Response of Leaf Non-Structural Carbohydrates to Elevation in Dioecious Plants, Populus cathayana and Hippophae rhamnoides
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling
2.3. Experimental Measurements
2.4. Statistical Analysis
3. Results
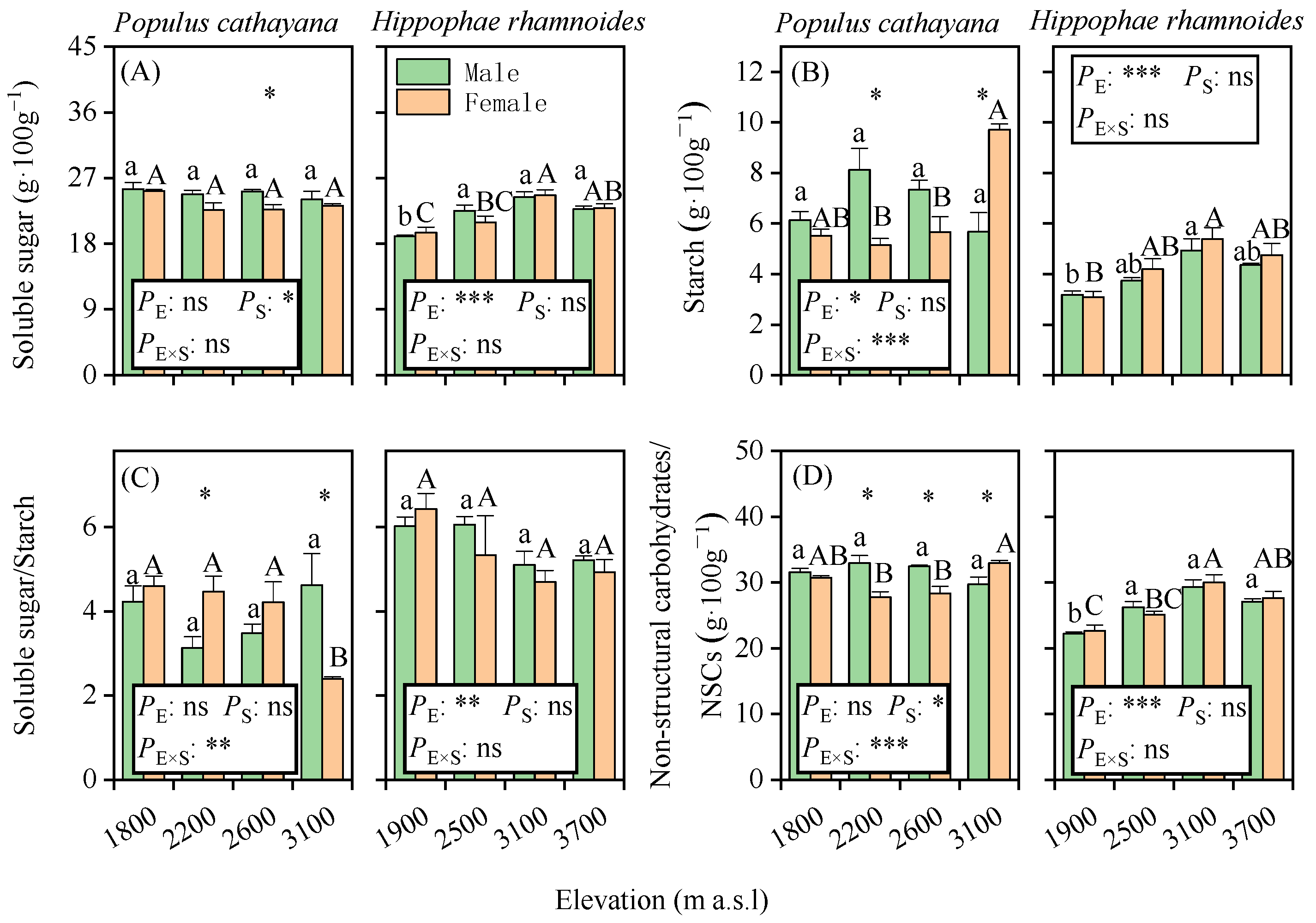
3.1. Elevational Patterns of Leaf NSCs, Starch, and Soluble Sugar Contents
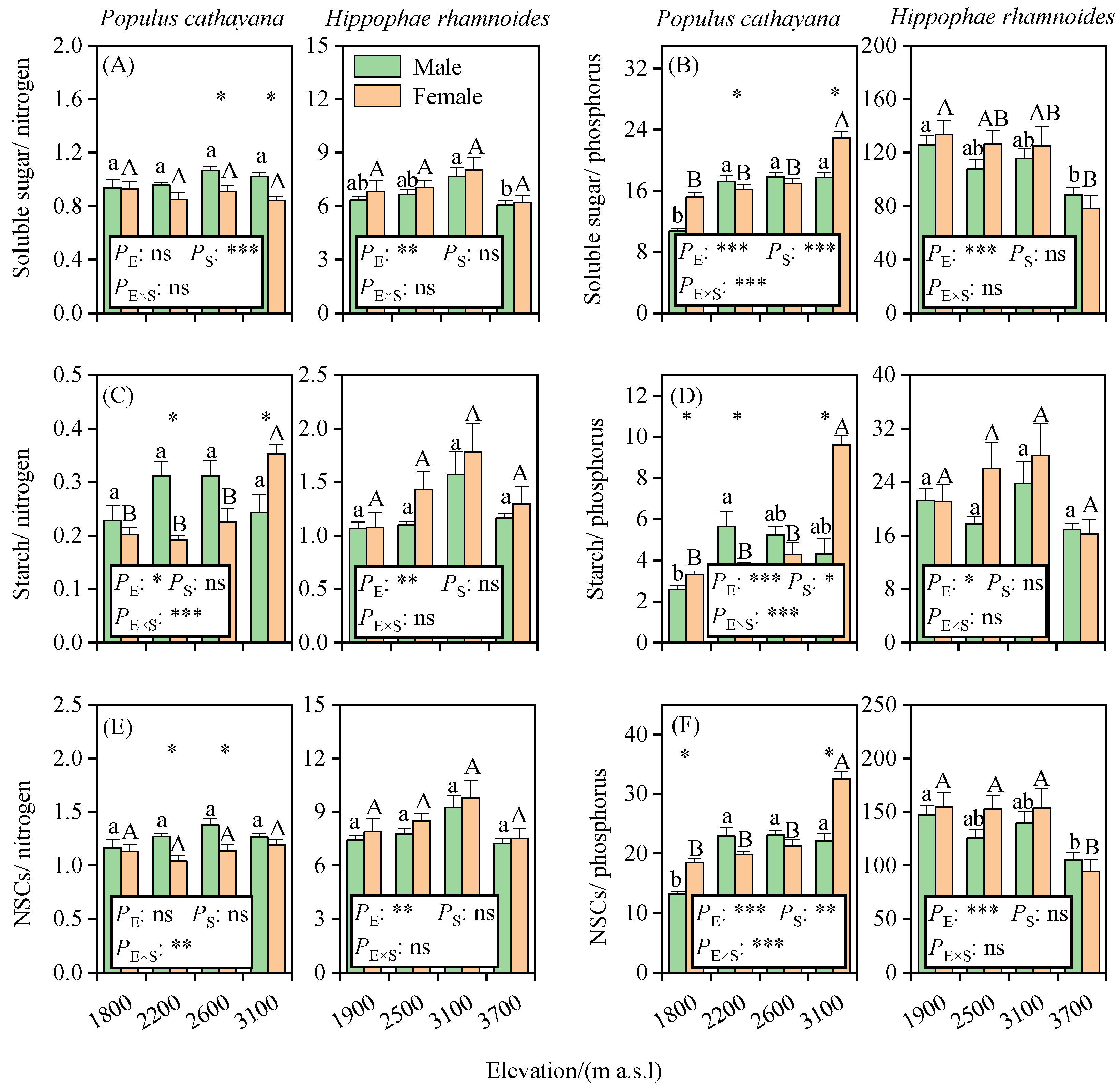
3.2. Elevational Patterns of Leaf Nitrogen and Phosphorus Concentrations
3.3. Elevational Patterns of the Ratios of Leaf Soluble Sugar, Starch, and NSCs to Nitrogen and Phosphorus
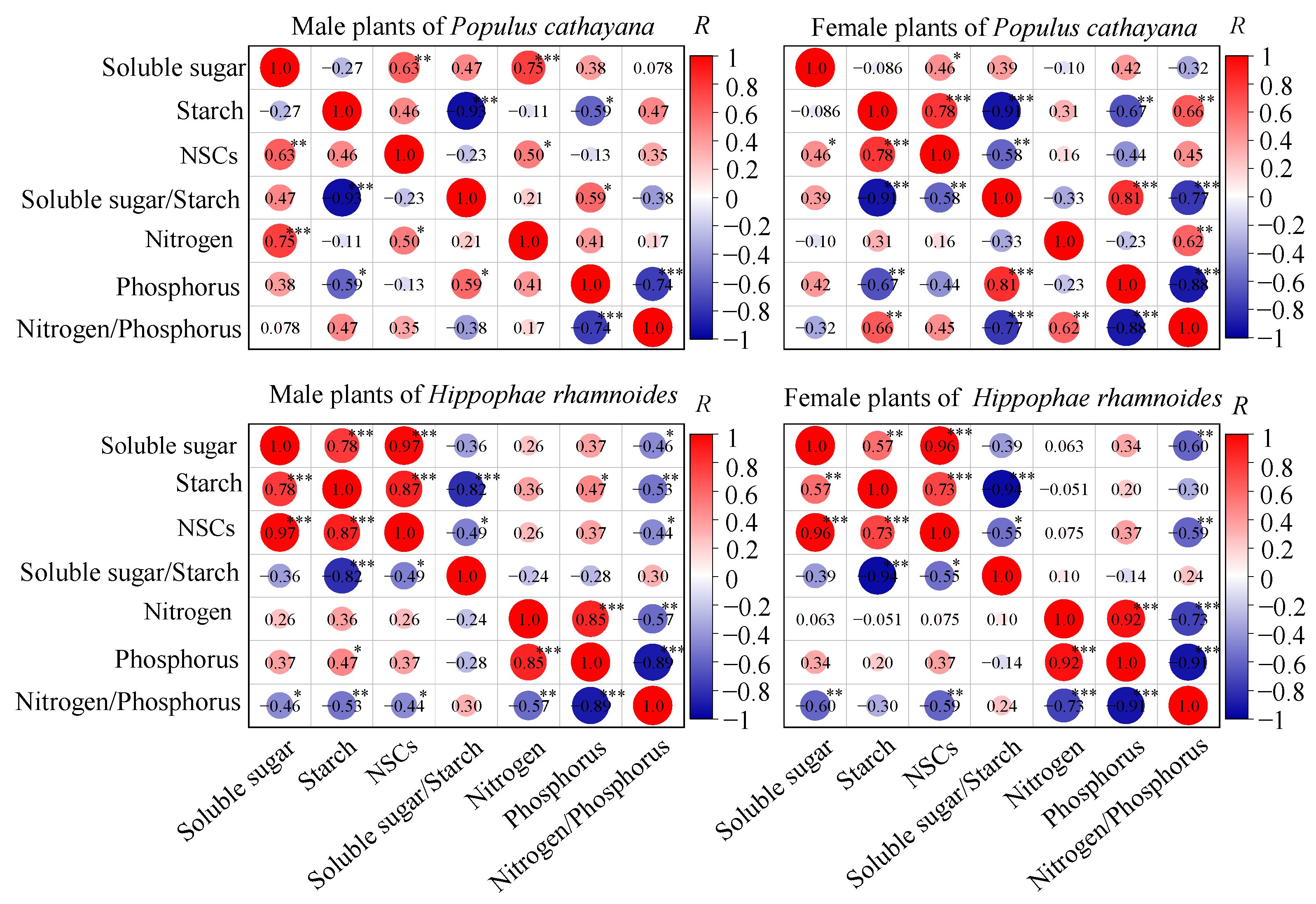
3.4. Correlations Between Soluble Sugar, Starch, and NSCs with Nitrogen and Phosphorus
4. Discussion
4.1. The Response of Leaf NSCs to Elevation Varied with Species
4.2. The Responses of Soluble Sugar and Starch to Elevation and Their Sex Differentiation Was Species-Specific
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.S.; Ding, H.H.; Li, J.R.; Fu, F.W.; Li, Y.Y.; Xiao, S.Y.; Xu, D.; Lu, J.; Fang, J.P. How do montane plants manage to survive? Inferring from non-structural carbohydrates. Trees-Struct. Funct. 2023, 37, 331–348. [Google Scholar] [CrossRef]
- Li, M.H.; Xiao, W.F.; Shi, P.L.; Wang, S.G.; Zhong, Y.D.; Liu, X.L.; Wang, X.D.; Cai, X.H.; Shi, Z.M. Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 2008, 31, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, H.; Zhang, Q.F.; Dang, H.S. Global patterns of mobile carbon partitioning in mountain trees in response to elevation. Environ. Exp. Bot. 2023, 208, 105248. [Google Scholar] [CrossRef]
- Zhang, P.P.; Ding, J.X.; Wang, Q.T.; McDowell, N.G.; Kong, D.L.; Tong, Y.D.; Yin, H.J. Contrasting coordination of non-structural carbohydrates with leaf and root economic strategies of alpine coniferous forests. New Phytol. 2024, 243, 580–590. [Google Scholar] [CrossRef]
- Jump, A.S.; Mátyás, C.; Peñuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef]
