Intra-Annual Growth Dynamics and Environmental Response of Leaves, Shoots and Stems in Quercus serrata on Lushan Mountain, Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Tree Selection
2.2. Measurements of Leaves’ and Shoots’ Growth and Phenology Observation
2.3. Monitoring of Stem Radius Variations
2.4. Microenvironment Data
2.5. Modelling of Intra-Annual Leaf, Shoot and Stem Growth and Determination of Phenological Phases
2.6. Statistical Analysis
3. Results
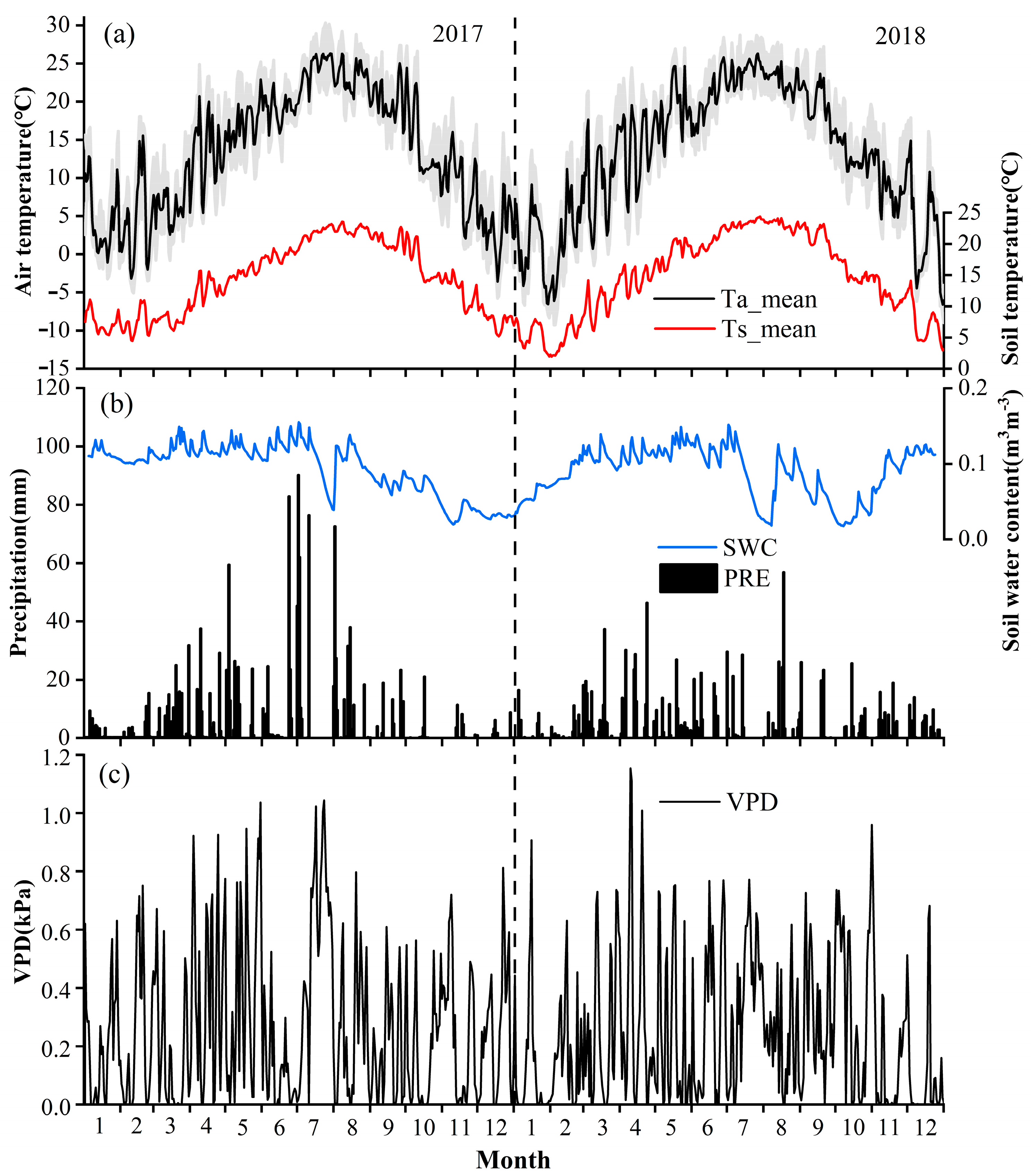
3.1. Environmental Conditions
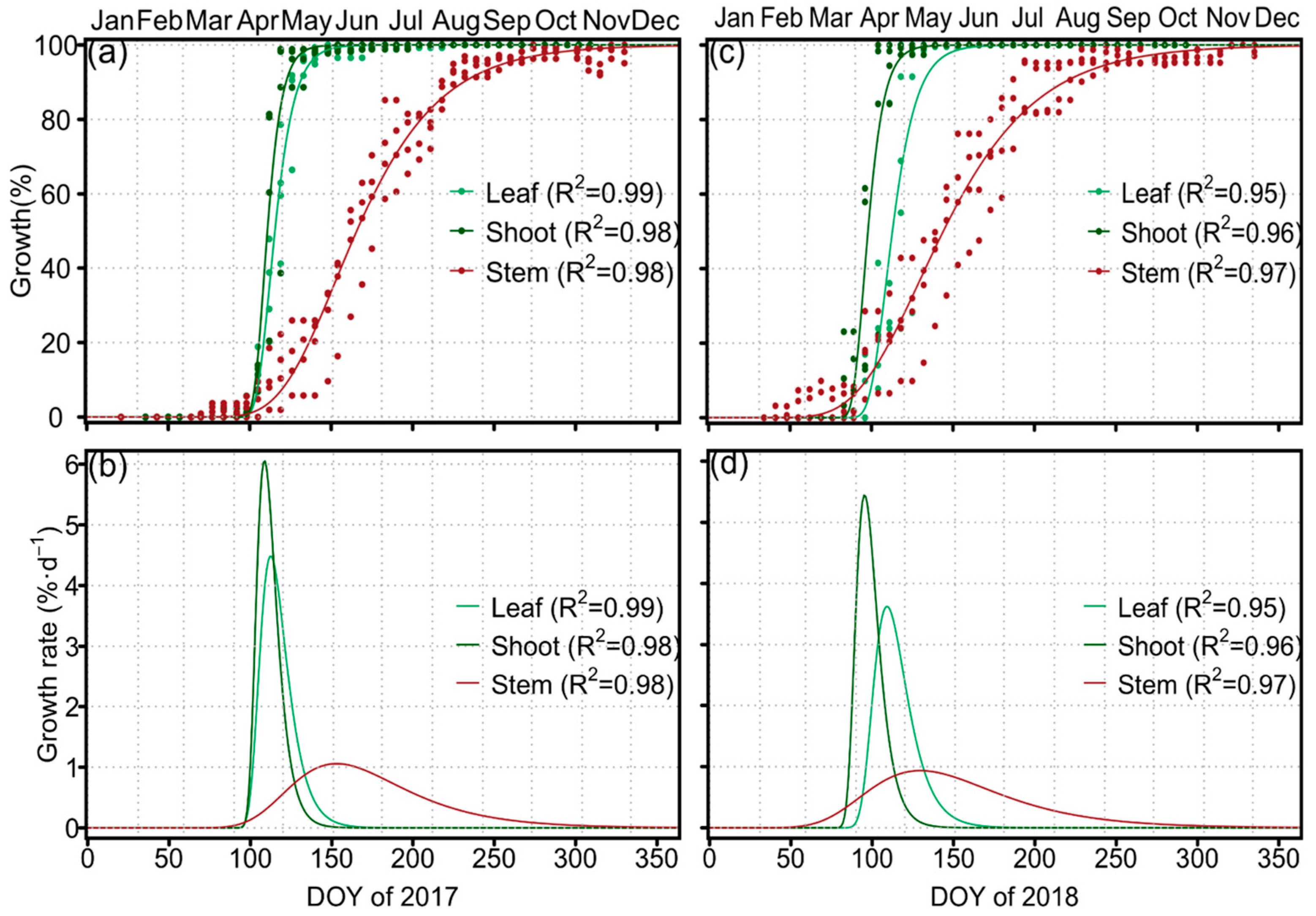
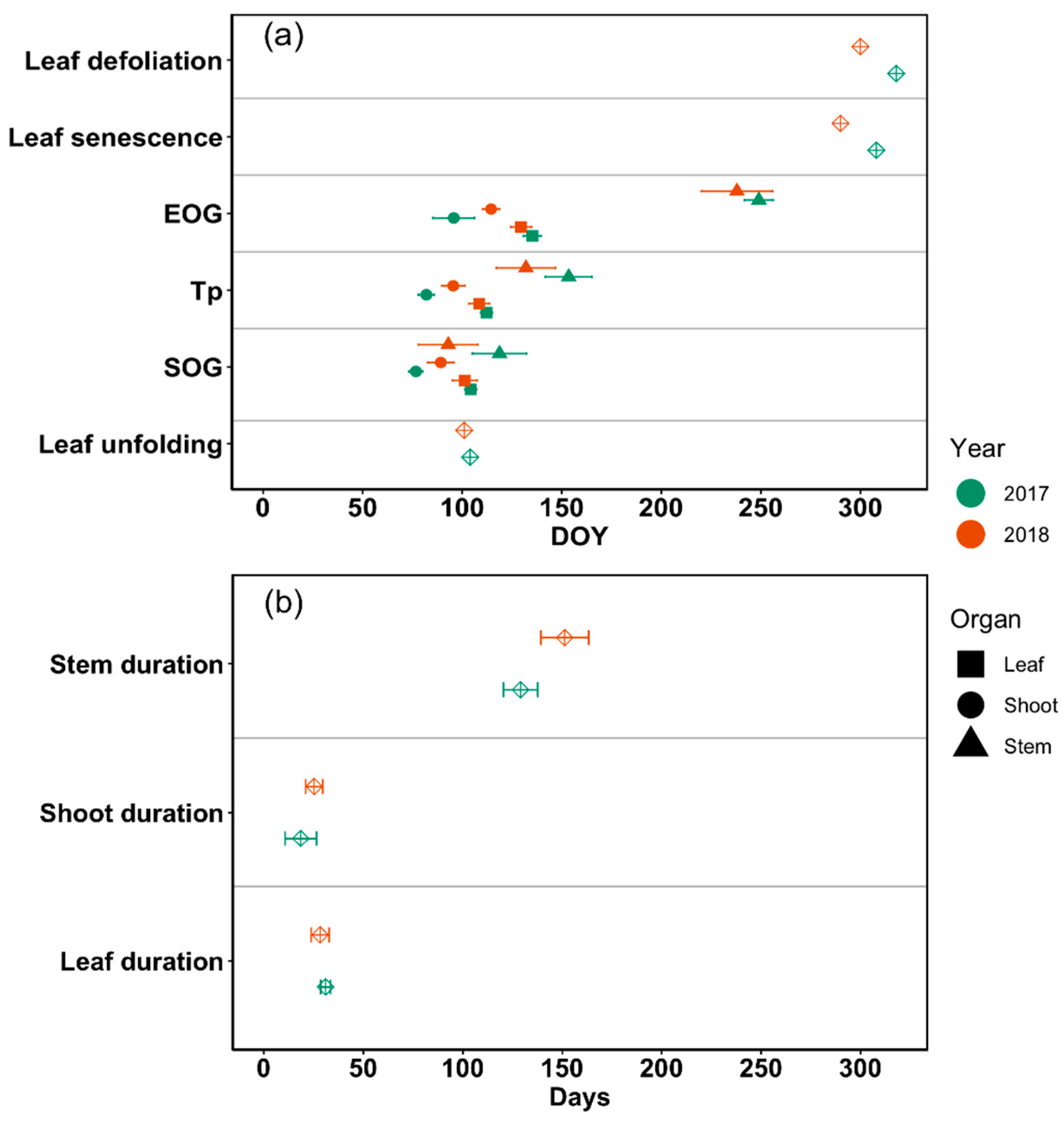
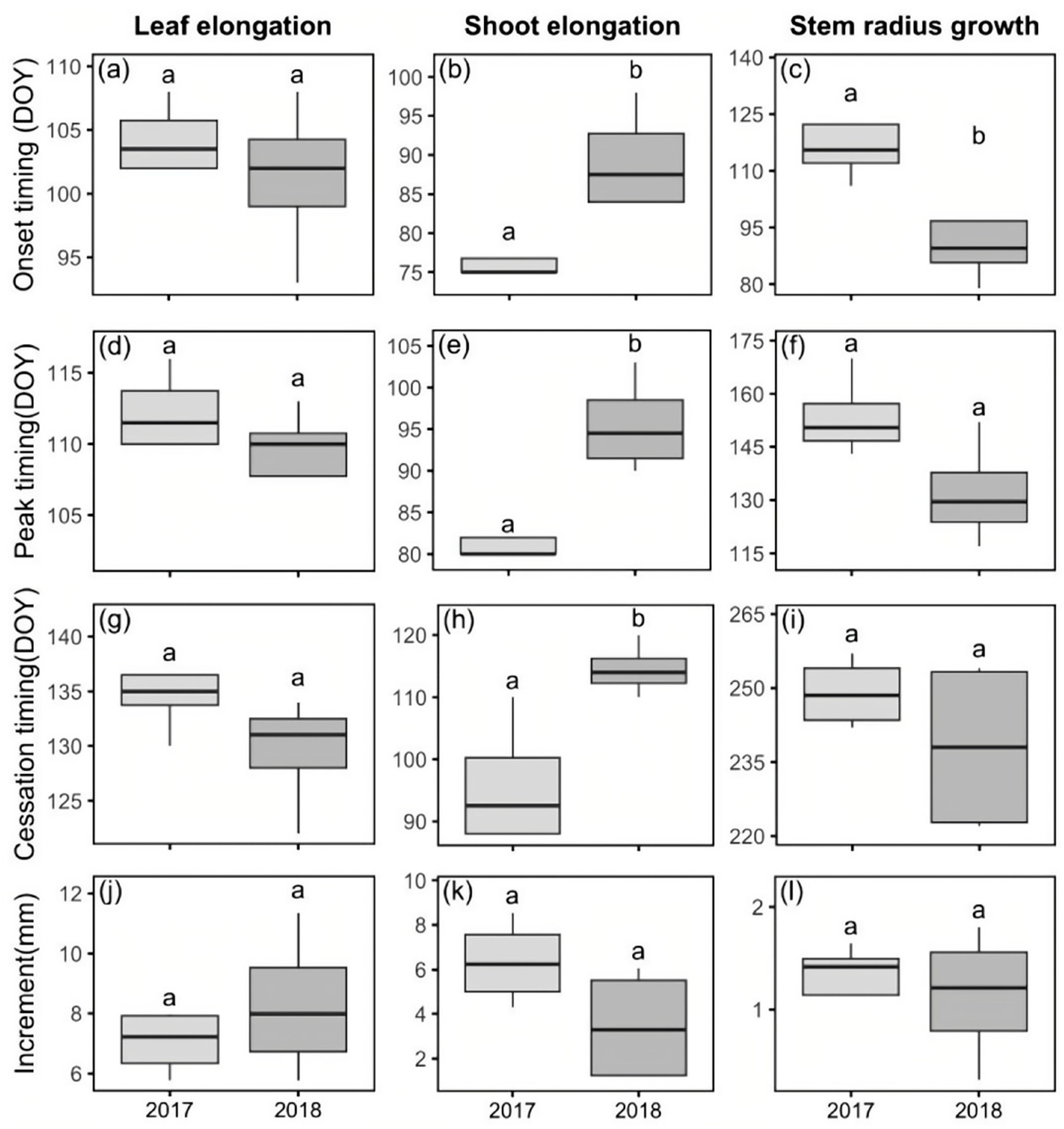
3.2. Seasonal Growth Dynamics of Leaf, Shoot and Stem and Organ Phenology
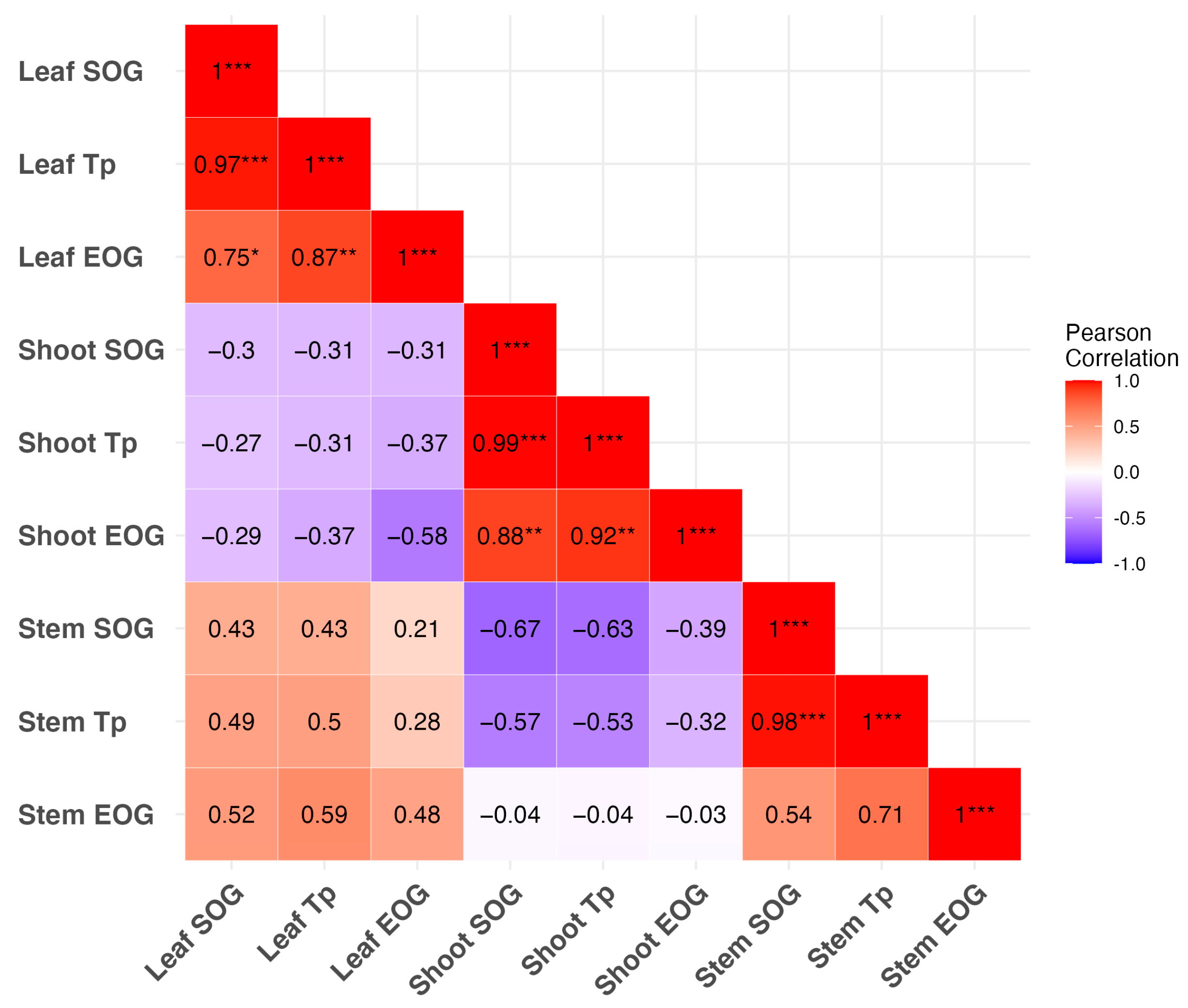
3.3. Temporal Correlations of Critical Phenological Phases Among Different Organs
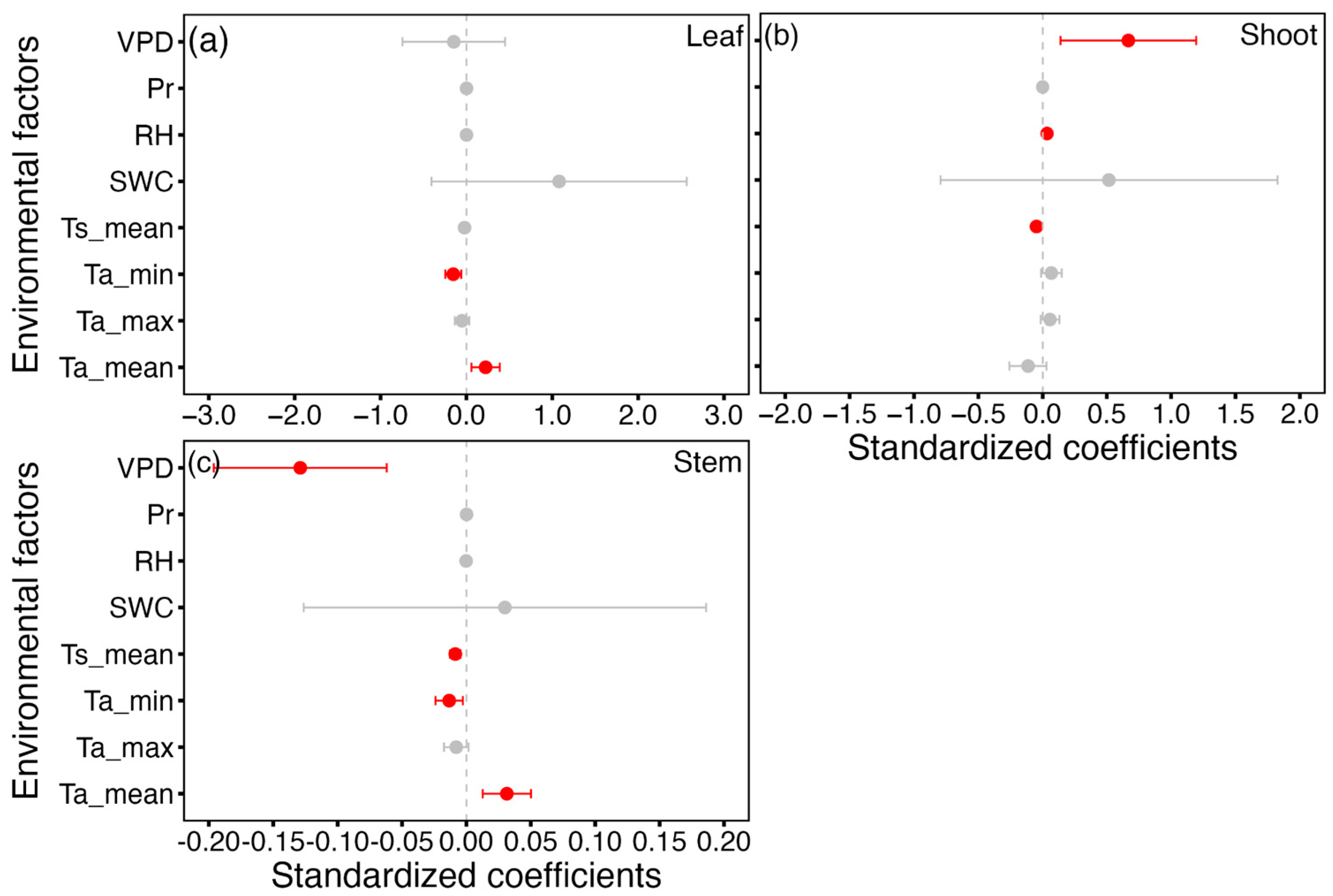
3.4. Response of Leaf, Shoot and Stem Growth to Environmental Variables
4. Discussion
4.1. Temporal Correlations of Phenological Events Differ Between Tree Organs
4.2. Inter-Annual Environment Variation and Growth Difference Between Years
4.3. Growth–Environment Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Deslauriers, A.; Rossi, S. Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytol. 2014, 203, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Delpierre, N.; Vitasse, Y.; Chuine, I.; Guillemot, J.; Bazot, S.; Rutishauser, T.; Rathgeber, C.B. Temperate and boreal forest tree phenology: From organ-scale processes to terrestrial ecosystem models. Ann. For. Sci. 2016, 73, 5–25. [Google Scholar] [CrossRef]
- Savidge, R. Intrinsic regulation of cambial growth. J. Plant. Growth. Regul. 2001, 20, 52–77. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Bégin, Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can. J. For. Res. 2003, 33, 190–200. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Camarero, J.; Liang, E.; Čufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ziaco, E.; Biondi, F.; Rossi, S.; Deslauriers, A. Environmental drivers of cambial phenology in Great Basin bristlecone pine. Tree Physiol. 2016, 36, 818–831. [Google Scholar] [CrossRef]
