1. Introduction
Soils represent a vast global carbon reservoir, storing three times more carbon than the atmosphere and two to four times more than terrestrial vegetation [
1]. Consequently, even minor fluctuations in the soil carbon pool can significantly influence the global carbon cycle, alter atmospheric CO
2 concentrations, and disrupt the planetary carbon balance [
2,
3,
4]. These disruptions have profound implications for the global climate, driving critical environmental issues such as global warming. Forests, as a core component of the terrestrial ecosystem, provide a crucial carbon sink and deliver indispensable services for maintaining ecological equilibrium [
5,
6,
7]. Therefore, accurately assessing soil carbon stocks and their dynamics across different regions and forest types is essential for designing and implementing targeted ecological conservation and restoration strategies.
Species diversity plays a critical role in regulating ecosystem stability, serving as a key determinant of both ecosystem productivity and stability [
8]. It influences soil carbon storage primarily through pathways such as litter input [
9], root exudate release [
10], and microbial interactions [
11]. Evidence indicates that ecosystems with high plant diversity typically exhibit greater productivity, supplying more organic matter and nutrients, which in turn enhances soil quality and promotes nutrient cycling [
9,
12,
13]. Research from the German Jena Experiment demonstrates that increased plant richness enhances carbon input from roots to soil microbial communities, thereby boosting microbial activity and soil carbon storage [
14]. Furthermore, highly diverse plant communities promote the formation of mineral–organic associations, which improves soil carbon retention capacity [
15]. In addition, variations in tree species composition directly affect recalcitrant compound dynamics—such as lignin content and polyphenols—and soil carbon-to-nitrogen ratios (C/N), consequently influencing the quality and stability of soil carbon sequestration [
16]. Unraveling the impact of plant diversity on forest carbon stocks is therefore essential for improving forest management and addressing global change.
The Loess Plateau, located in China’s semi-humid to semi-arid region, is the world’s largest loess deposit. It is a typically fragile ecosystem that has long been subjected to severe soil erosion [
17]. In this region, Soil Inorganic Carbon (SIC) is the dominant form of soil carbon [
18,
19]. Data indicate that the density of SIC in the plateau’s soils is approximately four times that of Soil Organic Carbon (SOC), making it one of China’s most significant SIC reservoirs [
20,
21]. Despite the predominance of soil inorganic carbon (SIC), the vast majority of research on forest carbon sequestration has focused on soil organic carbon [
22,
23]. Consequently, the dynamics of SIC and its relationship with plant diversity and total soil carbon in semi-arid forests remain poorly understood. Therefore, this study addresses this gap by focusing on the unique context of the Loess Plateau—a semi-arid, fragile ecosystem dominated by SIC. We specifically investigate the coupling relationships between forest plant diversity and both SIC and SOC, thereby providing novel insights into the species diversity-soil carbon dynamic in SIC-dominated semi-arid forests.
The Cuiying Mountain area in Yuzhong County, Gansu Province, exemplifies the typical loess ridge-mountain landscape of the plateau. The health of its forest ecosystems is crucial for maintaining local and regional ecological balance [
24,
25]. Historically, the area had sparse vegetation dominated by herbaceous plants, resulting in limited ecological functionality. In recent years, initiatives led by Lanzhou University and local authorities have combined artificial greening projects with the preservation of natural vegetation. This integrated approach has created a restoration model that merges human-assisted rehabilitation with natural regeneration, leading to significant ecosystem improvement [
26]. Consequently, the landscape now comprises a mosaic of natural and plantation forests, providing an ideal setting to investigate the relationship between forest naturalness and soil carbon sequestration [
27].
Total soil carbon plays a critical role in global ecological change. Unraveling its relationship with plant diversity is therefore vital for informing ecological restoration. However, research on these dynamics in semi-arid forests remains limited. Therefore, this study aims to address the following key questions: (1) to develop a multi-dimensional assessment framework for forest naturalness that integrates herb coverage, shrub coverage, tree biodiversity, and local stand structural attributes; (2) to examine the relationship between plant diversity and soil carbon content at different depths in the cinnamon soil profile [
28]. This research will advance our understanding of how species diversity in arid regions regulates soil nutrient cycling via carbon dynamics, providing a theoretical basis for optimizing plantation structures and enhancing soil carbon storage. Ultimately, these insights will offer practical guidance for regional ecological restoration and forest carbon management.