- Peng, Y.K.; Bloomfield, K.J.; Prentice, I.C. A theory of plant function helps to explain leaf-trait and productivity responses to elevation. New Phytol. 2020, 226, 1274–1284. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Wang, Q.W.; Qi, L.; Zhou, W.M.; Liu, C.G.; Yu, D.P.; Dai, L.M. Carbon dynamics in the deciduous broadleaf tree Erman’s birch (Betula ermanii) at the subalpine treeline on Changbai Mountain, Northeast China. Am. J. Bot. 2018, 105, 42–49. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Y.; He, Y.T.; Ma, S.Y.; Chu, Q.W.; Zhang, S. The ecological adaptability of dominant poplar species to alpine environments in the Yarlung Zangbo River Basin. J. Sichuan Univ. (Nat. Sci. Ed.) 2023, 60, 203–211. [Google Scholar]
- Zhou, Q.; Shi, H.; He, R.; Liu, H.K.; Zhu, W.T.; Yu, D.Y.; Zhang, Q.F.; Dang, H.S. Prioritized carbon allocation to storage of different functional types of species at the upper range limits is driven by different environmental drivers. Sci. Total Environ. 2021, 773, 145581. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, X.; Tognetti, R.; Lei, J.P.; Pan, H.L.; Liu, X.L.; Jiang, Y.; Wang, X.Y.; He, P.; Yu, F.H.; et al. Elevation alters carbon and nutrient concentrations and stoichiometry in Quercus aquifolioides in southwestern China. Sci. Total Environ. 2018, 622–623, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Arthur, F.M.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef]
- Charlesworth, B.; Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 1978, 112, 975–997. [Google Scholar] [CrossRef]
- Alvarez-Cansino, L.; Barradas, M.C.D.; Zunzunegui, M.; Esquivias, M.P.; Dawson, T.E. Gender-specific variation in physiology in the dioecious shrub Corema album throughout its distributional range. Funct. Plant Biol. 2012, 39, 968–978. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.H.; Duan, B.L.; Korpelainen, H.; Li, C.Y. Populus cathayana males exhibit more efficient protective mechanisms than females under drought stress. For. Ecol. Manag. 2012, 275, 68–78. [Google Scholar] [CrossRef]
- Chen, L.H.; Zhang, S.; Zhao, H.X.; Korpelainen, H.; Li, C.Y. Sex-related adaptive responses to interaction of drought and salinity in Populus yunnanensis. Plant Cell Environ. 2010, 33, 1767–1778. [Google Scholar] [CrossRef]
