 2014; from 5.95 × 103 to 187.89 × 103 CFU (27 July 2015, and 14 July 2015) in 2015; from 137.78 × 103 to 182.5 × 103 CFU (3 June 2016 and 24 June 2016) in 2016, and from 25.31 × 103 to 418.09 × 103 CFU (15 September 2017 and 30 June 2017) in 2017.
The most notable differences are observed in the numbers of bacteria in 2015 and 2017, actinomycetes in 2014, and fungi in 2015 and 2017 (
Figure 5).
The number of bacteria showed notably high levels in the fall of 2015 and throughout 2017, while all other data reflect close values. Actinomycetes are most numerous during the earliest and last measurements of each year, whereas their numbers are significantly lower during the June–September span. There is a strong correlation between changes in the overall number of actinomycetes and the levels of dehydrogenase enzyme activity. The number of fungi peaks in the earliest and latest measurements of each year, similar to the number of actinomycetes, and falls significantly during the hot and dry summer months. Seasonal changes in the environment have a significant impact on the number of fungi over the course of the study.
The dehydrogenase enzyme activity values during the study period varied between 80 and 300 µg TPF g
−1 24 h
−1, as presented in
Figure 6.
Analysis of dehydrogenase enzyme activity (DHA) has revealed significantly variable values over the course of the investigation: 45.71–771.43 µg TPF g−1 24 h−1 (site 10, 8 September 2014, and site 6, 11 July 2014, respectively) in 2014; 20.00–320.00 µg TPF g−1 24 h−1 (site 13, 10 August 2015, and site 19/20, 24 August 2015) in 2015; 22.86–494.29 µg TPF g−1 24 h−1 (site 21, 3 June 2016, and site 6, 9 September 2016) in 2016; and 40.00–800.00 µg TPF g−1 24 h−1 (site 3/4, 28 September 2017, and site 10, 12 May 2017) in 2017.
Figure 7 shows a visual representation of the correlation between all variables, including biotic and abiotic groups.
In 2014, the DHA had positive, strong correlations with the number of bacteria (0.55), actinomycetes (0.76) and fungi (0.47), and a significant negative correlation with the soil temperature (−0.41). The number of bacteria is positively correlated to the number of actinomycetes (0.36) and fungi (0.78) and negatively correlated to air humidity at 200 cm (−0.52). The number of actinomycetes has a strong negative correlation with soil humidity (−0.47), while the number of fungi has a strong negative correlation with air humidity at 200 cm (−0.52) and soil temperature (−0.53). The values of the abiotic factors only show a significant positive correlation among some of themselves like the air temperature at 30 cm with the air temperature at 200 cm (1.0) and the soil temperature (0.9), air humidity at 30 cm with air humidity at 200 cm (0.88), and air temperature at 200 cm with soil temperature (0.9).
In 2015, the DHA had strong, positive correlations with the air humidity at 30 cm (0.73) and 200 cm (0.68), and negative, strong correlations with the air temperature at 30 cm (−0.6) and 200 cm (−0.62) and the soil temperature (−0.55). The number of bacteria had a strong positive correlation with the number of actinomycetes (0.96) and a strong negative correlation with air temperature at 30 cm (−0.54) and 200 cm (−0.52) and the soil temperature (−0.65). The number of actinomycetes had a strong, negative correlation to air temperature at 30 cm (−0.59) and 200 cm (−0.59) and the soil temperature (−0.72). The number of fungi in 2015 did not have a strong correlation to any of the biotic or abiotic factors. Among the abiotic group of factors, the air temperature at 30 cm had a strong positive correlation with the air temperature at 200 cm (1.0) and the soil temperature (0.91), as well as a strong negative correlation to air humidity at 30 cm (−0.76) and 200 cm (−0.78); the air humidity at 30 cm had a strong positive correlation to the air humidity at 200 cm (0.99) and a strong negative correlation to the air temperature at 200 cm (−0.78) and the soil temperature (−0.66); air temperature at 200 cm had a strong negative correlation with the air humidity at 200 cm (−0.8) and a strong positive correlation with the soil temperature (0.89); and air humidity at 200 cm had a strong negative correlation with the soil temperature (−0.65).
In 2016, the DHA had strong positive correlations with the number of bacteria (0.62) and fungi (0.62) with actinomycetes (0.33), and negative correlations with the air humidity at 30 cm (−0.46) and 200 cm (−0.42) and soil humidity (−0.4). The number of bacteria had similar positive correlations with the number of actinomycetes (0.33), air humidity at 30 cm (0.33) and 200 cm (0.35), and a strong positive correlation with the number of fungi (1.0), as well as a negative correlation with the air temperature at 30 cm (−0.4) and at 200 cm (−0.41). The number of actinomycetes had a strong positive correlation with the soil humidity (0.72) and positive correlations with the number of fungi (0.33), the air humidity at 30 cm (0.37) and 200 cm (0.42), and strong negative correlations with the soil temperature (−0.94), air temperature at 30 cm (−0.84) and at 200 cm (−0.81). The number of fungi in 2015 did not have a strong correlation to any of the biotic or abiotic factors. The abiotic variables have multiple significant correlations among them, indicating their interdependence and diverse impact patterns.
