1. Introduction
Phellodendron chinense chinense is a deciduous tree belonging to the
Phellodendron chinense genus in the Rutaceae family. It is a nationally protected second-class plant and is endemic to Northeast Asia. This species is recognized as one of the “three major woody herbs” and is classified as endangered (EN) due to over-exploitation. It is also noted as one of the “three major woody medicinal herbs” and faces the same endangered classification for similar reasons [
1]. Its natural habitat is primarily found in cold–temperate to temperate coniferous forests and mixed forests between latitudes 39° to 52° north. In China, it is mainly found in regions such as Hubei, Hunan, Sichuan, and Chongqing. The tree is known for its rapid growth, strong shade tolerance, and good cold resistance, demonstrating a broad ecological adaptability. It has various uses, including medicinal applications, landscape gardening, timber production, and ecological restoration [
2,
3]. However, the rapid growth of the traditional Chinese medicine industry has led to a significant depletion of wild
Phellodendron chinense resources, and the limited scale of artificial cultivation has resulted in a shortage of medicinal supply. Consequently, large-scale cultivation and efficient farming techniques have become essential strategies to address the supply–demand imbalance of
Phellodendron chinense [
4].
Proportional fertilization, as an effective nutrient management approach, can greatly enhance plant growth and development by maintaining a balanced relationship between the nutrients available in the soil and the needs of the plants. In contrast to conventional fertilization methods, it offers benefits such as improved fertilizer efficiency, considerable economic advantages, and reduced environmental risks [
5,
6,
7]. Recent research has demonstrated that NPK fertilization greatly benefits a wide range of plants, as noted by Yin Mengya and colleagues [
8]. Yan Jiewei and colleagues discovered that appropriate NPK fertilization can enhance the growth traits and physiological processes of Gardenia jasminoides seedlings [
9]. Research indicated that NPK fertilization can greatly improve the growth of the ornamental peach variety ‘Yuanchun’ (Prunus persica ‘Yuanchun’), which is the most widely cultivated ornamental peach. This fertilization positively affects the biomass accumulation and photosynthetic efficiency of ‘Yuanchun’ (Prunus persica ‘Yuanchun’), according to Zhang Bin and colleagues [
10]. It was further noted that the combined use of NPK fertilizers could boost the photosynthetic ability and antioxidant enzyme activity in the leaf blades of the chestnut (Sloanea hemsleyana), thereby increasing the resilience of seedlings. However, only a small portion of current research on
Phellodendron chinense has concentrated on tissue culture [
11], extraction of active compounds [
12,
13], species identification [
14], and pharmacological properties [
15], with a lack of comprehensive studies on its fertilization strategies.
This study was carried out to assess the growth metrics (such as plant height, diameter, and biomass) and physiological factors (including chlorophyll content, photosynthetic rate, and antioxidant enzyme activity) of seedlings subjected to various fertilizer ratios in the Jiangjin region of Chongqing. The research utilized an L9(33) orthogonal experimental design and examined the effects of nitrogen, phosphorus, and potassium on seedling growth through correlation analysis and principal component evaluation. The findings of this research can inform the cultivation practices for Phellodendron chinense and offer a theoretical foundation and technical assistance for the effective fertilization and efficient management of Phellodendron chinense plantation forests.
2. Materials and Methods
2.1. Overview of the Test Site
The experiment was carried out at the West Garden Nursery of Central South Forestry University of Science and Technology, located in Changsha, Hunan Province, with geographical coordinates of 112°59′37″ E and 28°08′15″ N. This region is characterized by a subtropical monsoon climate, which features four distinct seasons, including hot and humid summers, mild winters with low precipitation, and a predominance of long summers and short winters. Notably, the majority of precipitation occurs between April and June, as indicated by data collected from the local meteorological station between 2020 and 2023.
2.2. Test Materials
The experimental subjects comprised 1-year-old live seedlings of
Phellodendron chinense (
Phellodendron chinense Schneid), sourced from the Jiangjin District of Chongqing, China. A selection of healthy seedlings exhibiting uniform plant height (15.3 ± 1.2 cm), ground diameter (2.8 ± 0.3 mm), and root development was conducted. These seedlings were transplanted in April 2023 into polyethylene plastic pots measuring 18 cm in upper diameter, 15 cm in lower diameter, and 20 cm in height, with each seedling being transplanted individually. The growth substrate was formulated as a homogeneous mixture of vermiculite, peat soil, and red loam in a volumetric ratio of 1:1:3. The substrate was filled to a height of 1 cm below the rim of the pots to facilitate irrigation. Prior to transplantation, the fundamental physicochemical properties of the substrate—including pH, organic matter content, total nitrogen, effective phosphorus, and readily available potassium—were assessed, as detailed in
Table 1 and
Table 2. The fertilizers utilized in the study included urea (CO(NH
2)
2, with a nitrogen content of 46%, classified as a nitrogen fertilizer calcium superphosphate (Ca(H
2PO
4)
2·H
2O, with a phosphorus pentoxide content of 16%, classified as a phosphate fertilizer), and potassium chloride (KCl, with a potassium oxide content of 60%, classified as a potash fertilizer).