- Chen, J.; Duan, B.L.; Wang, M.; Korpelainen, H.; Li, C.Y. Intra- and inter-sexual competition of Populus cathayana under different watering regimes. Funct. Ecol. 2014, 28, 124–136. [Google Scholar] [CrossRef]
- Li, C.Y.; Jian, R.; Luo, J.X.; Lu, R.S. Sex-specific physiological and growth responses to water stress in Hippophae rhamnoides L. populations. Acta Physiol. Plant. 2004, 26, 123–129. [Google Scholar] [CrossRef]
- Juvany, M.; Munne-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 2015, 66, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Shi, Z.M.; Liu, S.; Centritto, M.; Cao, X.W.; Zhang, M.M.; Zhao, G.D. Photosynthetic capacity of male and female Hippophae rhamnoides plants along an elevation gradient in eastern Qinghai-Tibetan Plateau, China. Tree Physiol. 2021, 41, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Wang, Y.H.; Peng, Y.H.; Korpelainen, H.; Li, C.Y. Genetic diversity of Populus cathayana Rehd populations in southwestern china revealed by ISSR markers. Plant Sci. 2006, 170, 407–412. [Google Scholar] [CrossRef]
- Li, C.Y.; Xu, G.; Zang, R.G.; Korpelainen, H.; Berninger, F. Sex-related differences in leaf morphological and physiological responses in Hippophae rhamnoides along an altitudinal gradient. Tree Physiol. 2007, 27, 399–406. [Google Scholar] [CrossRef]
- Xu, G.X.; Chen, H.H.; Shi, Z.M.; Liu, S.; Cao, X.W.; Zhang, M.M.; Chen, M.; Chen, J.; Xiong, K.; Yang, H.G.; et al. Mycorrhizal and rhizospheric fungal community assembly differs during subalpine forest restoration on the eastern Qinghai-Tibetan Plateau. Plant Soil 2021, 458, 245–259. [Google Scholar] [CrossRef]
- Deng, X.X.; Xiao, W.F.; Shi, Z.; Zeng, L.X.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana Lamb. seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef]
- Körner, C. Carbon investments. In Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer International Publishing: Cham, Switzerland, 2021; pp. 309–333. [Google Scholar]
