Effects of Stand Age and Environmental Factors on Soil Phytolith-Occluded Organic Carbon Accumulation of Cunninghamia lanceolata Forests in Southwest Subtropics of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area Overview
2.2. Sample Selection and Sample Collection
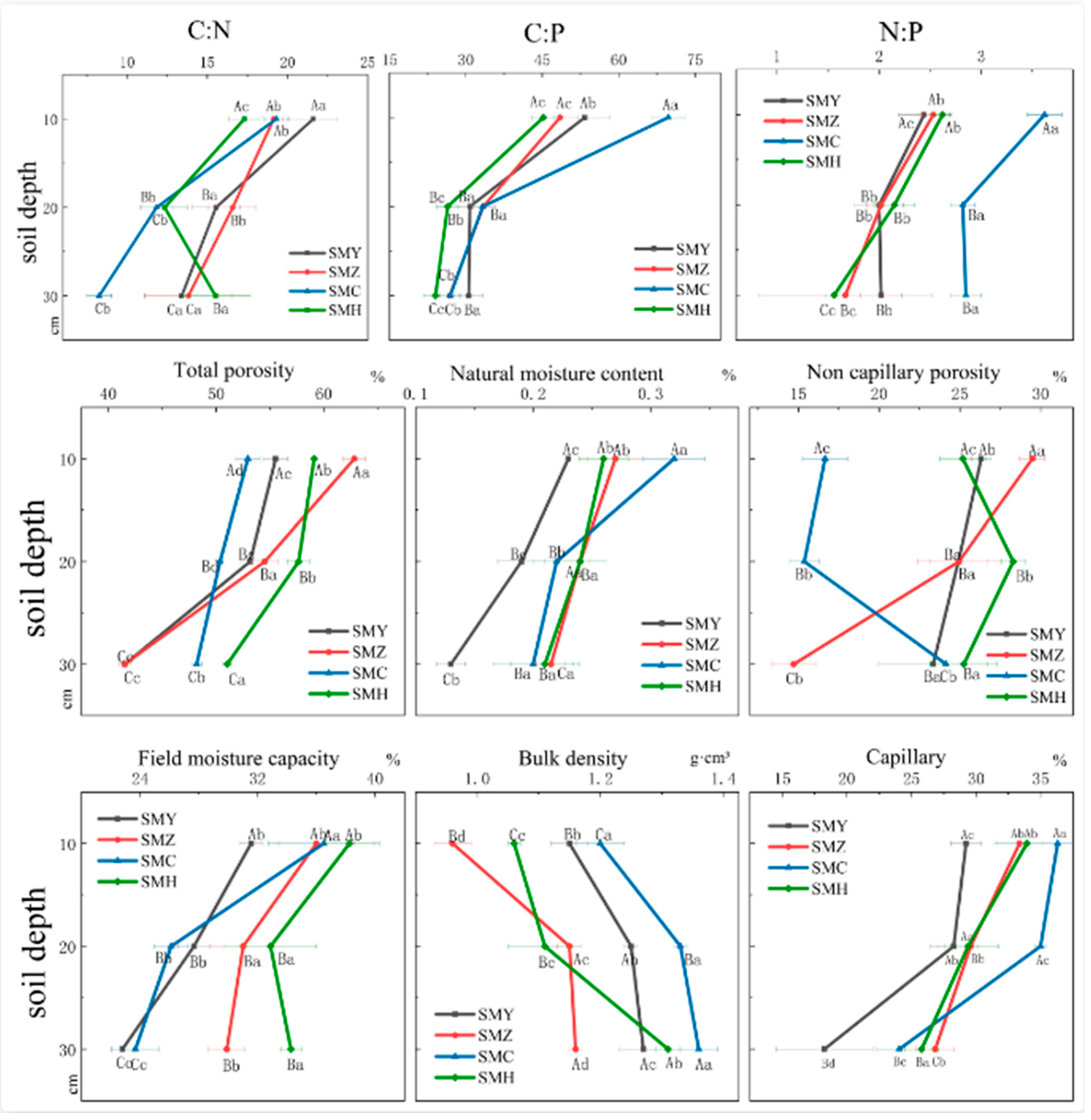
2.3. Measure of Soil Physicochemical Properties
2.4. Computational Formula of Phytolith and PhytOC
2.5. Statistical Analysis
3. Results
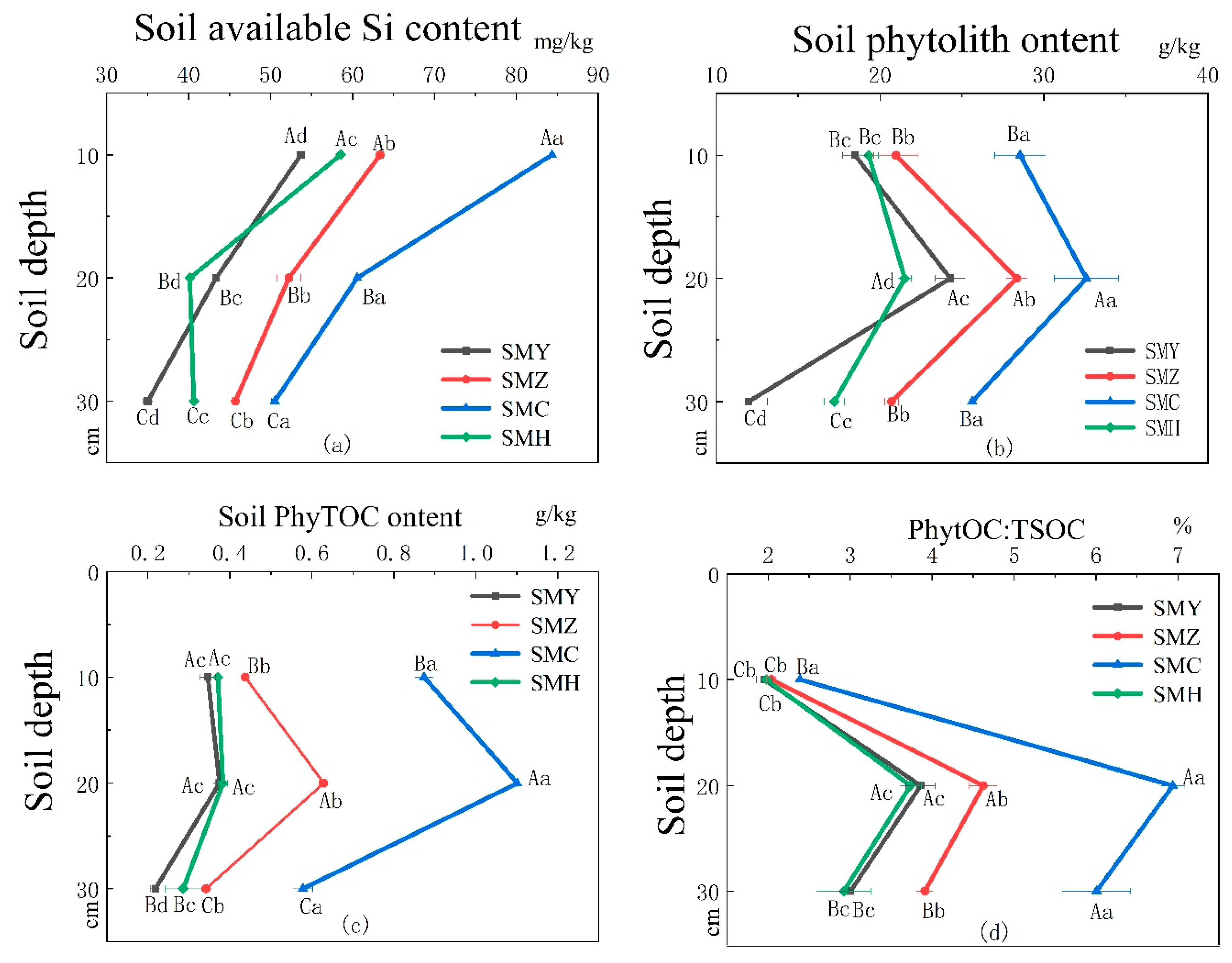
3.1. Soil Available Silicon Content and Phytolith Content
3.2. Soil PhytOC Content and the Ratio of Soil PhytOC Content to Total Soil Organic Carbon
3.3. Soil PhytOC Stock of Cunninghamia Lanceolata Stands
3.4. Correlation Between Soil PhytOC and Environmental Factors
3.5. RDA of Soil PhytOC Accumulation in Cunninghamia Lanceolata Stands
4. Discussion
4.1. Accumulation Characteristics of Soil PhytOC in Cunninghamia Lanceolata Forests of Different Stand Ages
4.2. Effects of High-Silicon, Carbon-Rich, and Acidic Soil Environments on the Accumulation of Soil PhytOC
4.3. Accumulation Potential of Soil PhytOC in Cunninghamia Lanceolata Forests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2014; Available online: http://www.ipcc.ch/report/ar5/wg3 (accessed on 10 September 2014).