In 2017, the DHA had strong positive correlations with the number of fungi (0.53) and the soil temperature (0.63), and negative correlations with the air humidity at 30 cm (−0.6) and 200 cm (−0.54). The number of bacteria had negative correlations with the number of fungi (−0.55) and actinomycetes (−0.48) with the soil humidity (−0.51). The number of actinomycetes had a strong positive correlation with the number of fungi (0.94). The number of fungi had a positive correlation with the soil humidity (0.58). The abiotic variables in 2017 had multiple strong positive and negative correlations among them, indicating their interdependence and diverse impact patterns.
4. Discussion
Soil microorganisms are impacting organic matter decomposition and as such are of exceptional importance for soil characteristics. Bacteria, fungi, algae, protozoa, viruses and lichens live in the soil, which actively participate in the creation and evolution of the soil and in achieving its fertility [
13]. Bacteria in forest soils contribute to the decomposition of organic matter and the recycling of nutrients in ecosystems and constitute one of the most diverse habitats on Earth [
29].
Due to the important role of microorganisms in the soil, it is important to monitor their number as well as the activity of the dehydrogenase enzyme. Soil microorganisms in temperate deciduous forests are manifesting large seasonal variations in environmental conditions, such as temperature and moisture [
29]. Enzymatic activity reflects the intensity of the processes taking place in the soil and is, therefore, used as an indicator of general biogenicity and soil fertility [
7,
15,
30]. The number of bacteria, actinomycetes and fungi in the soil are conditioned by ecological factors such as the air and soil temperature and humidity, and land management practices [
31]. All the previous findings listed above correspond with the results of this research.
In 2014, significant positive correlations were present within the group of abiotic variables, the number of bacteria, actinomycetes and fungi, which was negatively correlated to abiotic changes, and the DHA was strongly positively correlated to the number of microorganisms. These findings suggest that soil microorganisms are stressed by hot, dry weather, which lowers microbial activity and impacts DHA. The result corresponds with previous research findings [
17,
32].
Significant correlations between the abiotic group’s variables were observed in 2015. Only the strong positive correlation between the number of bacteria and actinomycetes is significant within the group of biotic variables. There is not a significant correlation between the number of fungi and any of the biotic or abiotic variables. The DHA is solely influenced by abiotic variables and does not significantly correlate with any microorganisms. This finding, which contradicts earlier studies conducted in the same region [
17,
22,
33], can be explained by the precipitation occurring more frequently during the vegetational period and in higher amounts each year.
In 2016, significant correlations were present within the abiotic group of variables, and within the biotic group number of fungi and bacteria, which were strongly positively correlated. The number of actinomycetes had strong negative correlations with air and soil temperatures, and a strong positive correlation with soil humidity, indicating their high variability due to natural conditions variations. There was no significant correlation between the number of fungi and any of the biotic or abiotic variables. Strong positive relationships between the DHA and the number of bacteria and fungi indicate that more bacteria lead to increased enzymatic activity, a hypothesis supported by other studies [
29,
34].
Similar to earlier years of this study, 2017 revealed a variety of significant correlations within the group of abiotic variables, with soil humidity having a particularly strong positive impact on the number of fungi. The number of fungi in the biotic factors category had a strong negative correlation with bacteria and a strong positive correlation with actinomycetes. This suggests that because fungi and actinomycetes can generate spores, a humid climate with fewer soil bacteria offers ideal circumstances for their emergence [
3,
9,
10]. Seasonal environmental variations have an impact on the fungal population and, in turn, the dehydrogenase activity, as evidenced by the DHA’s high positive correlations with the number of fungi and soil temperature and negative correlations with air humidity.
Previous research indicates that the interactions among bacteria, actinomycetes, and fungi in forest soils enhance DHA, which is an important indicator of microbial activity and soil health [
12]. Also, the bacteria are considered as most influential type of soil microorganisms for DHA, predominantly found in the uppermost layers of soil and exposed to various environmental conditions [
1,
2], and hence condition actinomycetes and fungi. Bacterial decrease in humid environment enables an increase in actinomycetes and fungi, due to their natural abilities, and promotes decomposing of organic materials resistant to bacterial degradation [
3,
10]. Strong correlations were found in this study between abiotic variables and soil microorganisms (bacteria, actinomycetes, and fungi), which together affect the dehydrogenase activity, corresponding with the outcomes of the above-mentioned previous studies.
5. Conclusions
In summary, the extensive analysis conducted over the four-year period from 2014 to 2017 has revealed intricate relationships between climate factors and soil microbial communities, as well as their respective activities.
The key findings indicate that climate conditions, particularly variations in air temperature and humidity, significantly impact the distribution and activity of soil microorganisms, including bacteria, actinomycetes, and fungi. The dehydrogenase enzyme activity, serving as an indicator of microbial function, underscores the vital role of environmental factors in shaping soil health and microbial dynamics. The activity of dehydrogenase enzymes is generally positively impacted by soil bacteria and actinomycetes, although the effect of fungi varies.
The observed correlations between microbial populations and abiotic conditions highlight the complexity of soil ecosystems and their sensitivity to climate variations. For instance, the notable variations in microbial number throughout the study period, particularly during extreme seasonal conditions, emphasize the need for ongoing monitoring to forecast ecological responses to climate change. Overall, this study not only enriches our understanding of soil biology but also calls for concerted efforts to preserve microbial diversity and soil health, which are critical for sustainable land management. By fostering a deeper connection between climate awareness and soil health, we can progress towards mitigating the impacts of climate change and ensuring the resilience of our ecosystems for future generations.