- Molina-Montenegro, M.A.; Gallardo-Cerda, J.; Flores, T.S.M.; Atala, C. The trade-off between cold resistance and growth determines the Nothofagus pumilio treeline. Plant Ecol. 2012, 213, 133–142. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Growth and carbon relations of tree line forming conifers at constant vs. variable low temperatures. J. Ecol. 2009, 97, 57–66. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2003; p. 337. [Google Scholar]
- Hoch, G.; Körner, C. Global patterns of mobile carbon stores in trees at the high-elevation tree line. Glob. Ecol. Biogeogr. 2012, 21, 861–871. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Deng, D.Z.; Guo, T.W.; Dmitrii, V. Response of nutrient content in the leaves and branchlets of Hippophae rhamnoides L. to altitude in western Sichuan PlateauII. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2019, 48, 392–397. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Technical report: Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavenderbares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Oleksyn, J.; Wright, I.J. Leaf phosphorus influences the photosynthesis-nitrogen relation: A cross-biome analysis of 314 species. Oecologia 2009, 160, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Yu, M.K.; Cheng, X.R. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Biochem. 2018, 124, 146–154. [Google Scholar] [CrossRef]
- He, J.S.; Fang, J.Y.; Wang, Z.H.; Guo, D.L.; Flynn, D.F.B.; Geng, Z. Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 2006, 149, 115–122. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Cao, P.H.; Xu, J.L.; Hai, X.Y.; Wu, W.X.; Jiao, B.W.; Shen, M.W.; Wang, R.F. Seasonal dynamics of non-structural carbohydrate contents in leaves of Quercus variabilis growing in the east Qinling Mountain range. Acta Ecol. Sin. 2019, 39, 7274–7282. [Google Scholar]
- Zhao, H.X.; Yan, L.; Duan, B.L.; Korpelainen, H.; Li, C.Y. Sex-related adaptive responses of Populus cathayana to photoperiod transitions. Plant Cell Environ. 2009, 32, 1401–1411. [Google Scholar] [CrossRef]
- Zhao, H.X.; Li, Y.P.; Zhang, X.L.; Korpelainen, H.; Li, C.Y. Sex-related and stage-dependent source-to-sink transition in Populus cathayana grown at elevated CO2 and elevated temperature. Tree Physiol. 2012, 32, 1325–1338. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, H.; Zhao, H.X.; Korpelainen, H.; Li, C.Y. Sexually different physiological responses of Populus cathayana to nitrogen and phosphorus deficiencies. Tree Physiol. 2014, 34, 343–354. [Google Scholar] [CrossRef]