- Ford, C.R.; Wurzburger, N.; Hendrick, R.L. Soil DIC uptake and fixat ion in Pinus taeda seedlings and its C contribution to plant tissues and ectomyc orrhizal fungi. Tree Physiol. 2007, 27, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Wilding, L.P. Radiocarbon dating of biogenetic opal. Science 1967, 156, 66–67. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F.; Sullivan, L.A.; Chen, B.H.; Gong, Y.; Zheng, W.P. Carbon bio-sequestratio n within the phytolith of economic bamboo species. Glob. Change Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Song, Z.L.; Liu, H.; Li, B.L.; Yang, X.M. The production of phytolith-occluded car bon in China’s forests: Implications to biogeochemical carbon sequestration. Glob. Change Biol. 2013, 19, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F.; Sullivan, L.A. Soil carbon sequestration in phytolith. Soil Biol. Biochem. 2005, 37, 117–124. [Google Scholar] [CrossRef]
- Gao, Y.; He, N.P.; Wang, Y.F. Carbon sequestration characteristics of ecosystems and research progress. J. Nat. Resour. 2013, 28, 1264–1274. [Google Scholar]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Zheng, X.J. Carbon Sequestration Characteristics and Influencing Factors of Phytosilica in Pinus masson Forest in Guizhou. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2024. [Google Scholar]
- Ma, J.F. Functions of silicon in higher plants. Prog. Mol. Subcell. Biol. 2003, 33, 127–147. [Google Scholar] [PubMed]
- Pan, W.J.; Yang, X.M.; Zhang, X.D. Research progress of phytopsitic carbon sinks in terrestrial ecosystems in China. Adv. Earth Sci. 2017, 32, 859–866. [Google Scholar]
- Zhu, H.Y.; Lu, C.; Gao, M. Soil phytopsilite carbon distribution in four stands of Jinyun Mountain. Acta Pedol. Sin. 2020, 57, 359–369. [Google Scholar]
- Song, Z.L.; Wang, H.L.; Strong, P.J. Plant impact on the coupled terrestrial biogeochemical cycles of silicon and carbon: Implications for biogeochemical carbon sequestration. Earth-Sci. Rev. 2012, 115, 319–331. [Google Scholar] [CrossRef]
- Blecker, S.W.; Mcculley, R.L.; Chadwick, O.A. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 2006, 20, 4253–4274. [Google Scholar] [CrossRef]
- Golyeva, A. Biomorphic analysis as a part of soil morphological investigations. Catena 2001, 43, 217–230. [Google Scholar] [CrossRef]
- Golyeva, A.; Andrič, M. Palaeoecological reconstruction of wetlands and Eneolithic land use in Ljubljansko barje (Slovenia) based on biomorphic and pollen analysis. Catena 2014, 112, 38–47. [Google Scholar] [CrossRef]
- Carnelli, A.L.; Madella, M.; Theurillat, J.P. Biogenic silica production in selected alpine plant species and plant communities. Ann. Bot. 2001, 87, 425–434. [Google Scholar] [CrossRef]
- Bartoli, F.; Wilding, L.P. Dissolution of biogenic opal as a function of its physical and chemical properties. Soil Sci. Soc. Am. J. 1980, 44, 873–878. [Google Scholar] [CrossRef]