2.3. Experimental Design
In this research, the L9(3
3) orthogonal experimental design was employed to establish three-factor and three-level fertilization treatments involving nitrogen (N), phosphorus (P
2O
5), and potassium (K
2O), as detailed in
Table 3. A no-fertilization treatment served as the control (CK). The experimental framework comprised ten treatment groups, including the control, with each group subjected to six biological replications, resulting in a total of 60 seedlings. The fertilization rates were determined based on the initial nutrient content of the potting substrate and the annual growth patterns of
Phellodendron chinense seedlings, with specific fertilization ratios outlined in
Table 4.
The experiment commenced in early May 2023 and concluded at the end of October, with a total duration of six months. The timing of fertilizer application was designed in accordance with the seasonal nutrient uptake characteristics of the seedlings, with dynamic fertilization implemented during the rapid growth phase from May to September 2023. Thirty days post-transplantation, the fertilizers for each treatment group were dissolved in 300 mL of deionized water, thoroughly mixed, and subsequently applied along a circular groove positioned 5 cm from the base of the main root. Fertilizer was applied at intervals of 30 days, totaling five applications, with each application constituting 20% (i.e., 1/5) of the total fertilizer amount to align with the specific nutrient demands of the seedlings at various growth stages.
2.4. Measurement of Indicators
Following a 150-day fertilization treatment, three seedlings were randomly selected from each treatment group to assess growth and physiological parameters. The ground diameter was measured using digital vernier calipers with an accuracy of 0.01 mm, while seedling height was determined with a laser altimeter, which has an accuracy of ±1 cm. The apical third of a mature leaf was collected for analysis, and the contents of chlorophylls a and b, as well as the total chlorophyll content, were quantified through acetone-ethanol extraction [
16].The soluble sugar content was assessed using the anthrone-sulfuric acid method [
17], and the soluble protein content was measured via the Thomas Brilliant Blue G-250 staining method [
18]. Additionally, the total nitrogen content in the roots, stems, leaves, and substrate was determined using the Kjeldahl method [
19]. The total phosphorus content was analyzed using the molybdenum-antimony colorimetric method [
20], and the total potassium content was measured using the flame photometric method [
21].
2.5. Data Analysis
All raw data generated from the experiment, including seedling growth indicators (height increment, ground diameter increment), biomass, root system parameters, physiological indices (chlorophyll, soluble sugar, soluble protein contents), and nutrient contents (total nitrogen, phosphorus, potassium in plant tissues and soil), were first collated and verified using Microsoft Excel 2022 (Microsoft Corporation, Redmond, WA, USA). Descriptive statistical indicators such as mean and standard deviation were calculated to characterize the data distribution.
Statistical analyses were primarily performed using IBM SPSS Statistics 27. Given the single variable of fertilization treatment in the study, one-way analysis of variance (ANOVA) was applied to assess the significance of differences in all measured indicators among different fertilization treatments. When significant differences were detected (p < 0.05), Duncan’s multiple comparison method was further employed for post hoc testing to identify specific differences between treatment groups. Additionally, Pearson correlation analysis was conducted to explore the strength and direction of associations among 25 key variables, with significance levels set at p < 0.05 and p < 0.01. Principal Component Analysis (PCA) was implemented for dimensionality reduction in the multi-index dataset, aiming to extract core principal components, clarify the contribution of each original variable to the principal components, and identify key comprehensive factors driving seedling growth and physiological responses.
All visualizations, including line charts for growth dynamics, bar charts for indicator comparisons, Pearson correlation network diagrams, and principal component analysis plots, were generated using Origin 2022 (OriginLab Corporation) to ensure clear and intuitive presentation of analytical results.