- Cartenì, F.; Deslauriers, A.; Rossi, S.; Morin, H.; De Micco, V.; Mazzoleni, S.; Giannino, F. The physiological mechanisms behind the earlywood-to-latewood transition: A process-based modeling approach. Front. Plant. Sci. 2018, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; van Groenigen, K.J.; Gillespie, M.A.; Hollister, R.D.; Post, E.; Cooper, E.J.; Welker, J.M.; Huang, Y.; Min, X.; Chen, J.; et al. Diminishing warming effects on plant phenology over time. New Phytol. 2024, 245, 2. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Rathgeber, C.B.K.; Deslauriers, A. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann. For. Sci. 2009, 66, 1–8. [Google Scholar] [CrossRef]
- Silvestro, R.; Mencuccini, M.; García-Valdés, R.; Antonucci, S.; Arzac, A.; Biondi, F.; Buttò, V.; Camarero, J.J.; Campelo, F.; Cochard, H.; et al. Partial asynchrony of coniferous forest carbon sources and sinks at the intra-annual time scale. Nat. Commun. 2024, 15, 6169. [Google Scholar] [CrossRef] [PubMed]
- Tumajer, J.; Kašpar, J.; Kuželová, H.; Shishov, V.V.; Tychkov, I.I.; Popkova, M.I.; Vaganov, E.A.; Treml, V. Forward modeling reveals multidecadal trends in cambial kinetics and phenology at treeline. Front. Plant. Sci. 2021, 12, 613643. [Google Scholar] [CrossRef] [PubMed]
- Cabon, A.; Peters, R.L.; Fonti, P.; Martínez-Vilalta, J.; De Cáceres, M. Temperature and water potential co-limit stem cambial activity along a steep elevational gradient. New Phytol. 2020, 226, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, S.; Rossi, S.; Deslauriers, A.; Lombardi, F.; Marchetti, M.; Tognetti, R. Synchronisms and correlations of spring phenology between apical and lateral meristems in two boreal conifers. Tree Physiol. 2015, 10, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Camarero, J.J.; Case, B.; Liang, E.; Rossi, S. The onset of xylogenesis is not related to distance from the crown in Smith fir trees from the southeastern Tibetan Plateau. Can. J. For. Res. 2016, 46, 885–889. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Xue, F.; Zhang, Y.; Wang, M.; Mang, M.; Xu, H. Intra-annual growth dynamics of Picea meyeri needles, shoots, and stems on Luya Mountain, North-central China. Trees 2021, 35, 637–648. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.; Lebourgeois, F.; Fortin, M.; Fournier, M. Life strategies in intra-annual dynamics of wood formation: Example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012, 32, 612–625. [Google Scholar] [CrossRef]
- Moser, L.; Buentgen, U.; Franzen, J.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010, 30, 225–233. [Google Scholar] [CrossRef]
