1. Introduction
Seed production by woody plants is an important mechanism for regulating the recovery and resilience of both natural and damaged forest communities [
1]. Because coniferous tree species are older in terms of evolution than deciduous ones, they have little or no vegetative reproduction. As a result, their competitiveness and ecological adaptability within the specific constraints of forest communities are determined by seed reproduction [
2].
Tree seed crops are influenced by both environmental (climatic, soil, and biotic conditions) and biological (ecobiological traits of tree species and stand characteristics) variables [
3]. Therefore, evaluating the periodicity and cyclicity of cone production is crucial for understanding conifer seed regeneration because it allows for the forecasting of cone crop peaks and declines at the stand level. Tree seed production may differ significantly from season to season [
4,
5,
6]. At the same time, cone (seed) crops can also range from completely absent cones (100% cone-free) to extreme bumper yields (known as mast years) [
7,
8]. Although determining the yearly cone production of tree stands by long-term monitoring is challenging, the results yield accurate data on the seed reproductive potential of forest communities [
9,
10,
11].
The annual dynamics of woody plant cone crops have long been the focus of research. Previously, several features of the annual crop of cones in the genera
Pinus [
12,
13,
14,
15],
Picea [
16,
17,
18,
19], and
Larix [
20,
21,
22] were investigated. In contrast, very little is known about the dynamics of the annual crop of cones in
Abies species [
23,
24,
25,
26]. Siberian fir (
Abies sibirica Ledeb.) grows in a cold boreal climate on moist soils in mountains or river basins at altitudes ranging from 1900 to 2400 m. This species is typical of the boreal region and is found throughout the Siberian taiga. Native to boreal (taiga) and mountain forests, Siberian fir can grow in pure or mixed stands with other hardwoods and conifers [
27,
28]. The range is located in northern and central Eurasia, extending from the southern Ural Mountains and northeastern European Russia across Siberia (thus the species name), north of the Chinese and Mongolian border, to the Uda and Amur Rivers, and to the central Heilongjiang Province (China), with an outlier in the Tian Shan (Kyrgyzstan) [
29]. They are slender, monecious evergreen trees that may grow up to 35 m high and 100 cm in diameter. They typically have a single stem and a conical crown; however, elderly trees sometimes have numerous crowns. They have been introduced as an amenity tree throughout Central and Eastern Europe (with various forms and cultivars) [
30]. Because the wood of Siberian fir is susceptible to fungal decay, the tree seldom survives for more than 200 years [
31]. Siberian fir is a commercially significant wood species.
General patterns of the geography, biology, and ecology of cone and seed production for Siberian fir have been established, as summarised in some monographs [
32,
33,
34]. The mast years of fir occur every 3–4 years, and every 2 years in favourable climatic zones, according to research [
35,
36]. The earliest generalisations of the dynamics of the annual crop of Siberian fir cones for the region where our research was conducted were based on data spanning 15–25 years of observations [
37,
38]. Our investigations extend the time of continuous observation by nearly twofold (up to 47 years), allowing us to make more reasonable inferences regarding the annual dynamics of the Siberian fir cone crop. Therefore, the current research focused on analysing data on the crop dynamics of Siberian fir cones that were collected via direct observations in conifer forests of the pre-Ural region (Russia) between 1975 and 2021 (47 years). Specifically, we aim to address the following questions: (1) What are the long-term dynamics of the yearly Siberian fir cone crop? (2) To what extent do the fir cone crop vary among forest types? (3) How do the current and previous year’s climatic conditions impact the fir cone crop? This study will contribute to a better understanding of the long-term dynamics of Siberian fir cone crop.
3. Results
Fir trees had a high mortality rate in the examined coniferous forests (
Table 5). At the end of the research period, only 28%–29% of the fir trees remained in the FSF (BFG) and FF (HWSG). FSF (SGM) had a greater survival rate, with 52% of the fir trees still standing at the end of the research.