3. Results and Analysis
3.1. Effects of Nitrogen, Phosphorus and Potassium on the Growth and Physiology of Phellodendron chinense Seedlings
3.1.1. Dynamic Response of Plant Height to Ground Diameter
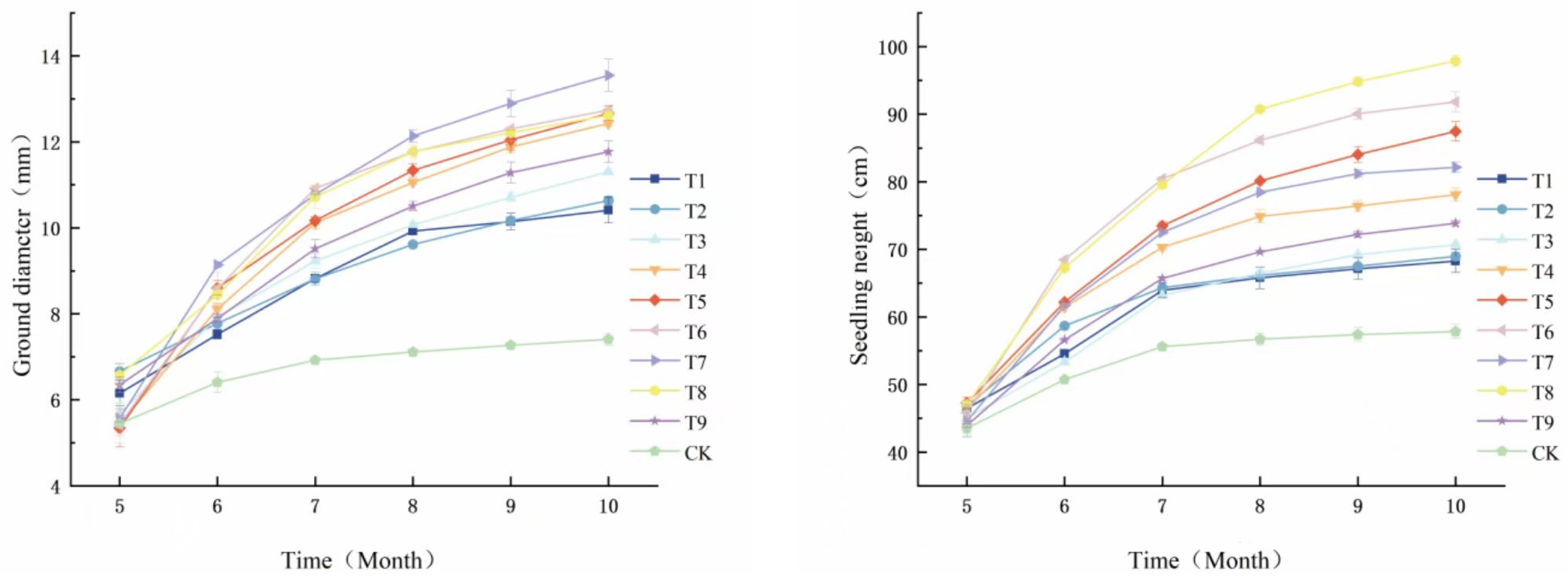
As illustrated in the accompanying figure (
Figure 1), the growth patterns of
Phellodendron chinense seedlings in relation to the timing of fertilizer application reveal a “slow-fast-slow” trend in both seedling height and diameter. The growth rate of seedling height for
Phellodendron chinense seedlings was found to be consistent with the growth rate of diameter, indicating a synchronized development of the aboveground parts. From May to August, the increase in plant height was observed to be relatively modest and gradual (
Figure 2). In contrast, from August onwards, both seedling height and diameter exhibited significant increases across all fertilization treatments when compared to the control group (CK). Notably, the treatment T7 resulted in the highest total increment in seedling height, measuring 46 cm, which represents a 261.5% increase relative to CK. Similarly, treatment T8 recorded the greatest total increment in diameter, reaching 7.98 mm.
The application of fertilizer treatments to seedlings resulted in significant increases in both plant height and diameter increments of
Phellodendron chinense, surpassing the measurements observed in the unfertilized control group (CK). Among the treatments, T5 exhibited the most substantial growth in both plant height and diameter, followed closely by T6, while CK demonstrated the least growth. Specifically, the increments in plant height for T5 and T6 were recorded at 161.5% and 146.9%, respectively, compared to CK, with T5 and T6 showing increases of 259.6% and 216.0% in diameter, respectively, over CK. Conversely, the lowest increments in both plant height and diameter were noted in the fertilized treatment T8, which were 25.2% and 34.0% higher than CK, respectively. These findings indicate that optimal growth outcomes were achieved with T5, followed by T6, suggesting that a specific concentration of phosphorus and potassium fertilizers can effectively enhance the growth of seedlings, particularly in
Phellodendron chinense, under the T5 formulation [
22,
23].
3.1.2. Regulatory Effects of Nitrogen, Phosphorus and Potassium Rationing on Biomass Accumulation
The accumulation of biomass exhibited a pronounced sensitivity to the fertilization ratio, as indicated in
Table 5. All biomass components, including aboveground, belowground, and total biomass, demonstrated significant increases (
p < 0.05) across all treatments when compared to the control (CK). Notably, the T1 treatment resulted in the most substantial enhancement of belowground biomass, with an increase of 92.37%. Conversely, the T9 treatment yielded the most pronounced improvement in aboveground biomass, achieving an increase of 319.69%. The T5 treatment recorded a peak total biomass of 47.67 g, reflecting a remarkable increase of 188.89% relative to CK. In the T6 treatment, which combined low potassium (K1) with high phosphorus (P3), there was no significant enhancement in aboveground biomass; however, total root length and root volume increased by 112.75% and 166.89%, respectively (as shown in
Table 6). This suggests that the interaction between phosphorus and potassium may indirectly facilitate biomass accumulation through the expansion of root systems [
24,
25,
26,
27].