- Bryant, K.N.; Fredericksen, B.W.; Rosenthal, D.M. Ring- and diffuse-porous species exhibit a spectrum of hydraulic behaviors from isohydry to anisohydry in a temperate deciduous forest. Trees 2022, 36, 485–495. [Google Scholar] [CrossRef]
- Leuschner, C.; Wedde, P.; Lübbe, T. The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann. For. Sci. 2019, 76, 60. [Google Scholar] [CrossRef]
- Čufar, K.; Prislan, P.; de Luis, M.; Gričar, J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees 2008, 22, 749–758. [Google Scholar] [CrossRef]
- Zhai, L.; Bergeron, Y.; Huang, J.; Berninger, F. Variation in intra-annual wood formation, and foliage and shoot development of three major Canadian boreal tree species. Am. J. Bot. 2012, 99, 827–837. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef]
- Yin, X.; Sass-Klaassen, U.; Hao, G.; Sterck, F. Ring-and diffuse-porous tree species coexisting in cold and humid temperate forest diverge in stem and leaf phenology. Dendrochronologia 2024, 86, 126220. [Google Scholar] [CrossRef]
- Downes, G.; Beadle, C.; Worledge, D. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees 1999, 14, 102–111. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Wen, F. Seasonal dynamics of stem radial increment of Pinus taiwanensis Hayata and its response to environmental factors in the Lushan Mountains, Southeastern China. Forests 2018, 9, 387. [Google Scholar] [CrossRef]
- Zweifel, R.; Sterck, F.; Braun, S.; Buchmann, N.; Eugster, W.; Gessler, A.; Matthias, H.; Richard, L.P.; Walthert, L.; Wilhelm, M.; et al. Why trees grow at night. New Phytol. 2021, 231, 2174–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sterck, F.; Kruijt, B.; Fan, Z.; Zuidema, P. Diel and seasonal stem growth responses to climatic variation are consistent across species in a subtropical tree community. New Phytol. 2023, 240, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, A.; Morin, H.; Krause, C.; Deslauriers, A.; Thibeault-Martel, M. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agric. Forest. Meteorol. 2009, 149, 1403–1409. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Deslauriers, A.; Bräuning, A. Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees 2015, 29, 25–34. [Google Scholar] [CrossRef]
- Luo, T.; Liu, X.; Zhang, L.; Li, X.; Pan, Y.; Wright, I.J. Summer solstice marks a seasonal shift in temperature sensitivity of stem growth and nitrogen use efficiency in cold-limited forests. Agric. For. Meteorol. 2018, 24, 469–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wen, Y.; Ding, X.; Wang, B.; Xu, J. Comparing Primary and Secondary Growth of Co-Occurring Deciduous and Evergreen Conifers in an Alpine Habitat. Forests 2019, 10, 574. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef]