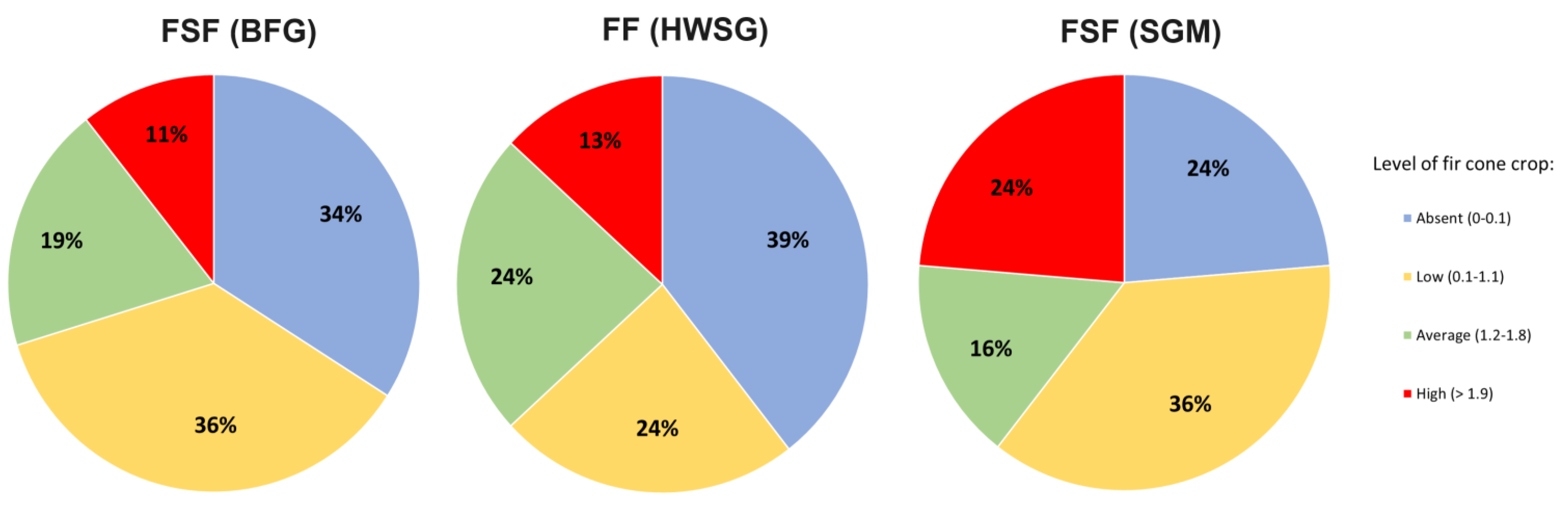
Fir cone crops are variable, unstable, and inconsistent from year to year. Fir cone crops vary from non-existent to highly level. In the FSF (BFG), the largest level of the fir cone crop was recorded in 1976. The average cone crop point in this year was 3.2 ± 0.23. A high and very high crop of cones was observed on more than 48% of the fir trees. Over 9% of the trees had an average level of fir cone crops. Furthermore, this year, only 2% of the fir trees lacked cones. The highest cone crop level in the FF (HWSG) was observed in 2005. In that year, the cone crop average point was 2.5 ± 0.18. More than 26% of the fir trees had high or very high crop of cones. Average levels of fir cone crops were present on more than 30% of the trees. Only 10% of the fir trees in that year had no cones. The cone crop in the FSF (SGM) peaked in 1994. The cone crop average point was 3.5 ± 0.13. A high or very high crop of cones was recorded on more than half of the fir trees. Approximately 30% of the trees had an average level of fir cone crops. There were no trees without cones this year because every other tree had cones.