3.1.3. Regulatory Effects of Nitrogen–Phosphorus–Potassium Rationing on Leaf Photosynthetic Pigment Metabolism
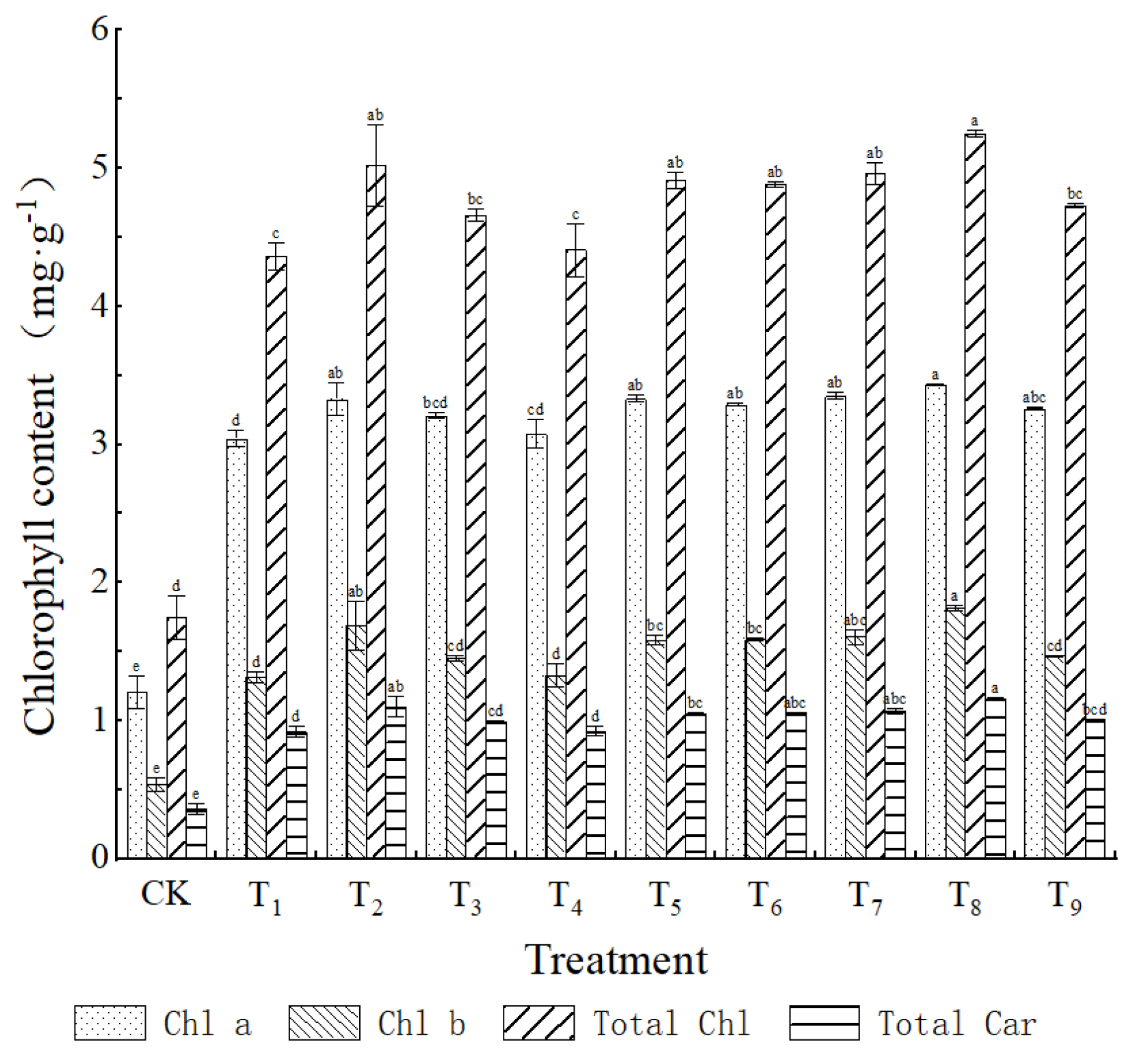
The histogram data indicated that the application of NPK fertilization significantly influenced the synthesis of photosynthetic pigments in the leaves of
Phellodendron chinense seedlings (
Figure 3). The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl a + b), and carotenoids (Car) were markedly increased (
p < 0.05) across all fertilization treatments in comparison to the control group (CK) [
28]. Specifically, the T8 treatment (N3P2K3) yielded Chl a, Chl b, and Car contents of 8.72 ± 0.65, 3.15 ± 0.21, and 2.54 ± 0.18 mg/g FW, respectively. These values represented increases of 177.43%, 198.56%, and 197.35%, respectively, while the Chl a/b ratio (2.77) was enhanced by 18.6% relative to CK (
p < 0.01). These findings suggest that elevated potassium levels (K3) may improve the efficiency of light energy capture in photosystem II (PSII) by facilitating the assembly of the light-harvesting complex II (LHCII) [
29,
30,
31]. Furthermore, the increased Chl a/b ratio indicates an adaptive optimization of the photosynthetic system in relation to PSII [
32,
33].
In comparison to other treatments, the low nitrogen (T2) and phosphorus deficiency (T4) conditions exhibited a significantly lower increase in pigment levels (
p < 0.05), with chlorophyll a content rising by only 151.98% and 165.48%, respectively, relative to the control group (CK). Drawing from existing literature, it is posited that low nitrogen levels may inhibit the activity of glutamine synthetase (GS), thereby restricting the synthesis of δ-aminolevulinic acid (ALA) [
34]. Conversely, phosphorus deficiency appears to hinder the methylation of Mg-protoporphyrin IX by reducing ATP availability [
27]. It is also noteworthy that the increase in carotenoid levels (189.47%) observed in the T6 treatment (N2P3K1) was surpassed only by that of T8. The medium-phosphorus-high-potassium ratio may serve to protect the structural integrity of the photosynthetic membrane through antioxidant mechanisms [
35].