- Huang, J.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeberi, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Zhao, J. Seasonal Drought Effects on Intra-Annual Stem Growth of Taiwan Pine along an Elevational Gradient in Subtropical China. Forests 2019, 10, 1128. [Google Scholar] [CrossRef]
- Zhang, J.; Gou, X.; Pederson, N.; Zhang, F.; Niu, H.; Zhao, S.; Wang, F. Cambial phenology in Juniperus przewalskii along different altitudinal gradients in a cold and arid region. Tree Physiol. 2018, 36, 840–852. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Rossi, S.; Čufar, K.; Ellison, A.M. Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Sci. Bull. 2017, 62, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Fu, X.; Zhao, B.; Dai, X.; Li, Q.; Yang, F.; Kou, L.; Wang, H. Intra-annual radial growth and its climate response for Masson pine and Chinese fir in subtropical China. Trees 2021, 35, 1817–1830. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, F.; Fonti, P.; Ren, P.; Li, X.; Miao, G.; Dong, Z.; Fang, K. Intra-Annual Wood Formation of Cryptomeria fortunei and Cunninghamia lanceolata in Humid Subtropical China. Front. Ecol. Evol. 2021, 9, 733974. [Google Scholar] [CrossRef]
- Dow, C.; Kim, A.; D’Orangeville, L.; Gonzalez-Akre, E.B.; Helcoski, R.; Herrmann, V.; Harley, G.L.; Maxwell, J.T.; McGregor, I.R.; McShea, W.J.; et al. Warm springs alter timing but not total growth of temperate deciduous trees. Nature 2022, 608, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wei, X.; Wu, Y.; Liu, S.; Huang, Y.; Yan, J.; Zhang, D.; Zhang, Q.; Liu, J.; Meng, Z.; et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Change Biol. 2011, 17, 3736–3746. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Yan, W.; Xia, J.; Xiang, W.; Wang, K.; Luo, Q.; Fu, W.; Yuan, W. Climate change and consequences on the water cycle in the humid Xiangjiang River Basin, China. Stoch. Environ. Res. Risk Assess. 2016, 30, 225–235. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, C.; Li, Y.; Liu, S.; Zhang, Q.; Tang, X.; Liu, J.; Yan, J.; Zhang, D.; Chu, G. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob. Change Biol. 2013, 19, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, Q.; Lv, L.; Zhang, C. Regional-scale winter-spring temperature variability and chilling damage dynamics over the past two centuries in southeastern China. Clim. Dyn. 2012, 39, 919–928. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y.; Duan, B.; Sun, C. Regional difference of the start time of the recent warming in Eastern China: Prompted by a 165-year temperature record deduced from tree rings in the Dabie Mountains. Clim. Dyn. 2018, 50, 2157–2168. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.; Wei, W.; Yu, S.; Zhang, T. Reconstructed temperature for Yong’an, Fujian, Southeast China: Linkages to the Pacific Ocean climate variability. Glob. Planet Change 2012, 86, 11–19. [Google Scholar] [CrossRef]
- Cao, X.; Kao, P.; Hu, H.; Zhou, F.; Zhang, D.; Fang, K. Reconstruction of seasonal precipitation anomalies from tree-ring latewood records in southeastern China. Clim. Dyn. 2024, 62, 2439–2454. [Google Scholar] [CrossRef]
- Aldea, J.; Ruiz-Peinado, R.; del Río, M.; Pretzsch, H.; Heym, M.; Brazaitis, G.; Jansons, A.; Metslaid, M.; Barbeito, I.; Bielak, K.; et al. Timing and duration of drought modulate tree growth response in pure and mixed stands of Scots pine and Norway spruce. J. Ecol. 2022, 110, 2673–2683. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Deng, Y.; Huang, Q.; Yuan, Y.; Shi, X.; Gou, X. Seasonal aridity regulates drivers and temporal variability of wood phenology: A meta-analysis of dendrometer monitoring data across the Northern Hemisphere. Dendrochronologia 2024, 85, 126201. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, Z.; Cherubini, P.; Cooper, D.J.; Zhu, L.; Lei, P. Tree-ring data reveal trees are suffering from severe drought stress in the humid subtropical forest. For. Ecol. Manag. 2023, 546, 121330. [Google Scholar] [CrossRef]
- Zhang, J. The Vertical Zonation of Vegetation on Mount Lushan. J. S. China Norm. Univ. (Nat. Sci. Ed.) 1982, 1, 1–14. Available online: https://kns.cnki.net/kcms2/article/abstract?v=YvzTZTPVeK44_kpvMTaoDCWKoYH84dyk_rDPh02C-fT-lJ4tsNADlcaOiobwXUvYu_-7BFEOsIFQwjPsjghcZeQZ7pOip-xzh67ZY-CGOvV854yNL81gKZCxOoAPnD9I8JcoKOICl_7wLGYBnOcd4K3nb5wML45XBTO-Hd-UdzCCw6LlmbOEuwahJaOaScyi&uniplatform=NZKPT&language=CHS (accessed on 2 July 2024). (In Chinese).
- Wang, J.; Lu, Z.; Wu, J.; Xiao, Q.; Zhu, M.; Wang, H. Characteristics and Distribution Patterns of Soil Types in Lushan Mountain. Acta Agr. Univ. Jiangxiensis 2010, 32, 1284–1290. (In Chinese) [Google Scholar] [CrossRef]