- Liu, M.; Korpelainen, H.; Li, C.Y. Sexual differences and sex ratios of dioecious plants under stressful environments. J. Plant Ecol. 2021, 14, 920–933. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Gibert, A.; Gray, E.F.; Westoby, M.; Wright, I.J.; Falster, D.S. On the link between functional traits and growth rate: Meta-analysis shows effects change with plant size, as predicted. J. Ecol. 2016, 104, 1488–1503. [Google Scholar] [CrossRef]
- Smith, W.K.; Germino, M.J.; Johnson, D.M.; Reinhardt, K. The altitude of alpine treeline: A bellwether of climate change effects. Bot. Rev. 2009, 75, 163–190. [Google Scholar] [CrossRef]
- Chen, L.H.; Dong, T.F.; Duan, B.L. Sex-specific carbon and nitrogen partitioning under N deposition in Populus cathayana. Trees-Struct. Funct. 2014, 28, 793–806. [Google Scholar] [CrossRef]
- Stitt, M.; Quick, W.P. Photosynthetic carbon partitioning: Its regulation and possibilities for manipulation. Physiol. Plant. 1989, 77, 633–641. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H.; Liu, X.L.; Wang, X.D.; Zhu, W.Z. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef]
- Du, J.H.; Shao, J.Y.; Li, S.F.; Qin, J. Non-structural carbohydrate content of trees and its influencing factors at multiple spatial-temporal scales: A review. Chin. J. Appl. Ecol. 2020, 31, 1378–1388. [Google Scholar]
- Pollock, C.J.; Lloyd, E.J. The effect of low temperature upon starch, sucrose and fructan aynthesis in leaves. Ann. Bot. 1987, 60, 231–235. [Google Scholar] [CrossRef]

| Elevation (m a.s.l) | Slope (°) | Slope Exposure | Mean Air Temperature (°C) * | Precipitation (mm) * | Height (m) | Diameter (cm) | Vegetation Type | ||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||||
| Populus cathayana | |||||||||
| 1800 | 15.2 | SE | 16.9 | 715.9 | 14 ± 2 | 15 ± 2 | 33.1 ± 4.9 | 35.8 ± 3.5 | BLF |
| 2200 | 12.6 | S | 14.2 | 636.0 | 18 ± 2 | 19 ± 2 | 36.1 ± 2.9 | 36.6 ± 3.3 | BLF |