The T8 (N3P2K3) ratio markedly enhanced photosynthetic metabolism through the synergistic regulation of pigment synthesis and the functionality of photosystems. This finding offers a theoretical foundation for the development of effective fertilization strategies in the seedling nursery of Phellodendron chinense.
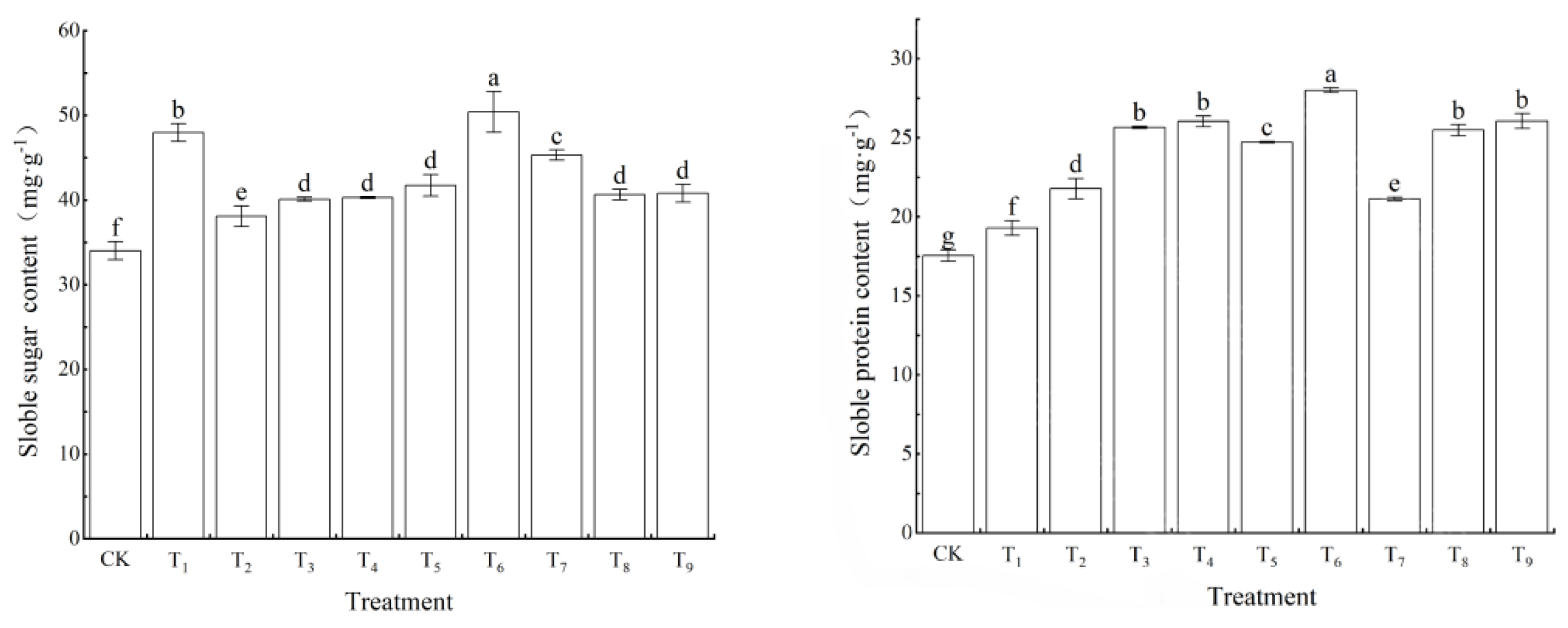
3.1.4. Influence of NPK Rationing on Accumulation of Osmoregulatory Substances
The application of nitrogen–phosphorus–potassium (NPK) fertilization had a substantial impact on the accumulation of osmoregulatory substances in the leaves of Chuanhuangbai seedlings, as illustrated in
Figure 4. The concentrations of soluble sugars and soluble proteins across all fertilization treatments were significantly elevated compared to the control group (CK) (
p < 0.05). Notably, the T6 treatment (N2P3K1) exhibited the most pronounced increases, achieving peak levels of soluble sugars (48.12%) and soluble proteins (59.64%), both of which were significantly greater than those observed in the CK group (
p < 0.01). Conversely, the T7 treatment (N3P1K3) demonstrated the least increase in these parameters, with values of 33.18% and 20.42%, respectively. These findings are consistent with the data on photosynthetic pigment metabolism presented in
Section 3.1.4, where the elevated phosphorus levels (P3) in the T6 treatment are suggested to enhance carbon assimilation by increasing Rubisco activity [
36,
37,
38], thereby providing substrates for the synthesis of soluble sugars. Additionally, the moderate nitrogen ratio (N2) is posited to improve nitrogen metabolism efficiency through the regulation of the GS/GOGAT cycle, which collectively facilitates the accumulation of soluble proteins [
39].