- Tang, Z.; Chambers, J.L.; Guddanti, S.; Yu, S.; Barnett, J.P. Seasonal shoot and needle growth of loblolly pine responds to thinning, fertilisation, and crown position. For. Ecol. Manag. 1999, 120, 117–130. [Google Scholar] [CrossRef]
- Xing, P.; Zhang, Q.; Baker, P.J. Age and radial growth pattern of four tree species in a subtropical forest of China. Trees 2012, 26, 283–290. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and microclimate: A quantitative approach to environmental plant physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Rathgeber, C.B.; Rossi, S.; Bontemps, J.D. Cambial activity related to tree size in a mature silver-fir plantation. Ann. Bot. 2011, 108, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, Z.; Lin, Y.; Kaewmano, A.; Wei, X.; Fu, P.; Grießinger, J.; Bräuning, A. Impact of extreme pre-monsoon drought on xylogenesis and intra-annual radial increments of two tree species in a tropical montane evergreen broad-leaved forest, southwest China. Tree Physiol. 2024, 44, tpae086. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2022, 13, 2. Available online: https://www.R-project.org/ (accessed on 2 June 2024).
- Yin, X.; Hao, G.; Sterck, F. A trade-off between growth and hydraulic resilience against freezing leads to divergent adaptations among temperate tree species. Funct. Ecol. 2022, 36, 739–750. [Google Scholar] [CrossRef]
- Palacio, S.; Camarero, J.J.; Maestro, M.; Alla, A.Q.; Lahoz, E.; Montserrat-Martí, G. Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees 2018, 32, 777–790. [Google Scholar] [CrossRef]
- Gričar, J.; Jevšenak, J.; Hefner, P.; Prislan, P.; Ferlan, M.; Lavrič, M.; Vodnik, D.; Eler, K. Climatic regulation of leaf and cambial phenology in Quercus pubescens: Their interlinkage and impact on xylem and phloem conduits. Sci. Total. Environ. 2022, 802, 149968. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.Y.; Louvet, J.M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–774. [Google Scholar] [CrossRef]
- Rossi, S.; Girard, M.J.; Morin, H. Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Glob. Change Biol. 2014, 20, 2261–2271. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Kocher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.; Frank, D.C. Twentieth century redistribution in climatic drivers of global tree growth. Sci. adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef]