| 2600 | 16.3 | SE | 12.4 | 645.0 | 19 ± 1 | 20 ± 2 | 38.9 ± 6.6 | 35.2 ± 3.3 | CBF |
| 3100 | 13.7 | S | 9.4 | 667.7 | 18 ± 1 | 20 ± 3 | 35.7 ± 2.8 | 34.7 ± 4.4 | CBF |
| Hippophae rhamnoides | |||||||||
| 1900 | 4.0 | S | 14.7 | 770.0 | 7 ± 1 | 7 ± 1 | 12.6 ± 3.5 | 15.7 ± 4.3 | BLF |
| 2500 | 8.5 | S | 11.7 | 651.7 | 6 ± 2 | 8 ± 3 | 12.9 ± 1.9 | 14.7 ± 3.0 | CBF |
| 3100 | 9.4 | SE | 8.6 | 675.7 | 7 ± 1 | 7 ± 2 | 13.6 ± 5.0 | 12.5 ± 2.8 | CBF |
| 3700 | 6.8 | S | 6.5 | 696.0 | 6 ± 2 | 5 ± 3 | 14.9 ± 4.8 | 13.8 ± 5.5 | SL |
| Populus cathayana | Hippophae rhamnoides | ||||||
|---|---|---|---|---|---|---|---|
| p | R2 | Regression Equations | p | R2 | Regression Equations | ||
| Soluble sugar | Male | - | - | - | 0.001 | 0.384 | y = 15.985 + 0.002x |
| Female | - | - | - | 0.003 | 0.381 | y = 15.490 + 0.002x | |
| ANCOVA | - | ||||||
| Starch | Male | - | - | - | 0.003 | 0.330 | y = 1.849 + 0.001x |
| Female | <0.001 | 0.516 | y = −0.619 + 0.003x | 0.006 | 0.335 | y = 1.432 + 0.0016x | |
| ANCOVA | - | ||||||
| NSCs | Male | - | - | - | 0.001 | 0.402 | y = 17.835 + 0.003x |
| Female | - | - | - | 0.001 | 0.421 | y = 16.922 + 0.003x | |
| ANCOVA | - | ||||||
| Soluble sugar/starch | Male | - | - | - | 0.007 | 0.287 | y = 7.131 − 0.001x |
| Female | 0.002 | 0.423 | y = 7.417 − 0.001x | 0.037 | 0.209 | y = 7.770 − 0.001x | |
| ANCOVA | - | ||||||
| Populus cathayana | Hippophae rhamnoides | ||||||
|---|---|---|---|---|---|---|---|
| p | R2 | Regression Equations | p | R2 | Regression Equations | ||
| Nitrogen | Male | 0.040 | 0.253 | y = 32.909 − 0.003x | <0.001 | 0.446 | y = 23.641 + 0.003x |
| Female | - | - | - | 0.004 | 0.361 | y = 19.761 + 0.004x | |
| ANCOVA | - | ||||||
| Phosphorus | Male | <0.001 | 0.594 | y = 3.281 − 0.001x | <0.001 | 0.619 | y = 0.562 + 0.001x |
| Female | <0.001 | 0.841 | y = 2.486 − 0.0005x | <0.001 | 0.577 | y = −0.311 + 0.001x | |
| ANCOVA | <0.001 | ||||||
| Nitrogen/phosphorus | Male | 0.012 | 0.351 | y = 7.398 + 0.003x | <0.001 | 0.580 | y = 24.365 − 0.003x |
| Female | <0.001 | 0.631 | y = 1.803 + 0.008x | <0.001 | 0.732 | y = 27.447 − 0.004x | |
| ANCOVA | - | ||||||