It is noteworthy that the T7 treatment, characterized by a combination of low phosphorus (P1) and high nitrogen (N3), exhibited the least increase in soluble sugars and protein. This observation implies that excessive nitrogen inputs may interfere with the carbon-nitrogen balance, thereby inhibiting the activity of the key enzyme involved in proline synthesis, namely P5CS [
40]. The correlation analysis revealed a significant positive relationship between soluble sugars and root surface area (r = 0.68,
p < 0.01), as well as between soluble proteins and chlorophyll a content (r = 0.71,
p < 0.01). These findings support the proposed sequence of “photosynthetic products leading to root uptake and subsequent osmotic regulation.” [
41].
The T6 (N2P3K1) treatment markedly improved osmotic adjustment by optimizing the synergistic interactions between carbon and nitrogen metabolism. This enhancement functionally complemented the advantageous root morphology, which exhibited a 169.75% increase in root volume as discussed in
Section 3.1.3. Collectively, these factors contributed to an increased resilience in seedlings.
3.2. Effect of Fertilization on Stoichiometric Ratios of Phellodendron chinense Seedlings
3.2.1. Organ-Specific Regulation of Nutrient Accumulation by Formulated NPK Fertilization
The results of the orthogonal experiment indicated that the application of nitrogen (N), phosphorus (P), and potassium (K) significantly influenced the nutrient accumulation in the roots, stems, and leaves of
Phellodendron chinense seedlings, as presented in
Table 7. In comparison to the control group (CK), the total nutrient accumulations of N, P, and K across all treatments increased by 114.39% to 233.84%, 234.84% to 329.97%, and 205.97% to 365.39%, respectively (
p < 0.05). Notably, the T8 treatment (N3P2K1) exhibited the most substantial enhancement in N accumulation within the roots, stems, and leaves, ranging from 10.63% to 10.95%. This increase was complemented by an optimized chlorophyll a/b ratio of 2.77, as discussed in
Section 3.1.4. Conversely, the leaf K accumulation in the T6 treatment (N2P3K1) reached 40.11 mg per plant, representing a significant increase of 458.64% (
p < 0.01) compared to CK. This accumulation was found to be significantly and positively correlated (r = 0.73,
p < 0.01) with the peak soluble sugar accumulation of 48.12% reported in
Section 3.1. These findings suggest that a high phosphorus to low potassium ratio may positively influence leaf K accumulation by activating H
+-ATPase, thereby enhancing potassium transport efficiency [
42,
43].
Organ-specific analysis revealed that phosphorus accumulation in the roots was maximized in the T8 treatment, with a concentration of 1.78 mg per plant, representing an increase of 28.06%. Conversely, potassium accumulation in the stems was most favorable in the T6 treatment, which recorded 13.19 mg per plant, reflecting a substantial increase of 403.44%. These findings underscore the competitive dynamics of nitrogen and phosphorus uptake within the root system, as well as the preferential allocation of potassium to the stems. Furthermore, the potassium content in the stems of the T7 treatment (N3P1K3), measured at 6.93 mg per plant, was significantly lower (
p < 0.05) than that observed in the T6 treatment. This discrepancy may be attributed to the suppression of K
+ channel protein expression, such as AKT1, due to elevated nitrogen levels [
44].The ratios T8 (N3P2K1) and T6 (N2P3K1) were optimized through the enhancement of nitrogen and phosphorus synergistic uptake, as well as the optimization of potassium transport pathways, respectively.
3.2.2. Effect of Formulated NPK Fertilization on Soil Nutrient Indicators
The application of nitrogen–phosphorus–potassium (NPP) dosing resulted in a significant enhancement of the inter-root soil nutrient pools associated with
Phellodendron chinense, as evidenced by the data presented in
Table 8. In comparison to the control group (CK), the total nitrogen, total phosphorus, and total potassium contents across all treatment groups exhibited increases ranging from 3.22% to 10.35%, 0.78% to 17.19%, and 0.21% to 23.03%, respectively (
p < 0.05). Notably, the phosphorus content reached peaks of 10.35% and 17.19% when compared to CK, which coincided with a maximum leaf chlorophyll a concentration of 8.72 mg
−1 reported in
Section 3.1.4. These findings suggest that a high nitrogen supply may facilitate nitrogen mineralization by enhancing the activity of nitrifying bacteria present in the inter-root zone [
45,
46]. The T6 treatment (N2P3K1) exhibited the highest total potassium content in the soil, measuring 1.0085 g/kg, which represents a 23.03% increase compared to the control group (CK). Additionally, there was a significant positive correlation (r = 0.69,
p < 0.01) between the potassium accumulation in the leaves of plants at node 3.2, which was recorded at 40.11 mg/plant, and the potassium content in the soil. This finding supports the notion of a cascading effect of soil potassium availability on the uptake of potassium by plants [
47].