- Helcoski, R.; Tepley, A.J.; Pederson, N.; McGarvey, J.C.; Meakem, V.; Herrmann, V.; Thompson, J.R.; Anderson-Teixeira, K.J. Growing season moisture drives interannual variation in woody productivity of a temperate deciduous forest. New Phytol. 2019, 223, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.; Guo, Y.; Kang, M.; Wang, M.; Wang, B. Intra-annual xylem growth of Larix principis-rupprechtii at its upper and lower distribution limits on the Luyashan mountain in North-Central China. Forests 2015, 6, 3809–3827. [Google Scholar] [CrossRef]
- Tumajer, J.; Scharnweber, T.; Smiljanic, M.; Wilmking, M. Limitation by vapor pressure deficit shapes different intra-annual growth patterns of diffuse and ring-porous temperate broadleaves. New Phytol. 2022, 233, 2429–2441. [Google Scholar] [CrossRef]
- Zhang, X.; Rademacher, T.; Liu, H.; Wang, L.; Manzanedo, R. Fading regulation of diurnal temperature ranges on drought-induced growth loss for drought-tolerant tree species. Nat. Commun. 2023, 14, 6916. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Biondi, F. Inter-specific transpiration differences between aspen, spruce, and pine in a sky-island ecosystem of the North American Great Basin. For. Ecol. Manage 2021, 491, 119157. [Google Scholar] [CrossRef]
- Liu, X.; Ziaco, E.; Biondi, F. Stomatal regulation and xylem hydraulics of limber pine and Engelmann spruce in Great Basin sky-island ecosystems. Sci. Total Environ. 2023, 892, 164351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hai, X.; Xu, J.; Wu, W.; Cao, P.; An, W. Seasonal dynamics of non-structural carbohydrate content in branch of Quercus variabilis growing in east Qinling Mountain range. Chin. J. Plant Ecol. 2019, 43, 521–531. (In Chinese) [Google Scholar] [CrossRef]
- Shi, J.; Cook, E.R.; Lu, H.; Li, J.; Wright, W.; Li, S. Tree-ring based winter temperature reconstruction for the lower reaches of the Yangtze River in southeast China. Clim. Res. 2010, 41, 169–175. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Fang, K.; Zhang, P.; Chen, D.; Brauning, A.; Grießinger, J.; Yang, B.; Liang, C.; Zhang, H.; et al. Deciphering the transfer of hydroclimate signals to tree-ring δ18O using a proxy system model in East Asia’s Meiyu region. Chem. Geol. 2024, 668, 122350. [Google Scholar] [CrossRef]

| Species | Tree no. | DBH (cm) | Height (m) | Age (yr.) c |
|---|---|---|---|---|
| Quercus serrata | 1 a | 16.4 | 8.2 | 64 |
| 2 a | 16.3 | 6.7 | 63 | |