| Populus cathayana | Hippophae rhamnoides | ||||||
|---|---|---|---|---|---|---|---|
| p | R2 | Regression Equations | p | R2 | Regression Equations | ||
| Soluble sugar/nitrogen | Male | - | - | - | - | - | - |
| Female | - | - | - | - | - | - | |
| ANCOVA | - | ||||||
| Starch/nitrogen | Male | - | - | - | - | - | - |
| Female | <0.001 | 0.600 | y = −0.052 + 0.0001x | - | - | - | |
| ANCOVA | - | ||||||
| NSCs/nitrogen | Male | - | - | - | - | - | - |
| Female | - | - | - | - | - | - | |
| ANCOVA | - | ||||||
| Soluble sugar/phosphorus | Male | <0.001 | 0.537 | y = 4.467 + 0.005x | 0.006 | 0.294 | y = 158.539 − 0.017x |
| Female | <0.001 | 0.692 | y = 3.511 + 0.006x | 0.010 | 0.303 | y = 192.596 − 0.027x | |
| ANCOVA | 0.008 | ||||||
| Starch/phosphorus | Male | - | - | - | - | - | - |
| Female | <0.001 | 0.711 | y = −6.520 + 0.005x | - | - | - | |
| ANCOVA | - | ||||||
| NSCs/phosphorus | Male | 0.008 | 0.380 | y = 6.711 + 0.006x | 0.021 | 0.221 | y = 181.875 − 0.018x |
| Female | <0.001 | 0.720 | y = −3.009 + 0.011x | 0.028 | 0.229 | y = 221.154 − 0.029x | |
| ANCOVA | - | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liu, S.; Feng, Q.; Cao, X.; Xing, H.; Shi, Z. Response of Leaf Non-Structural Carbohydrates to Elevation in Dioecious Plants, Populus cathayana and Hippophae rhamnoides. Forests 2025, 16, 246. https://doi.org/10.3390/f16020246
Wu J, Liu S, Feng Q, Cao X, Xing H, Shi Z. Response of Leaf Non-Structural Carbohydrates to Elevation in Dioecious Plants, Populus cathayana and Hippophae rhamnoides. Forests. 2025; 16(2):246. https://doi.org/10.3390/f16020246
Chicago/Turabian StyleWu, Jiamei, Shun Liu, Qiuhong Feng, Xiangwen Cao, Hongshuang Xing, and Zuomin Shi. 2025. "Response of Leaf Non-Structural Carbohydrates to Elevation in Dioecious Plants, Populus cathayana and Hippophae rhamnoides" Forests 16, no. 2: 246. https://doi.org/10.3390/f16020246
APA StyleWu, J., Liu, S., Feng, Q., Cao, X., Xing, H., & Shi, Z. (2025). Response of Leaf Non-Structural Carbohydrates to Elevation in Dioecious Plants, Populus cathayana and Hippophae rhamnoides. Forests, 16(2), 246. https://doi.org/10.3390/f16020246