2. Materials and Methods
2.1. Study Area
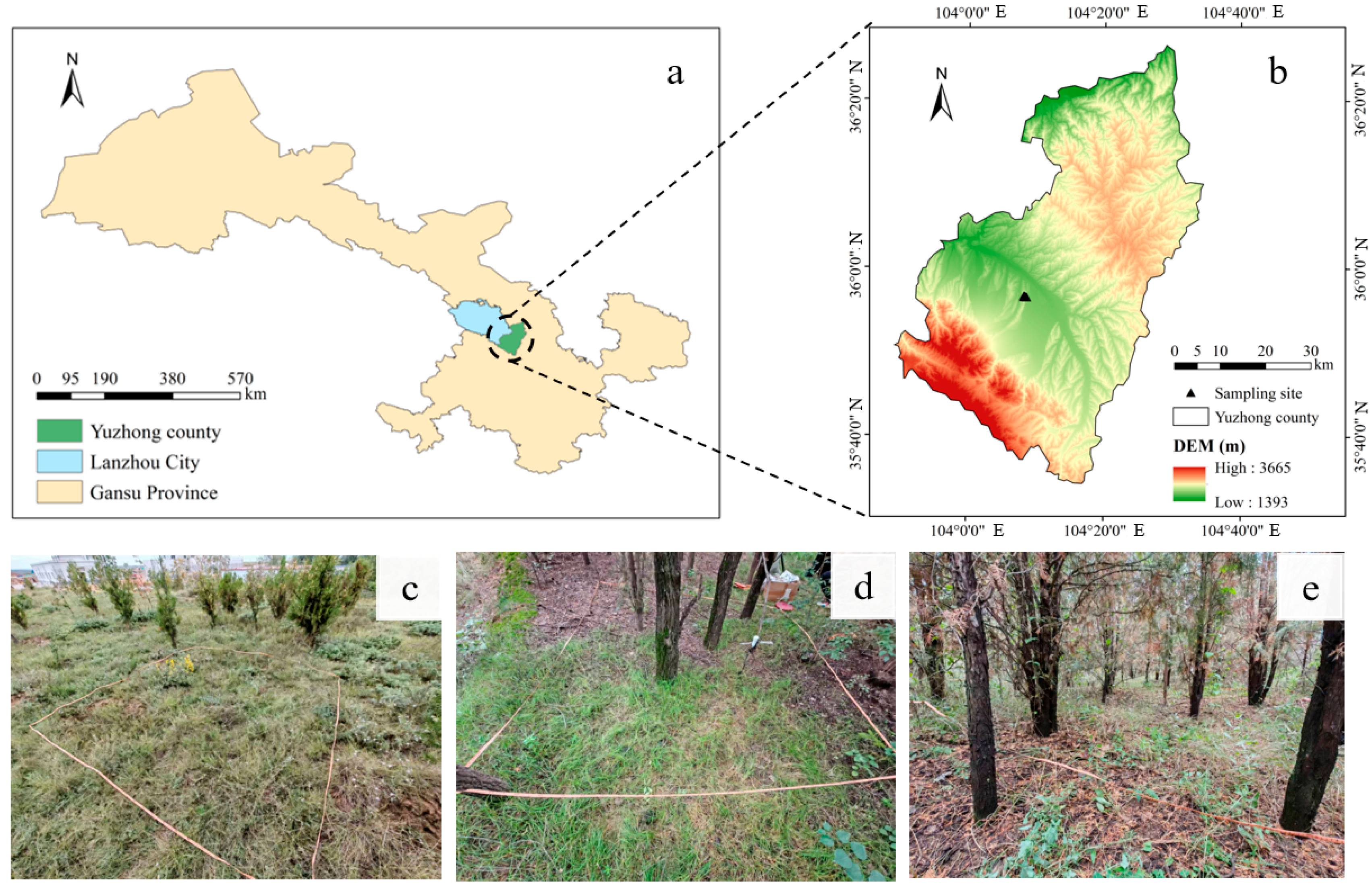
The study area is located on Cuiying Mountain in Yuzhong County, Lanzhou City, Gansu Province, China (104°08′14″ E–104°08′37″ E, 35°56′36″ N–35°56′40″ N), covering a total area of 3.49 hectares. Cuiying Mountain features a typical loess ridge landform of the Loess Plateau, located at the convergence of the Loess Plateau, the Inner Mongolian Plateau, and the northeastern Tibetan Plateau. The region has a temperate semi-arid continental climate, with a mean annual temperature ranging from 6 to 10 °C and a long-term average precipitation of 350 mm. The soils in the study area are classified as Gray-cinnamon soil according to the Classification and Codes for Chinese Soil, which corresponds to Calcisols in the World Reference Base for Soil Resources (WRB) system. These soils are characterized by an alkaline pH and a distinct subsurface layer of calcium carbonate accumulation (calcic horizon), formed under the arid and semi-arid conditions of the region. The natural vegetation is predominantly composed of xerophytic, psammophytic, and halophytic perennial herbs, shrubs, and tree species. Representative native plants include conifers (e.g., Pinus spp.), sagebrush (e.g., Artemisia spp.), needlegrass (Achnatherum splendens), and goosegrass (Eleusine indica). These species form diverse plant communities with associated companion species, creating an integrated ecosystem that plays a vital role in windbreaking, sand fixation, soil and water conservation, and maintaining ecological balance.
2.2. Sample Collection and Processing
In September 2024, ten sampling sites (Y1–Y10) were established along a hillslope transect from the hilltop to the footslope within the Cuiying Mountain study area (
Figure 1). These sites were strategically placed away from human settlements and distributed to represent the area’s primary features. At each sampling site, a 10 m × 10 m plot was established, resulting in a total sampled area of 1000 m