In the examined coniferous forests, high crops of fir cones are rare (
Table 6 and
Table 7). For example, only 5 out of 47 years (approximately once every ten years) saw high crops of cones in the FSF (BFG). In the FF (HWSG), a comparable trend was noted. Here, only 5 of the 38 years had high cone crops, or approximately once every 10 years. Compared to other types of forests, the FSF (SGM) had more fir cone crops more frequently. Further, 9 years out of 38 years were distinguished by high cone crops, i.e., high cone crops were distinguished approximately twice every 10 years. Coniferous forest studies show statistically significant differences in cone crops; these differences are more common in years with high cone crops than in years with average cone crops. No statistically significant differences were detected between the examined coniferous forests in the years with low levels of cone crops.
In two of the three forest types examined, the number of years with an average cone crop was approximately twice the number of years with a high crop. The average crop of cones in the FSF (BFG) was recorded for 9 of 47 years, or approximately twice every 10 years. In the FF (HWSG), the average crop was reported for 9 of the 38 years, or twice in 10 years. The number of years in the FSF (SGM) with an average cone crop throughout the studied period is half the number of years with high crops. Here, the average cone crop was recorded for 5 of the 38 years, or once every 10 years. The remaining years were characterised by low or non-existent fir cone crops. Cone crops were completely absent in the following sequences: FF (HWSG), 15 years out of 38 (39%); FSF (BFG), 16 years out of 47 (34%); and FSF (SGM), 9 years out of 38 (23%). In all other years, low levels of cone crops were recorded in the studied coniferous forests.
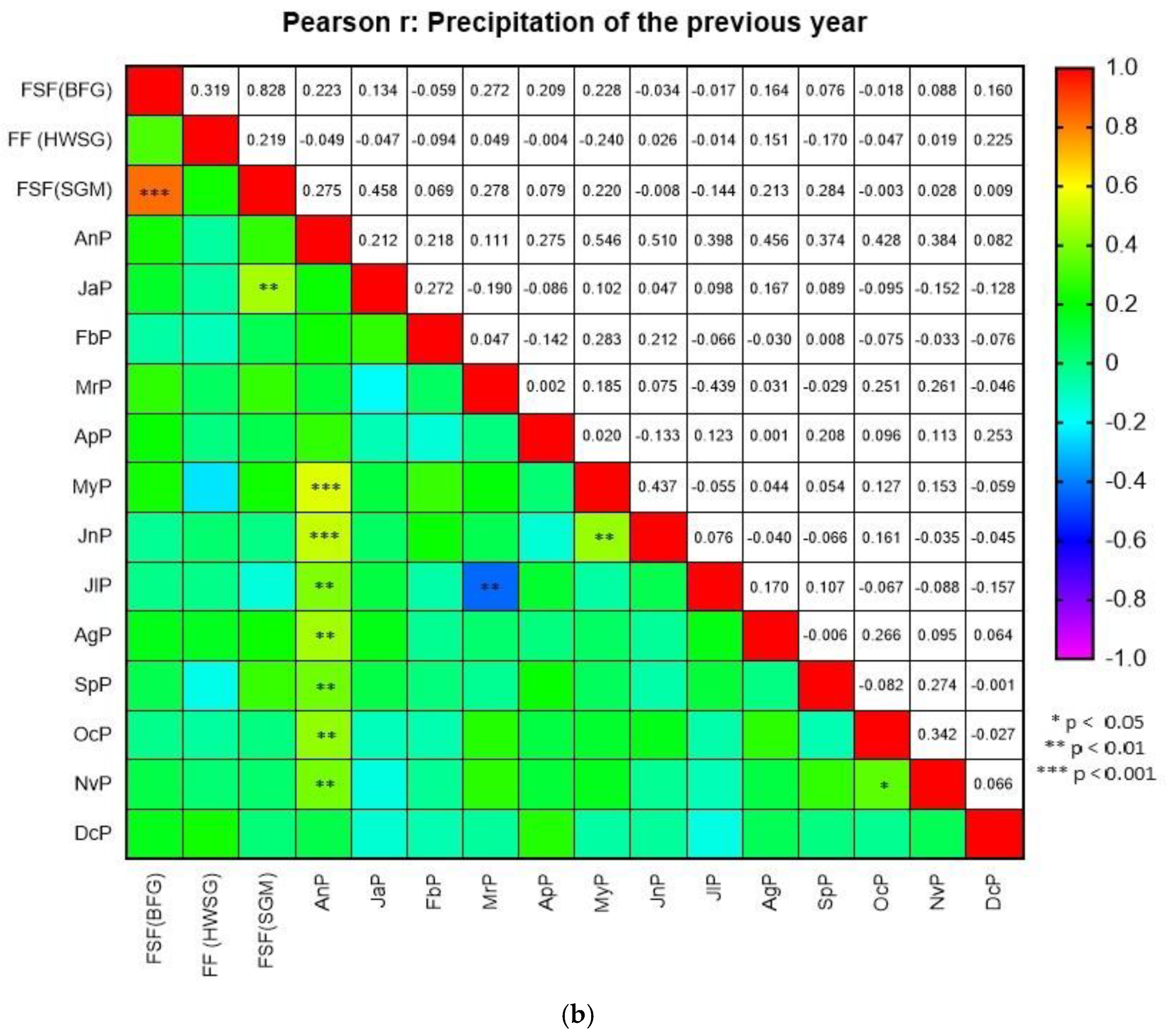
There were no evident trends in the meteorological factors influencing the fir cone crops. In the FF (HWSG), the level of fir cone crops had a moderate negative correlation with the temperature in February (