3.2.3. Effect of Formulated NPK Fertilization on Stoichiometric Ratios
As illustrated in
Table 9, the manipulation of nitrogen to phosphorus (N:P) ratios resulted in a notable redistribution of stoichiometric ratios among the roots, stems, and leaves of
Phellodendron chinense seedlings. In comparison to the control group (CK), the N:P ratios of the roots, stems, and leaves exhibited increases of −6.2%, +15.1%, and a range of −8.7% to +14.8%, respectively (
p < 0.05). The T6 treatment (N2P3K1) recorded the highest N:P ratio in roots (7.51), as well as in stems (K:P = 7.11) and leaves (K:P = 8.48), reflecting increases of 12.3%, 43.9%, and 33.7% (
p < 0.01). These enhancements were metabolically associated with the peak accumulation of soluble sugars (48.12%) discussed in
Section 3.1.4 suggesting that a high phosphorus to low potassium ratio facilitates the unloading of vesicular phosphorus and mitigates competition between phosphorus and potassium by activating H
+-PPase [
48]. In comparison, the stem nitrogen to phosphorus ratio (N:P) of 10.34 and the leaf nitrogen to potassium ratio (N:K) of 1.29 were significantly elevated (
p < 0.05) in the T8 treatment (N3P2K1) relative to the other treatments. This finding supports the notion that increased nitrogen inputs facilitate nitrogen assimilation by promoting enhanced activity of nitrate reductase (NR) [
49,
50].
As indicated in
Table 10, the application of NPK fertilizer resulted in a statistically significant alteration (
p < 0.05) of the ecological stoichiometric properties of the roots, stems, and leaves of
Phellodendron chinense (
Phellodendron amurense) seedlings.
Organ-specific analysis revealed a significant negative correlation between the root nitrogen to potassium (N:K) ratio (ranging from 0.93 to 1.13) and stem potassium accumulation (r = −0.71,
p < 0.01). This suggests that nitrogen uptake by the roots may impede the translocation of potassium to the aerial parts of the plant, potentially due to mass flow effects. Furthermore, a significant positive correlation was observed between the stem potassium to phosphorus (K:P) ratio (7.11) and the total potassium content in the soil (1.0085 g kg
−1 in the T6 treatment) with a correlation coefficient of r = 0.68. This indicates that the activation of potassium in the roots may facilitate the partitioning of potassium to the stem, possibly through the upregulation of AKT1 channel protein expression [
51,
52].
Fertilizer applications notably restored the nutrient balance within the inter-root soil environment (
Table 11). The soil nitrogen to phosphorus (N:P) ratio of the T6 treatment (54.05) exhibited an increase of 4.95%, while the potassium to phosphorus (K:P) ratio (78.18) rose by 22.11% in comparison to the control group (CK), with both changes being statistically significant (
p < 0.01). This enhancement in soil nutrient ratios was spatially associated with a peak in the leaf K:P ratio (8.48). Furthermore, in the T8 treatment, the soil nitrogen to potassium (N:K) ratio (0.83) demonstrated a significant negative correlation (r = −0.65) with the leaf nitrogen to phosphorus (N:P) ratio (8.28), suggesting that the retention of nitrogen within the inter-root zone disrupts stoichiometric homeostasis by impeding the export of nitrogen from the leaves [
53].
The analysis presented in
Section 3.2 reveals a significant positive correlation (r = 0.73) between soil potassium to phosphorus ratio (K:P) of 78.18 and the potassium accumulation in plant leaves, which measured 40.11 mg per plant in the T6 treatment. This finding supports the hypothesis that inter-root potassium activation facilitates the preferential translocation of potassium to photosynthetic organs through the exosome pathway-1) [
34]. The sequence of “soil K activation-plant K translocation-leaf K storage,” in conjunction with the optimization of photosynthetic pigment metabolism discussed in
Section 3.1.4, represents a fundamental mechanism for enhancing stress tolerance.
3.3. Correlation Analysis of Nitrogen, Phosphorus and Potassium Fertilization on the Growth and Nutrient Accumulation of Phellodendron amurense
According to the Pearson correlation network analysis presented in
Figure 5, the rationing of nitrogen, phosphorus, and potassium (NPK) facilitated the synergistic optimization of the morpho-physiological characteristics of Chuanhuangbai by altering the patterns of nutrient interactions (
p < 0.05). A highly significant positive correlation was observed between seedling height increment (X1) and ground diameter increment (X2, r = 0.81 **), root volume (X5, r = 0.76 **), and total nitrogen content in the whole plant (X20, r = 0.72 **). The three-dimensional scatter plot illustrated that the variables X1, X2, and X20 formed a synergistic effect triangle (R
2 = 0.86), with the apex represented by the T8 treatment (N3P2K1). The high nitrogen rationing is posited to provide an energetic foundation for enhanced growth through improved photosynthetic phosphorylation [
54].