2, which accounted for 2.9% of the total study area. Within each plot, we documented plant community characteristics, including species identity, individual count, plant height, and tree diameter at breast height (DBH). Each plot was subdivided into four 5 m × 5 m quadrats. A soil profile was excavated at the center of each quadrat, and a one-meter deep soil sample was collected using a profile sampler. A quartering method was employed to obtain a representative sub-sample, which was then sealed and transported to the laboratory. Prior to analysis, the soils were air-dried, and any stones, plant residues, and roots were removed. The samples were subsequently ground and sieved through 2 mm and 0.149 mm meshes for physicochemical analysis. Basic information for the sampling sites is provided in
Table 1. Soil types were identified and classified based on the Classification and Codes for Chinese Soil [
28].
2.3. Development of the Forest Naturalness Assessment Framework
Community composition in this study was characterized by three key indicators: herb coverage, shrub coverage, and tree layer biodiversity. Herb coverage, defined as the proportion of ground covered by herbaceous plants, is expressed as a percentage. Similarly, shrub coverage represents the areal extent of shrub vegetation and is also quantified as a percentage [
29]. Biodiversity within the tree layer was quantified using the Shannon Index, which incorporates both species richness and the evenness of individual distribution among species. The index was calculated as follows [
30]:
where H′ is the tree layer biodiversity index (Shannon Index), S is the total number of tree species, and P
i is the proportion of individuals belonging to the i-th species relative to the total number of trees in the stand.
The structural characteristics of the tree layer were characterized by the coefficient of variation of diameter at breast height (CV
DBH) and the mean mingling degree (
). The CV
DBH measures the relative variability in tree diameters at breast height (DBH, measured at 1.3 m above ground) within a given area and is expressed as the relative standard deviation. It was calculated as follows [
31]:
where CV
DBH is the coefficient of variation for diameter at breast height,
is the standard deviation of DBH, and
is the mean DBH.