| 3 b | 20.4 | 12.2 | 80 | |
| 4 b | 21.1 | 7.5 | 82 |
| Months | Year | Ta (°C) | Ts (°C) | Pr (mm) | SWC (m3 m−3) | VPD (kPa) |
|---|---|---|---|---|---|---|
| Jan–Dec | 2017 | 13.3 | 0.17 | 1481 | 0.17 | 0.26 |
| 2018 | 13.1 | 0.17 | 1167 | 0.15 | 0.24 | |
| Mar–Jun | 2017 | 14.1 | 0.20 | 710 | 0.21 | 0.26 |
| 2018 | 15.9 | 0.19 | 559 | 0.18 | 0.27 | |
| Jul–Sep | 2017 | 22.5 | 0.17 | 595 | 0.15 | 0.33 |
| 2018 | 22.1 | 0.16 | 280 | 0.11 | 0.30 |
| Organ | Year | A | β | κ | y0 | R2 | Rmax (mm d−1) | Rmean (mm d−1) |
|---|---|---|---|---|---|---|---|---|
| Leaf | 2017 | 7.033 ± 1.078 | 1.164 ± 0.024 | 0.129 ± 0.009 | 0.009 ± 0.012 | 0.999 | 0.333 ± 0.059 | 0.203 ± 0.035 |
| 2018 | 8.221 ± 2.404 | 1.199 ± 0.077 | 0.147 ± 0.024 | 0.051 ± 0.042 | 0.997 | 0.449 ± 0.160 | 0.270 ± 0.010 | |
| Shoot | 2017 | 7.995 ± 5.843 | 1.336 ± 0.219 | 0.265 ± 0.114 | 0.426 ± 0.071 | 0.997 | 0.894 ± 0.778 | 0.546 ± 0.475 |
| 2018 | 3.472 ± 2.593 | 6.881 ± 1.318 | 0.164 ± 0.024 | 0.329 ± 0.055 | 0.985 | 0.225 ± 0.183 | 0.138 ± 0.112 | |
| Stem | 2017 | 1.226 ± 0.552 | 2.082 ± 0.293 | 0.031 ± 0.002 | 1.806 ± 1.336 | 0.994 | 0.014 ± 0.007 | 0.006 ± 0.006 |
| 2018 | 1.180 ± 0.509 | 1.615 ± 0.207 | 0.026 ± 0.005 | 2.974 ± 1.136 | 0.994 | 0.012 ± 0.006 | 0.007 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, D.; Zhang, W.; Liu, X.; Zhao, Y.; Sun, L.; Zhang, S.; Chen, Z. Intra-Annual Growth Dynamics and Environmental Response of Leaves, Shoots and Stems in Quercus serrata on Lushan Mountain, Subtropical China. Forests 2025, 16, 305. https://doi.org/10.3390/f16020305
Fu D, Zhang W, Liu X, Zhao Y, Sun L, Zhang S, Chen Z. Intra-Annual Growth Dynamics and Environmental Response of Leaves, Shoots and Stems in Quercus serrata on Lushan Mountain, Subtropical China. Forests. 2025; 16(2):305. https://doi.org/10.3390/f16020305
Chicago/Turabian StyleFu, Dina, Wenpeng Zhang, Xinsheng Liu, Yesi Zhao, Lian Sun, Sirui Zhang, and Zilong Chen. 2025. "Intra-Annual Growth Dynamics and Environmental Response of Leaves, Shoots and Stems in Quercus serrata on Lushan Mountain, Subtropical China" Forests 16, no. 2: 305. https://doi.org/10.3390/f16020305
APA StyleFu, D., Zhang, W., Liu, X., Zhao, Y., Sun, L., Zhang, S., & Chen, Z. (2025). Intra-Annual Growth Dynamics and Environmental Response of Leaves, Shoots and Stems in Quercus serrata on Lushan Mountain, Subtropical China. Forests, 16(2), 305. https://doi.org/10.3390/f16020305