- Yang, X.M.; Song, Z.L.; Liu, H.Y.; Lukas, V.Z.; Song, Z.L.; Li, Z.M.; Hao, Q.; Zhang, X.D.; Wang, H.L. Phytolith accumulation in broadleaf and conifer forests of no rthern China: Implications for phytolith carbon sequestration. Geoderma 2018, 312, 36–44. [Google Scholar] [CrossRef]
- Feng, X.Z. Soil Silicon Carbon Evolution Characteristics of Bamboo Forest with Different Planting Years. Master’s Thesis, Zhejiang A&F University, Zhejiang, China, 2018. (In Chinese). [Google Scholar]
- Lu, K.S.; Li, L.; Lin, J.W.; Mao, Y.S. Effects of stand age and bedrock on soil phytolith-occluded organic carbon accumulation of large-diameter bamboo forests in Southwest China. Soil Ecol. Lett. 2025, 7, 1–15. [Google Scholar]
- Wang, X.; Hu, H.B.; Cheng, C. Study on soil phytopsilite carbon storage in North subtropical Quercus acorn forest. J. Zhejiang A F Univ. 2021, 38, 1–9. [Google Scholar]
- Zhang, Y.; Wang, L.; Gong, Z. Soil PhytOC accumulation of Masson pine forests in Southwest China. Eur. J. For. Res. 2024, 143, 1135–1147. [Google Scholar] [CrossRef]
- Feng, Z.W. Biomass and Productivity of Forest Ecosystems in China; Science Press: Beijing, China, 1999. [Google Scholar]
- State Forestry Administration. China Forest Resources Report; State Forestry Administration: Beijing, China, 2019.
- Lin, W.L.; Ying, Y.Q.; Jiang, P.K. Study on phytosilite carbon in subtropical forest soil in southern Zhejiang Province. Acta Pedol. Sin. 2015, 52, 1365–1373. [Google Scholar]
- Zhang, X.D. Study on Phytolite Accumulation and Silicon Morphology Distribution in Forest Soil of Eastern China. Master’s Thesis, Zhejiang A & F University, Hangzhou, China, 2016. [Google Scholar]
- He, S.Q. Study on the Carbon Variation and Stability of Phytosilica in Typical Forest-Soil System in Tropical and Subtropical Regions. Master’s Thesis, Zhejiang A & F University, Hangzhou, China, 2016. [Google Scholar]
- Cheng, B. Research on Optimization of Protection Material Ratio of Red Sand Mudstone Admixture Slope in Chishui. Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Wang, D.J. Research on Weathering Characteristics of Red Layer in Danxia Landform Area of Chishui. Master’s Thesis, Guizhou University, Guiyang, China, 2020. [Google Scholar]
- Li, S. Classification and Comparison of Purple Soil in ST and WRB. Master’s Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Tian, Y.C. Ecological and Hydrological Processes in Chishui River Basin; Science Press: Beijing, China, 2020. [Google Scholar]
- He, Y.R. Purple Soil in China (Part 2); Science Press: Beijing, China, 2003. [Google Scholar]
- Huang, W.L.; Tu, Y.L.; Yang, L. Vegetation in Guizhou; Guizhou People’s Publishing House: Guizhou, China, 1988. [Google Scholar]
- State Forestry Administration. Age Class and Age Group Classification of Major Tree Species; State Forestry Administration: Beijing, China, 2018.