The degree of species segregation in the mixed forest was characterized using a spatial species mingling metric, calculated as the mean mingling degree for all trees in the stand [
32]. The formula is as follows:
where M
i is the mingling degree of the i-th subject tree, n is the number of its nearest neighbors, and v
ij is an indicator variable that equals 1 if the j-th neighboring tree is a different species from the subject tree i, and 0 otherwise. The mean mingling degree for the entire stand,
, was calculated as the average of M
i across all N subject trees surveyed in the stand.
Within each plot, herb coverage was measured by randomly placing five 1 m × 1 m quadrats. The vertical projected area of herbaceous plants was determined in each quadrat, and the average value across the five quadrats was calculated to represent the plot’s herb coverage. Similarly, shrub coverage was assessed using three randomly placed 2 m × 2 m quadrats. The tree layer diversity index was calculated by recording all trees with a diameter at breast height (DBH) ≥ 5 cm, including their species identity and individual counts. The proportion of each species relative to the total number of trees was then computed and used to calculate the Shannon index (H′). The standard deviation of DBH was calculated from all measured trees across the plots. The coefficient of variation of DBH (CVDBH) was then derived as the ratio of this standard deviation to the mean DBH. Finally, the mean mingling degree () was determined for each plot. For every tree, its mingling degree was calculated based on the species of its n nearest neighbors (awarding 1 point for a different species and 0 for the same species), as defined in Formula (3). The mean mingling degree for the plot was the average of all individual tree values.
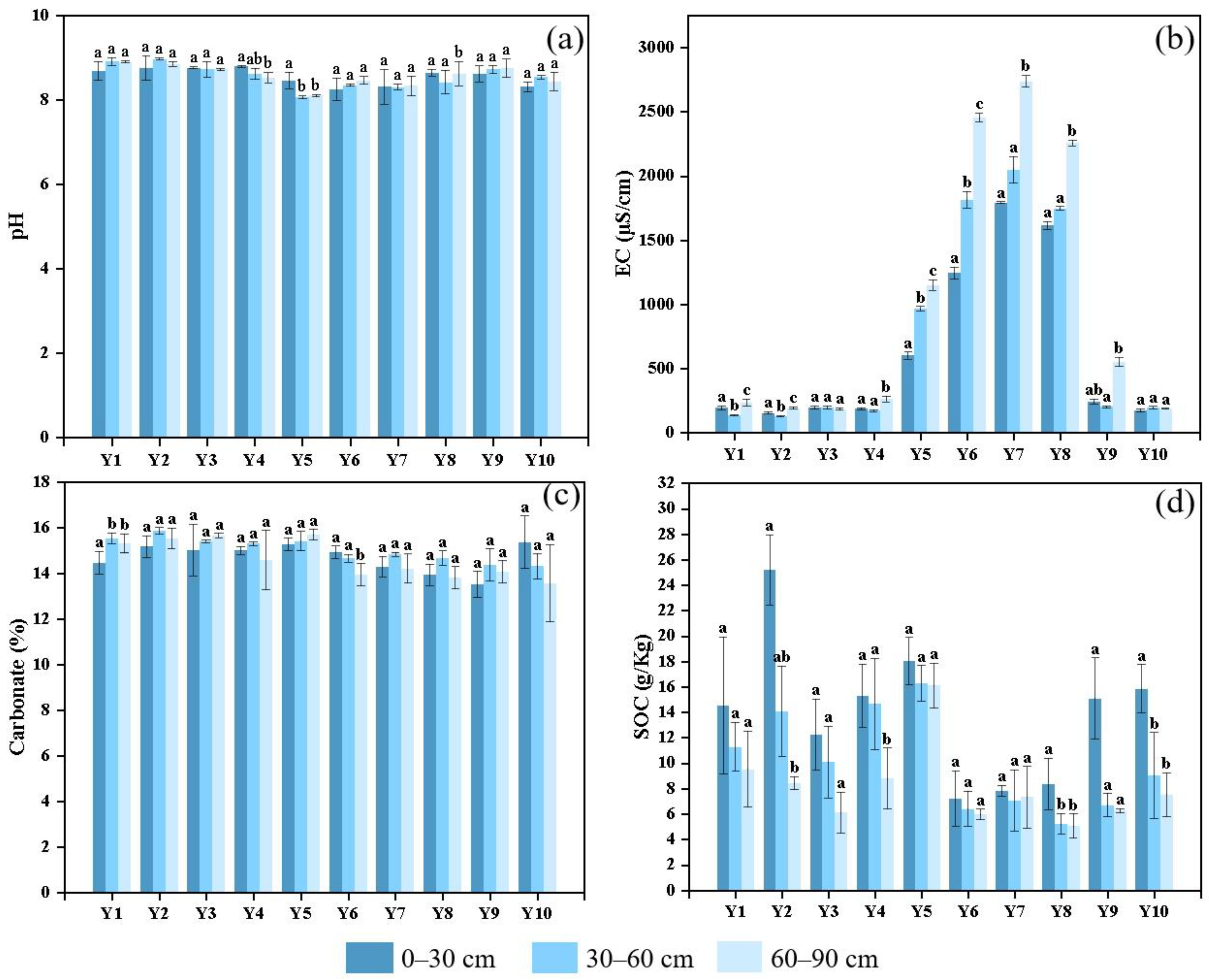
2.4. Laboratory Analysis of Soil Properties
Soil physicochemical properties were analyzed following the procedures outlined in “Soil Agricultural Chemistry Analysis” [
33]. Soil pH and electrical conductivity (EC) were measured in a 1:2.5 soil-to-water suspension at room temperature using a pH meter (PHS-3E, Leici, INESA, Shanghai, China) and an EC meter (HI 98311, HANNA Instruments, Villafranca Padovana, Italy), respectively. Soil organic carbon (SOC) was determined by the potassium dichromate oxidation method [
34], and soil carbonate (CaCO
3) content was measured via volumetric titration [
35]. Triplicate measurements were conducted for each specified parameter on every soil sample to ensure analytical precision.
2.5. Data Processing
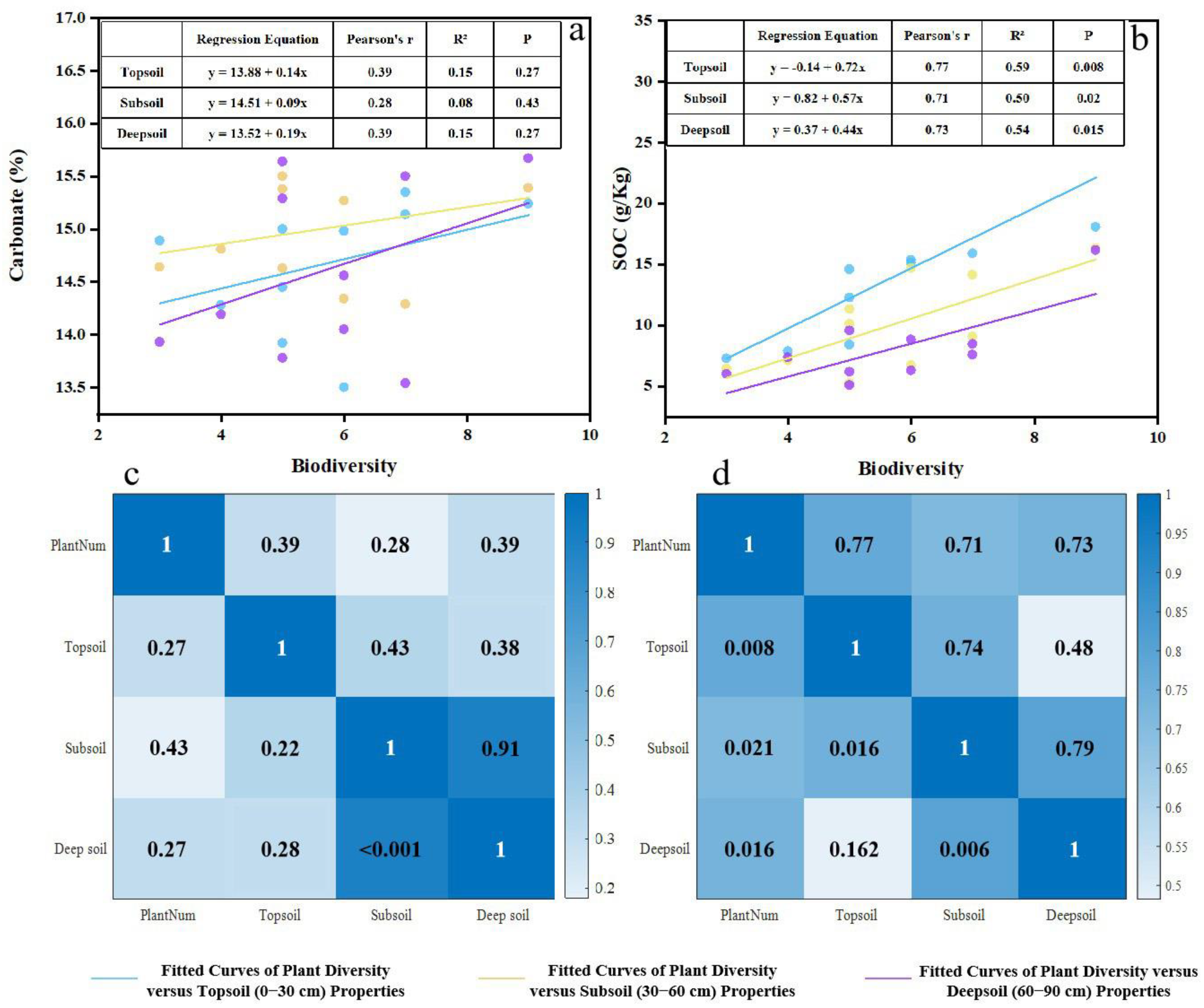