r = −0.418,
p < 0.01) and March (
r = −0.400,
p < 0.05) of the current year (
Figure 2a) and a weak negative correlation (
r = −0.334,
p < 0.05) with precipitation in April of the current year (
Figure 2b). In the FSF (SGM), the temperature in February of the previous year (
Figure 3a) and the fir cone crop had a weak positive correlation (
r = 0.332,
p < 0.05), whereas the precipitation in January of the previous year (
Figure 3b) and the crop had a moderate positive correlation (
r = 0.458,
p < 0.01). In the FSF (BFG), the temperature in February of the previous year (
Figure 3a) and the fir cone crop had a weak positive correlation (
r = 0.334,
p < 0.05).
Cone production was unaffected by precipitation during the growing season, even in years with abnormally low precipitation (dry years). Throughout the growing season (May to August), the average precipitation based on records spanning 47 years was 251 mm (
Table S1). In all the years of observation, 2010 was the driest year. During the growing season this year, there was only 92 mm of precipitation, or 37% of the average amount. In this year, average and high levels of cone crops were recorded (
Table 6). Following the driest year in 2011, average and high levels of cone crops were recorded, indicating that the crop was at the same level. In the growing season, 2012 was the second year with the least amount of precipitation. Only 109 mm of precipitation was recorded during the growing season this year, which is 42% of the norm. Low levels of cone crops were recorded during the observation period, in contrast to 2010. However, average and high levels of cone crops were observed during the next year. In contrast, the cones were totally absent in 1989 and 1990, or two consecutive years. In 1989 and 1990, 249 and 294 mm of precipitation, respectively, occurred throughout the growing season (with an average level of 251 mm).