- Chinese Fir Ecology Laboratory, Institute of Forestry Science, Ministry of Forestry, PRC. Chinese Fir Forestation; China Forestry Publishing House: Beijing, China, 1958. [Google Scholar]
- Li, K.; Li, Z.J. Soil Chemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2019; pp. 86–88. [Google Scholar]
- Zhang, Y. Study on carbon sequestration characteristics of phytopsilites in underforest system (root and soil) of Masson pine in karst area of Guizhou Province. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2024. [Google Scholar]
- Ma, X.H. Methodological Guidelines for Forest Ecosystem Location Research; Chinese Science and Technology Press: Beijing, China, 1994. [Google Scholar]
- Sheng, M.; Xiong, K.; Wang, L.; Li, X.; Li, R.; Tian, X. Response of soil physical and chemical properties to Rocky desertification succession in South China Karst. Carbonates Evaporites 2018, 33, 15–28. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1982, 9, 539–579. [Google Scholar]
- Walkey Black, C.A. An examination of Digtiareff method for determining soil organic matter and proposed modification for the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Agricultural Chemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Li, S.; Sheng, M.; Yuan, F. Effect of land cover change on total SOC and soil PhytOC accumulation in the karst subtropical forest ecosystem, SW China. J. Soils Sediments 2021, 21, 2566–2577. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.F.; Huang, Z.T.; Jiang, P.K.; Xiang, T.T.; Ying, Y.Q. Determination of carbon content in phytolith by alkaline solution spectrophotometry. Chin. J. Anal. Chem. 2014, 42, 1389–1390. [Google Scholar]
- Alexandre, A.; Meunier, J.D.; Colin, F. Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim. Cosmochim. Acta 1997, 61, 677–682. [Google Scholar] [CrossRef]
- Schaller, J.; Turner, B.L.; Weissflog, A. Silicon in tropical forests: Large variation across soils and leaves suggests ecological significance. Biogeochemistry 2018, 140, 161–174. [Google Scholar] [CrossRef]
- Ding, X.Q.; Liu, M.C.; Yan, C.X. Analysis of influencing factors on carbon accumulation potential of soil phytophilite. Chin. J. Soil Sci. 2014, 45, 749–753. [Google Scholar]
- Ma, X.Q.; Liu, C.J.; Hannu, I.; Westman, C.; Liu, A.Q. Biomass, litter and nutrient distribution of Chinese fir forests at different developmental stages in subtropical regions of China. J. For. Res. 2002, 165–170, 250. [Google Scholar]
- Tombeur, F.; Etienne, L.; Lambers, H.; Michelccerre, F.; Gregory, M. A shift from phenol to silica-based leaf defences during long-term soil and ecosystem development. Ecol. Lett. 2021, 24, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Song, Z.; Sullivan, L. Enhancing phytolith carbon sequestration in rice ecosystems through basalt powder amendment. Sci. Bull. 2015, 60, 591–597. [Google Scholar] [CrossRef]
- Li, Z.M.; Song, Z.L.; Parr, J.F. Occluded C in rice phy-toliths: Implications to biogeochemical carbon sequestration. Plant Soil 2013, 370, 615–623. [Google Scholar] [CrossRef]
- Song, Z.; Parr, J.F.; Guo, F. Potential of global cropland phytolith carbon sink from optimization of cropping system and fertilization. PLoS ONE 2013, 8, e73747. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Z.M.; Dai, L.M. Research progress of silicon cycling in forest ecosystem. Acta Ecol. Sin. 2007, 7, 3010–3017. [Google Scholar]