Data processing, statistical analysis, and standardization were performed using Microsoft Excel 2021. A one-way ANOVA was used to assess differences in soil properties across depths, while the Kruskal–Wallis test was employed to evaluate variations among plots. The relationships between plant species number and soil carbon content were examined using regression analysis, while Pearson’s correlation analysis was used to assess the association between plant diversity and soil carbon. Post hoc multiple comparisons were conducted using the Least Significant Difference (LSD) test, with a significance level of p < 0.05. Graphs and charts were generated using OriginPro 2021, and the sampling location map was created with ArcMap 10.8.1.
5. Conclusions
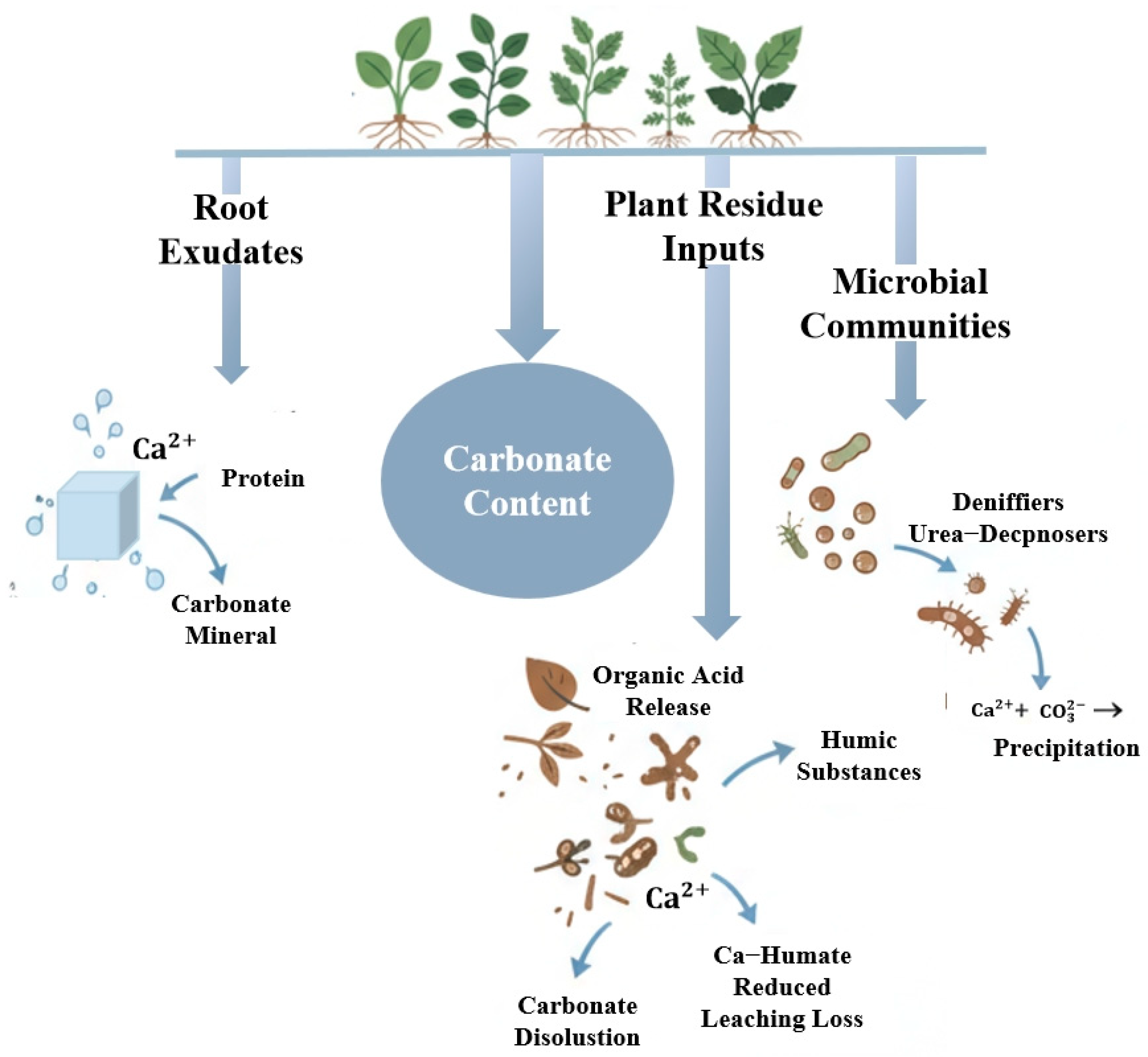
This study evaluated forest naturalness in the Cuiying Mountain area and investigated its relationship with soil carbon content in an arid region of Northwest China. Our findings indicate that (1) the forest exhibited a low level of naturalness, characterized by high herbaceous coverage but minimal shrub coverage (2.1%), low tree diversity (Shannon index = 0.09), a wide range of tree sizes indicating multiple age classes, and a simple, single-layer structure (mean mingling degree = 0.14). (2) Forest naturalness positively influenced both soil inorganic and organic carbon, with the strongest correlation observed with surface soil organic carbon accumulation. The more pronounced effect on SOC can be attributed to distinct accumulation mechanisms. While SIC dynamics are primarily governed by dissolution–precipitation processes influenced by root exudates and litter decomposition, SOC accumulation is directly regulated by plant-derived inputs. These inputs, through litterfall and root secretions, along with the consequent increase in microbial diversity and abundance, directly enhance SOC storage and distribution.
This study provides initial insights into the relationship between forest naturalness and soil carbon content through a single sampling event. A primary limitation is that this single-time-point measurement may not fully capture interannual dynamics in soil carbon and plant–soil interactions, potentially affecting the long-term robustness and generalizability of our findings. Future work should include multi-year monitoring to track how vegetation dynamics influence soil carbon stocks, thereby verifying the persistence and stability of our results. Incorporating additional ecological factors (e.g., microbial community structure, litter decomposition rates) will further elucidate the underlying mechanisms through which plant diversity drives soil carbon accumulation, ultimately providing a more comprehensive theoretical basis for ecosystem carbon management in the Loess Plateau.