4. Discussion
The water protection zone of the Pavlovka Reservoir (pre-Ural, Russia) contains mature forests with complex compositions. Almost all species of woody plants found in the South Urals and surrounding territories can be found in this small area, which is bounded by a riverside that is 500–2000 m wide [
38]. As a dominant tree species and co-dominant species with other conifers (mainly Siberian spruce), Siberian fir grows in almost all types of forests. The study site is an ideal test region for tracking the crop dynamics of Siberian fir cones because it lacks sources of industrial pollution, recreational load, wildfires, and insect outbreaks throughout the examined period. The evaluation of the three coniferous forest types under study revealed that these forests are growing and developing steadily based on changes in key tree stand characteristics. However, it is worth noting the high mortality rate of mature fir trees. By the end of the 47-year observation period, only 28%–29% of the initial number of fir trees survived at the two test sites. This observation can be explained by the fact that 70%–90% of fir undergrowths form xylorhizomes, and that undergrowths may exhibit long-term depressed states throughout the early phases of ontogeny [
39]. As a result, the age at which depressed undergrowth has reached the first layer of stand during growth may vary significantly from that of the fir individuals that did not form a xylorhizome by 40 to 50 years. Because this fir growth feature occurs during the early ontogeny stages, the sample size required for long-term studies must be carefully considered. To ensure that there will be enough living trees remaining at the end of the experiment, at least 50 fir trees should be used to track the long-term dynamics of the cone crop.
The overall number of years with average and high levels of fir cone crops throughout the 47-year observation period ranged from 30% to 40% in all observed stands in the coniferous forests of the water protection zone of the Pavlovka Reservoir (pre-Ural region) (
Figure 4). Simultaneously, the percentage of years with low or non-existent fir cone crops varied from 60% to 70%. Notably, high levels of fir cone crops, which can occur once or twice every 10 years depending on the type of forest, rarely occur. This information partially contradicts the findings of some scholars who, based on far less factual data, concluded that high-level fir cone crops recur every four, three, or even two years (on the southern edge of the range) [
35]. Research on Siberian fir cone crops in the Urals revealed a three- to four-year cycle of high cone production [
57]. For seven years of study, there were two years of high crops (about every three years) in the fir forests in the southeast part of the West Siberian Plain [
35]. During a 10-year study, two high crops of fir cones southw(about every five years) were observed in the Salair Ridge fir forests [
36]. Annual fir cone crops were found in the southwestern Altai during a 10-year investigation [
58], although the level of crops differed significantly from year to year. In the Pyrenees, silver fir (
Abies alba Mill.) cone crops varied greatly from year to year, but no distinct cone crop cycle was identified [
59]. There was a significant difference between the production of cones and seeds in three-year trials of the silver fir cone crop in the Carpathian foothills [
60]. A 36-year count of Douglas fir (
Pseudotsuga menziesii (Mirbel) Franco) and grand fir (
Abies grandis (Douglas ex D. Don) Lindley) cone crops in the Cowichan Lake area (Vancouver Island, British Columbia) revealed a cyclical pattern of good cone crops occurring every four years [
61]. Thus, it should be noted that the periodicity of high cone crops changes significantly not only within different environmental conditions of Siberian fir growth but also in other species of the genus
Abies.
Up to 80% of the seeds a stand produces in a particular period come from years with high cone production [
62]. Fir seeds are poorly stored in the soil (almost no seed bank is formed) and germination rates are low (up to 65%) [
34,
36]. Based on the collected data, it is possible to conclude that there is a general paucity of seeds available for successful natural regeneration of fir under a forest canopy.
Annual variations in cone crops have been documented for many conifer species [
63,
64]. The causes of these changes include fluctuations in climate, the availability of resources, variations in pollination, the effects of animals and pests, and other variables [
7,
65,
66]. The production of cones from Siberian fir was influenced by climatic conditions in both positive and negative correlations. Cones, including Siberian spruce, are produced by many conifers over a two-year cycle [
67,
68]. In the first year, cone buds are formed, and in the following year, cones grow and mature. Therefore, climatic conditions in the current (year of observation) and preceding years affect the creation of the fir cone crop. Low temperatures in February affect cone buds (damage from frost), which explains the positive correlation between the level of cone crops and the temperature in February of the preceding year (the first year of the cone production cycle). Previously, similar effects were observed in other conifers [
69,
70]. A process akin to the formation of frost cracks on tree stems can explain the negative correlation between the level of cone crops and the temperature in February and March of the current year. Typically, frost cracks appear in late winter and early spring [
71,
72]. The bark warms on bright days in late winter and early spring, causing the cells in the bark and the wood just beneath it to enlarge. The temperature decreases rapidly when the sun settles, which causes the bark to cool and contract. The bark becomes damaged as the wood beneath it cools more slowly. The cones undergo an analogous damaging process. To minimise self-pollination, conifer seed cones usually form in the upper third of the tree, whereas pollen buds form in the lower third [
73,
74]. Because the upper parts of trees receive more heat on bright days, the crop of fir cones decreases because seed cones sustain damage first.