- Gao, Z.; Jie, D.M.; Liu, L.D.; Liu, H.Y.; Lu, M.J.; Gao, G.Z.; Liu, H.T. Spatial and temporal variation of soil available silicon and its influence on the formation of reed phytolith in Northeast China. Quat. Sci. 2015, 35, 967–976. [Google Scholar]
- Bremond, L.; Bodin, S.; Bebtaleb, I. Past tree cover of the Congo Basin recovered by phytolith and δ13C along soil profiles. Quat. Int. 2017, 434, 91–101. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J. Surface chemistry and reactivity of plant phytolith in aqueous solutions. Chem. Geol. 2009, 258, 197–206. [Google Scholar] [CrossRef]
- Karkanas, P. Preservation of anthropogenic materials under different geochemical processes: A mineralogical approach. Quat. Int. 2010, 214, 63–69. [Google Scholar] [CrossRef]





| Sample Quadratsnumbers | Environment of Sample Quadrats | Basic Information of Cunninghamia lanceolata Forests | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elevation (m) | Slope Location | Slope (°) | Stand Ages (a) | Group of Stand Age | Mean Diameter at Breast Height (cm) | Basal Area (cm2) | Mean Tree Height (m) | Stand Density (Individual hm−2) | Canopy Density (%) | |
| SMY-H1 | 949 | Middle | 21 | 9 | Young | 9.90 | 7555.30 | 8.60 | 2455 | 85 |
| SMY-H2 | 952 | Middle | 21 | 9 | Young | 9.97 | 7709.34 | 8.64 | 2470 | 88 |
| SMY-H3 | 950 | Middle | 22 | 9 | Young | 10.00 | 7802.90 | 8.69 | 2485 | 86 |
| SMY-G1 | 955 | Middle | 23 | 9 | Young | 10.03 | 7897.17 | 8.73 | 2500 | 87 |
| SMY-G2 | 951 | Middle | 24 | 9 | Young | 10.40 | 8541.50 | 8.78 | 2515 | 88 |
| SMY-G3 | 953 | Middle | 22 | 9 | Young | 10.28 | 8445.08 | 8.82 | 2545 | 90 |
| SMY-T1 | 960 | Middle | 21 | 9 | Young | 10.41 | 8608.98 | 8.87 | 2530 | 87 |
| SMY-T2 | 956 | Middle | 21 | 9 | Young | 10.31 | 8544.51 | 8.91 | 2560 | 88 |
| SMY-T3 | 966 | Middle | 21 | 9 | Young | 10.26 | 8494.88 | 8.96 | 2570 | 89 |
| SMZ-H1 | 1021 | Middle | 27 | 17 | Middle-aged | 12.40 | 3992.81 | 16.34 | 827 | 74 |
| SMZ-H2 | 1022 | Middle | 27 | 17 | Middle-aged | 12.46 | 4090.04 | 16.35 | 839 | 71 |
| SMZ-H3 | 1024 | Middle | 27 | 17 | Middle-aged | 12.44 | 4047.77 | 16.50 | 833 | 72 |
| SMZ-G1 | 1016 | Middle | 28 | 17 | Middle-aged | 12.49 | 4139.15 | 16.49 | 845 | 75 |
| SMZ-G2 | 1014 | Middle | 28 | 17 | Middle-aged | 12.53 | 4224.86 | 16.69 | 857 | 74 |
| SMZ-G3 | 1016 | Middle | 28 | 17 | Middle-aged | 12.55 | 4208.69 | 16.63 | 851 | 73 |
| SMZ-T1 | 1026 | Middle | 27 | 17 | Middle-aged | 12.71 | 4377.55 | 16.74 | 863 | 78 |
| SMZ-T2 | 1030 | Middle | 29 | 17 | Middle-aged | 12.52 | 4306.72 | 16.50 | 875 | 77 |
| SMZ-T3 | 1033 | Middle | 29 | 17 | Middle-aged | 12.41 | 4202.36 | 16.63 | 869 | 76 |
| SMC-H1 | 1005 | Middle | 20 | 28 | Mature | 18.40 | 7175.78 | 20.41 | 675 | 75 |
| SMC-H2 | 1010 | Middle | 20 | 28 | Mature | 18.34 | 7393.10 | 20.39 | 700 | 68 |
| SMC-H3 | 1008 | Middle | 22 | 28 | Mature | 18.62 | 7620.56 | 20.38 | 700 | 72 |
| SMC-G1 | 957 | Middle | 21 | 28 | Mature | 18.64 | 6818.70 | 20.40 | 625 | 71 |
| SMC-G2 | 958 | Middle | 22 | 28 | Mature | 18.45 | 6947.62 | 20.41 | 650 | 72 |
| SMC-G3 | 957 | Middle | 21 | 28 | Mature | 18.76 | 6906.78 | 20.43 | 625 | 70 |
