Morphological and Molecular Characterization of Botryosphaeria wangensis Causing Branch Blight of Acer saccharum in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen Isolation and Morphological Identification
2.2. Phylogenetic Analyses
2.3. Pathogenicity Test
3. Results
3.1. Symptom Description and Morphological Characteristics
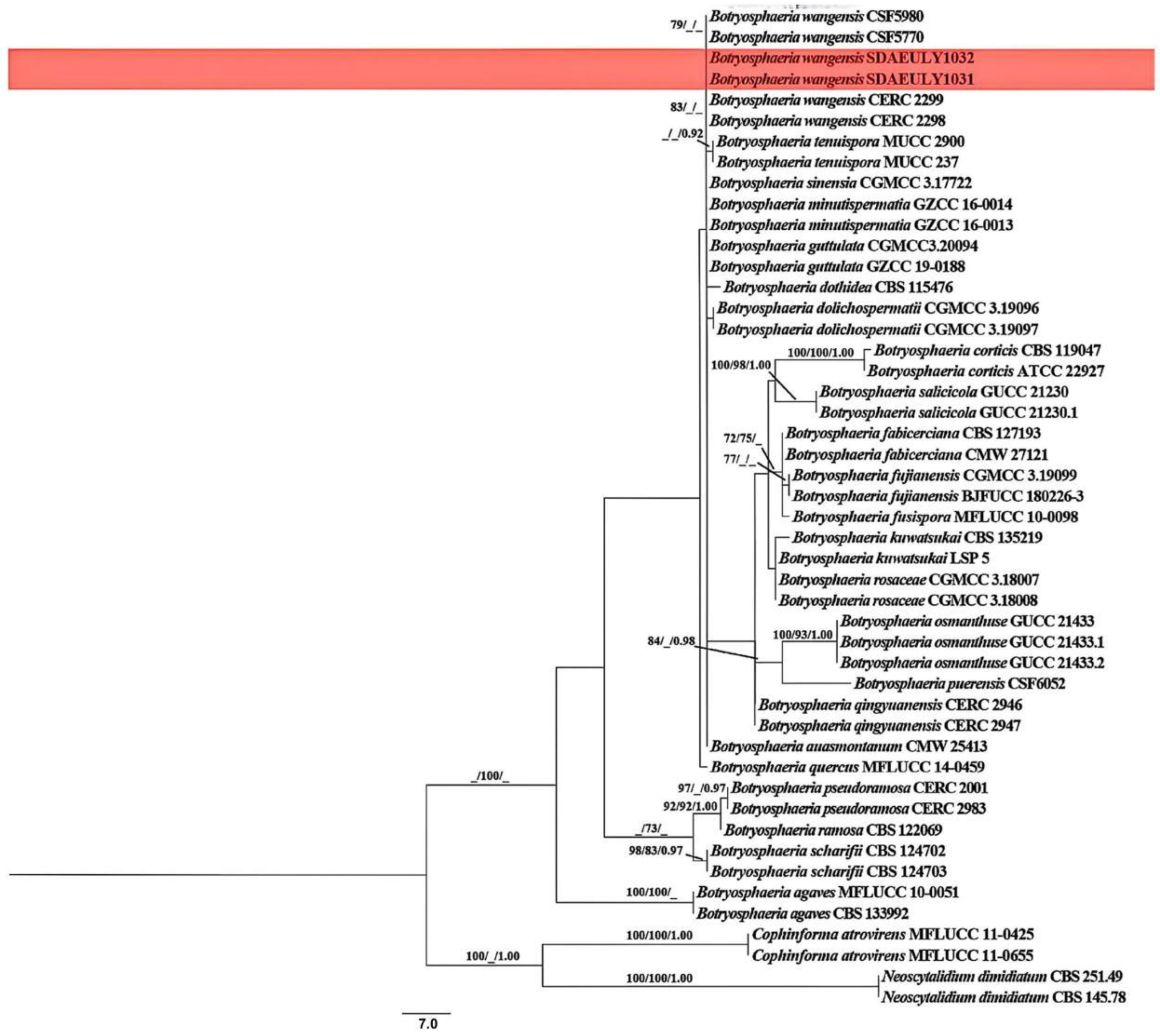
3.2. Molecular Characterization and Phylogenetic Analyses
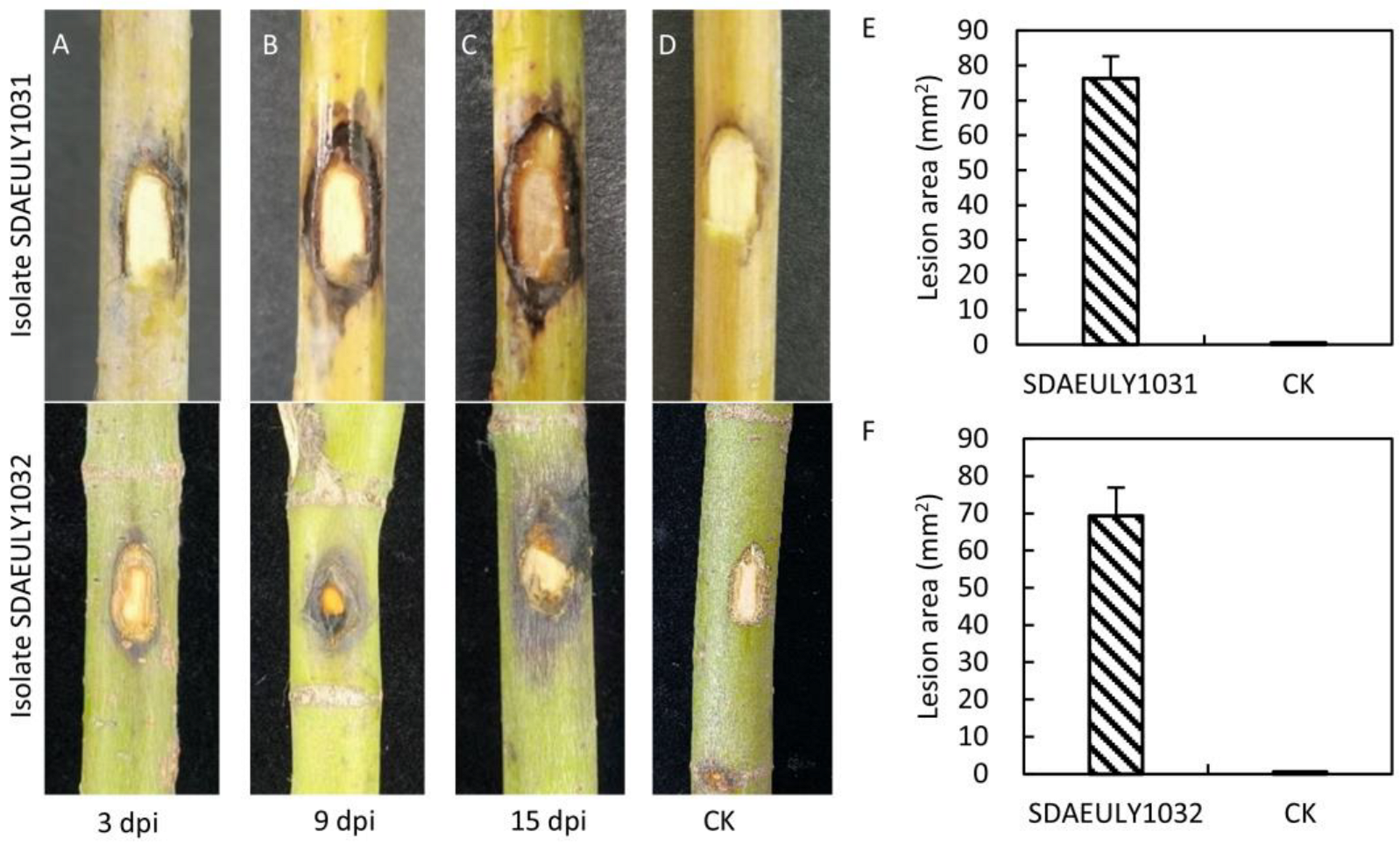
3.3. B. wangensis Pathogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Gelderen, D.M.; Jong, P.C.; Oterdoom, H.J. Maples of the World, 1st ed.; Timber Press: Portland, OR, USA, 1994; pp. 15–20. [Google Scholar]
- Dey, D.C.; Dwyer, J.; Wiedenbeck, J. Relationship between tree value, diameter, and age in high-quality sugar maple (Acer saccharum) on the Menominee reservation. Wis. J. For. 2017, 115, 397–405. [Google Scholar] [CrossRef]
- Bhatta, S.; Ratti, C.; Poubelle, P.E.; Stevanovic, T. Nutrients, antioxidant capacity and safety of hot water extract from sugar maple (Acer saccharum M.) and red maple (Acer rubrum L.) Bark. Plant Food Hum. Nutr. 2018, 73, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.; Hebda, R.; Johns, T. Use of plants for food and medicine by native peoples of eastern Canada. Can. J. Bot. 1981, 59, 2189–2325. [Google Scholar] [CrossRef]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wan, C.; González-Sarrías, A.; Kandhi, V.; Cech, N.B.; Seeram, N.P. Phenolic glycosides from sugar maple (Acer saccharum) Bark. J. Nat. Prod. 2011, 74, 2472–2476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, H.; Wu, Z. North American trees grown in China. Acta Geogr. Sin. 1997, 52, 169–176. [Google Scholar]
- Zhang, Y.; Chen, X.; Liu, H.; Zuo, H.; Zhang, H. Efficacy tests on fungicides of Phyllosticta negundinis in laboratory. J. Northeast Agric. Univ. 2011, 42, 76–80. [Google Scholar]