Isolating the influence of a single environmental factor on the fir cone crops is challenging because of the complex interactions among environmental factors, which either boost or diminish each other’s degree of effect [
75]. Thus, the following series of favourabilities for the production of fir cones may be constructed by analysing data on the level of fir cone crops in various types of coniferous forests. Fir cone crops are highest in coniferous forests (FSF (SGM)) growing on south-facing slopes with sustainably wetted humus-carbonate mountain-forest soil. The second-highest level of fir cone crops is found in coniferous forests (FF (HWSG)), which grow at the base of slopes with grey bleached mountain-forest soils. The fir cone crop level of coniferous forests (FSF (BFG)), which grow on a broad plateau with grey mountain-forest soils, was the lowest in this series.
Most frequently, research on cone crop frequency (seed production) is conducted to create forecasting techniques for cone crops, which in turn helps determine the likelihood that forest communities will regenerate [
17,
33]. However, research conducted over short observation periods often forms the basis for these estimations of cone crops [
17,
76]. Any trends that previous researchers, e.g., [
32,
36], may have noticed, for instance, the frequency of high cone crops after a specific number of years, are not evident as a consequence of this study. For instance, in various coniferous forests, average fir cone crop levels may alternate with low crop levels for 12 years (1977–1988), after which there may be a three-year period during which there are no cones at all (1989–1991). In addition, during the period of 1992 to 2006, high fir cone crop levels were followed after five years by low crops for four years and then high crops after one year again during the next five years. In other words, it is very difficult to predict fir cone crop dynamics because the dynamics of the crop are unexpected.
Low cone crops are frequently repeated in the patterns of Siberian fir cone crop dynamics that have been recorded, which helps to expand our understanding of the ecological and biological traits of this tree species. These data are valuable for forest management because they remind foresters that they cannot rely exclusively on natural Siberian fir regeneration. There are also some issues with the artificial regeneration of this tree species. Rare years with high levels of cone crops, low-quality seeds, and challenging seed collecting make it challenging to cultivate planting material and promote artificial reforestation. Therefore, it is recommended to employ cutting techniques that conserve as many natural undergrowths as possible while managing forests that are mostly composed of Siberian fir trees. Practical foresters may have a false image of fir biology and ecology if we ignore the frequent presence of low-level fir cone crops. This may result in the deployment of inappropriate forest management techniques. From the perspective of forest management, this may lead to a disastrous reduction in valuable fir forests and their replacement by low-value birch and aspen forests.
5. Conclusions
In the absence of harmful influences (industrial pollution sources, recreational loads, wildfires, and insect outbreaks), the coniferous forests in the study area grow and develop steadily. The dynamics of changes in the key tree stand characteristics support these findings.
Because of the low level of cone crops in all three types of coniferous forests, it is feasible to forecast a lack of seeds that will be necessary for the effective natural regeneration of fir under the forest canopy. Moreover, the long-term crop dynamics of fir cones are unpredictable. In various types of coniferous forests, statistically significant variations in the level of fir cone crops mostly occurred in years with medium- and high-cone crops. The coniferous forests growing on south-facing slopes with sustainably wetted humus-carbonate mountain-forest soil had the highest crop of cones during the same period.
The impact of meteorological factors on the fir cone crop did not appear to follow any clear trends. The fir cone crop and weather indications for the previous year and the current year for late winter and early spring (February and March) were positively and negatively correlated.