| SMC-T1 | 945 | Middle | 24 | 28 | Mature | 18.63 | 7083.84 | 20.41 | 650 | 66 |
| SMC-T2 | 945 | Middle | 24 | 28 | Mature | 18.65 | 7645.14 | 20.40 | 700 | 65 |
| SMC-T3 | 944 | Middle | 24 | 28 | Mature | 18.86 | 7539.05 | 20.40 | 675 | 64 |
| SMH-H1 | 1023 | Upper | 40 | 2 | Huitou-sha | 2.05 | 126.02 | 1.80 | 955 | 85 |
| SMH-H2 | 1024 | Upper | 40 | 2 | Huitou-sha | 2.08 | 131.77 | 1.95 | 970 | 84 |
| SMH-H3 | 1027 | Upper | 40 | 2 | Huitou-sha | 2.13 | 142.46 | 2.10 | 1000 | 86 |
| SMH-G1 | 1002 | Upper | 42 | 2 | Huitou-sha | 2.14 | 141.64 | 2.25 | 985 | 86 |
| SMH-G2 | 1002 | Upper | 42 | 2 | Huitou-sha | 2.09 | 139.22 | 2.40 | 1015 | 89 |
| SMH-G3 | 1005 | Upper | 42 | 2 | Huitou-sha | 2.21 | 162.56 | 2.55 | 1060 | 88 |
| SMH-T1 | 990 | Upper | 40 | 2 | Huitou-sha | 2.23 | 160.83 | 2.70 | 1030 | 86 |
| SMH-T2 | 993 | Upper | 40 | 2 | Huitou-sha | 2.27 | 173.94 | 2.85 | 1075 | 88 |
| SMH-T3 | 1000 | Upper | 40 | 2 | Huitou-sha | 2.30 | 173.58 | 3.01 | 1045 | 87 |
| Environmental Factors | RDA Analysis | Monte Carlo Test | ||||
|---|---|---|---|---|---|---|
| RDA1 | RDA2 | RDA3 | Order of Importance | r2 | p | |
| ASi | 1.486 | −0.146 | −0.025 | 1.000 | 0.983 | 0.001 *** |
| TSOC | 1.471 | −0.124 | 0.192 | 2.000 | 0.962 | 0.001 *** |
| CD | −1.384 | 0.287 | 0.387 | 3.000 | 0.871 | 0.001 *** |
| TN | 1.374 | 0.277 | 0.382 | 4.000 | 0.857 | 0.001 *** |
| TP | 1.311 | −0.245 | 0.161 | 5.000 | 0.778 | 0.001 *** |
| TH | 1.289 | −0.220 | −0.474 | 6.000 | 0.750 | 0.001 *** |
| DBH | 1.274 | 0.064 | −0.343 | 7.000 | 0.720 | 0.001 *** |
| N:P | 1.265 | 0.472 | 0.371 | 8.000 | 0.771 | 0.001 *** |
| NCP | −1.168 | 0.097 | −0.134 | 9.000 | 0.607 | 0.001 *** |
| C:P | 1.104 | 0.281 | 0.186 | 10.000 | 0.562 | 0.001 *** |
| pH | 1.094 | −0.320 | −0.070 | 11.000 | 0.558 | 0.001 *** |
| C:N | −1.063 | −0.408 | −0.351 | 12.000 | 0.546 | 0.001 *** |
| SDI | −0.825 | 0.703 | 0.027 | 13.000 | 0.440 | 0.001 *** |
| BD | 0.724 | 0.942 | 0.675 | 14.000 | 0.480 | 0.001 *** |
| NMC | 0.718 | −0.538 | −0.032 | 15.000 | 0.310 | 0.001 *** |
| STP | −0.605 | −0.529 | 0.170 | 16.000 | 0.240 | 0.001 *** |
| FMC | −0.424 | −0.669 | 0.144 | 17.000 | 0.204 | 0.001 *** |
| CP | −0.015 | −0.485 | 0.128 | 18.000 | 0.066 | 0.029 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Sheng, M. Effects of Stand Age and Environmental Factors on Soil Phytolith-Occluded Organic Carbon Accumulation of Cunninghamia lanceolata Forests in Southwest Subtropics of China. Forests 2025, 16, 240. https://doi.org/10.3390/f16020240
Huang Q, Sheng M. Effects of Stand Age and Environmental Factors on Soil Phytolith-Occluded Organic Carbon Accumulation of Cunninghamia lanceolata Forests in Southwest Subtropics of China. Forests. 2025; 16(2):240. https://doi.org/10.3390/f16020240
Chicago/Turabian StyleHuang, Qifen, and Maoyin Sheng. 2025. "Effects of Stand Age and Environmental Factors on Soil Phytolith-Occluded Organic Carbon Accumulation of Cunninghamia lanceolata Forests in Southwest Subtropics of China" Forests 16, no. 2: 240. https://doi.org/10.3390/f16020240
APA StyleHuang, Q., & Sheng, M. (2025). Effects of Stand Age and Environmental Factors on Soil Phytolith-Occluded Organic Carbon Accumulation of Cunninghamia lanceolata Forests in Southwest Subtropics of China. Forests, 16(2), 240. https://doi.org/10.3390/f16020240