- Dodds, K.J.; Hull-Sanders, H.M.; Siegert, N.W.; Bohne, M.J. Colonization of three maple species by Asian longhorned beetle, Anoplophora glabripennis, in two mixed-hardwood forest stands. Insects 2013, 5, 105–119. [Google Scholar] [CrossRef]
- Aljawasim, B.; Vincelli, P. Evaluation of polymerase chain reaction (PCR)-based methods for rapid, accurate detection and monitoring of Verticillium dahliae in woody hosts by real-time PCR. Plant Dis. 2015, 99, 866–873. [Google Scholar] [CrossRef]
- Ma, X.; Ge, J.; Wang, Q.; Zhao, H.; Sun, Y.; Gao, Y.; Shen, G.; Yu, W. EAG and olfactory behavioral responses of Anoplophora glabripennis (Coleoptera: Cerambycidae) to volatiles of Acer saccharum. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 123–130. [Google Scholar]
- Petronek, H.M.; Kasson, M.T.; Metheny, A.M.; Stauder, C.M.; Lovett, B.; Lynch, S.C.; Garnas, J.R.; Kasson, L.R.; Stajich, J.E. Draft genome sequences for Neonectria magnoliae and Neonectria punicea, canker pathogens of Liriodendron tulipifera and Acer saccharum in West Virginia. Microbiol. Resour. Announ. 2025, 14, e0104224. [Google Scholar] [CrossRef]
- Osorio, J.A.; Crous, C.J.; De Beer, Z.W.; Wingfield, M.J.; Roux, J. Endophytic Botryosphaeriaceae, including five new species, associated with mangrove trees in South Africa. Fungal Biol. 2017, 121, 361–393. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Slippers, B.; Wingfield, M.J.; Chen, S. Variation in Botryosphaeriaceae from Eucalyptus plantations in YunNan Province in southwestern China across a climatic gradient. IMA Fungus 2020, 11, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, B.; Usman, H.M.; Javed, K.; Al-Otibi, F.; Hyde, K.D.; Wang, Y. First report of Botryosphaeria wangensis, Colletotrichum nymphaeae, Diaporthe eres, and Geotrichum candidum causing postharvest fruit rot of plums (Prunus salicina) in China. Crop Prot. 2025, 190, 107089. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, L.; Mondino-Hintz, P.; Alaniz-Ferro, S. Diversity of Botryosphaeriaceae species causing stem canker and fruit rot in olive trees in Uruguay. J. Phytopathol. 2022, 170, 264–277. [Google Scholar] [CrossRef]
- Hattori, Y.; Ando, Y.; Sasaki, A.; Uechi, N.; Nakashima, C. Taxonomical study of noteworthy species of Botryosphaeria in Japan. Mycobiology 2021, 49, 122–132. [Google Scholar] [CrossRef]
- Li, G.; Liu, F.; Li, J.; Liu, Q.; Chen, S. Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia 2018, 40, 63–95. [Google Scholar] [CrossRef]
- Chen, Y.; Dissanayake, A.J.; Liu, Z.; Liu, J. Additions to Karst Fungi 4: Botryosphaeria spp. associated with woody hosts in Guizhou Province, China including B. guttulata sp. nov. Phytotaxa 2020, 454, 186–202. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Liu, Y.; Wu, Z.; Li, C.; Pan, H. First report of Botryosphaeria dothidea causing leaf blight on Aesculus chinensis. J. Phytopathol. 2025, 173, e70085. [Google Scholar] [CrossRef]
- Lin, L.; Bai, Y.; Pan, M.; Tian, C.; Fan, X. Morphology and molecular analyses reveal three new species of Botryosphaeriales isolated from diseased plant branches in China. MycoKeys 2023, 97, 1–19. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 12, 1799. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Meng, C.; Phillips, A.J.L.; Wang, Y. Two new Botryosphaeria (Botryosphaeriales, Botryosphaeriaceae) species in China. MycoKeys 2022, 94, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, A.; Jiang, N. Identification of the nut rot pathogen affecting Castanopsis carlesii based on morphological and phylogenetic analyses. Forests 2025, 16, 627. [Google Scholar] [CrossRef]
- Garcia, J.F.; Laurence, D.P.; Morales-Cruz, A.; Travadon, R.; Minio, A.; Hernandez-Mertinez, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Phylogenomics of Plant-Associated Botryosphaeriaceae Species. Front. Microbiol. 2021, 12, 652802. [Google Scholar] [CrossRef]
- Nagel, J.H.; Wingfield, M.J.; Slippers, B. Increased abundance of secreted hydrolytic enzymes and secondary metabolite gene clusters define the genomes of latent plant pathogens in the Botryosphaeriaceae. BMC Genom. 2021, 22, 589. [Google Scholar] [CrossRef]
- Phillips, A.J.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Moral, J.; Morgan, D.; Trapero, A.; Michailides, T.J. Ecology and epidemiology of diseases of nut crops and olives caused by Botryosphaeriaceae fungi in California and Spain. Plant Dis. 2019, 103, 1809–1827. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Chen, W.; Liu, X.; Wu, M.; Liu, Y. Morphological and Molecular Characterization of Botryosphaeria wangensis Causing Branch Blight of Acer saccharum in China. Forests 2025, 16, 1786. https://doi.org/10.3390/f16121786
Shao C, Chen W, Liu X, Wu M, Liu Y. Morphological and Molecular Characterization of Botryosphaeria wangensis Causing Branch Blight of Acer saccharum in China. Forests. 2025; 16(12):1786. https://doi.org/10.3390/f16121786
Chicago/Turabian StyleShao, Chenxi, Wenxian Chen, Xiaojia Liu, Mutao Wu, and Yun Liu. 2025. "Morphological and Molecular Characterization of Botryosphaeria wangensis Causing Branch Blight of Acer saccharum in China" Forests 16, no. 12: 1786. https://doi.org/10.3390/f16121786
APA StyleShao, C., Chen, W., Liu, X., Wu, M., & Liu, Y. (2025). Morphological and Molecular Characterization of Botryosphaeria wangensis Causing Branch Blight of Acer saccharum in China. Forests, 16(12), 1786. https://doi.org/10.3390/f16121786