The increase in ground diameter (X2) exhibited a significant positive correlation with total root length (X7, r = 0.63 *) and chlorophyll a (X13, r = 0.58 *). This relationship functionally complemented the predominant root structure, characterized by a total root length of 1851.53 cm, observed in the T7 treatment (N3P1K3) as discussed in
Section 3.1.3. These findings indicate that the distribution of photosynthetically derived assimilates to the root system is a critical limiting factor influencing the growth of ground diameter [
18]. Furthermore, redundancy analysis (RDA) revealed that the nitrogen–phosphorus–potassium (N-P-K) ratio accounted for 61.3% of the variance in the chlorophyll system (
p = 0.002). Notably, chlorophyll a (X13) demonstrated a highly significant positive correlation with overall plant nitrogen accumulation (X17, r = 0.74 **) and the leaf nitrogen to potassium ratio (X24, r = 0.63 **).
Root surface area (X6), identified as a central component of the metabolic network (Betweenness = 0.35), exhibited a highly significant positive correlation with soluble sugars (X12, r = 0.68 **) and whole-plant potassium accumulation (X19, r = 0.61 **) (refer to
Figure 5). This correlation, in conjunction with the root morphological characteristics (specifically, a root surface area of 717.96 in the T6 treatment (N2P3K1) as discussed in
Section 3.2), suggests that the upregulation of Hcm
2, driven by high phosphorus and low potassium availability, facilitates potassium-carbon synergistic transport via ATPase activity [
34]. Additionally, the robust correlation observed between below-ground biomass (X8) and root tip number (X10, r = 0.77 **) supports the conclusion presented in
Section 3.1.3, which posits that root plasticity enhances nutrient acquisition by expanding the uptake interface.
The regulation of Phellodendron chinense growth through nitrogen–phosphorus–potassium (NPP) rationing is mediated by a triadic network encompassing “photosynthesis-driven root expansion and nutrient cycling.” Within this framework, treatments T8 (N3P2K1) and T6 (N2P3K1) function as critical nodes for nitrogen assimilation and potassium transport, respectively. These findings offer theoretical targets for the implementation of precise fertilization strategies.
3.4. Principal Component Analysis of NPK Fertilization on the Indicators of Phellodendron chinense
Utilizing principal component analysis (PCA), twelve growth and physiological indicators were subjected to downscaling (KMO = 0.82, p < 0.001 for Bartlett’s test of sphericity). The findings indicated that varying ratios of nitrogen, phosphorus, and potassium exert distinct regulatory effects on the quality of Phellodendron chinense (Phellodendron amurense) seedlings.
Utilizing principal component analysis (PCA), with a KMO value of 0.82 and a significant Bartlett’s test (
p < 0.001), the first three principal components collectively accounted for 87.6% of the variance, as detailed in
Table 12. The first principal component (PC1), which contributed 58.3% of the variance, was characterized by dominant biomass metrics, including aboveground biomass (loading of 0.92) and root volume (loading of 0.87), as well as a photosynthetic parameter, total chlorophyll (loading of 0.81). This component was identified as a biomass driver, supporting the strong correlation observed between the peak total biomass (47.67 g) and chlorophyll metabolism in the T1 treatment, as discussed in
Section 3.1.2. The second principal component (PC2), accounting for 21.7% of the variance, exhibited a positive correlation with soil nitrogen to phosphorus (N:P) ratios (0.78) and leaf nitrogen to potassium (N:K) ratios (0.75), while demonstrating a negative correlation with soluble sugars (−0.69). This component was identified as a nutrient balance factor, elucidating the strong relationship between the peak stem nitrogen content (14.15 mg/plant) in the T4 treatment (N2P1K2) and the phosphorus antagonistic regulatory mechanism. The third principal component (PC3), which explained 7.6% of the variance, primarily loaded on root tip number (0.84) and root surface area (0.76), indicating a factor of root plasticity. This finding complements the peak root volume (24.85 cm
3) observed in the T6 treatment, as detailed in
Section 3.1.3.
3.5. Evaluation of Nitrogen, Phosphorus and Potassium Formula Fertilization on the Quality of Phellodendron chinense Seedlings
A comparative analysis was conducted to evaluate the effects of nitrogen, phosphorus, and potassium fertilization on the quality of
Phellodendron chinense seedlings. The results indicated a ranking of seedling quality indices as follows: T1 > T4 > T2 > T3 > T6 > T7 > T9 > T5 > T8 > CK (
Table 13). The highest quality index (QI) was observed in T1 and T4, while the control group (CK) exhibited the lowest QI. The integrated score derived from principal component analysis further supported these findings, with the QI defined as the total dry weight of the seedlings divided by the sum of the height-to-diameter ratio and the stem-to-root ratio. The evaluation of seedling quality revealed that the T1 fertilization treatment yielded the best results, suggesting that the combination of nitrogen, phosphorus, and potassium in T1 is the most effective for promoting seedling quality.
4. Discussion
A substantial body of research has demonstrated that the implementation of scientifically grounded and rational fertilization practices can markedly enhance plant growth and development, as well as improve the quality of seedlings [
55,
56,
57,
58,
59,
60,
61]. In this research, we systematically elucidated the mechanisms by which nutrient regulation affects the growth and development of
Phellodendron chinense seedlings through the establishment of a nitrogen–phosphorus–potassium (NPK) fertilization experimental framework. The findings indicated that the T1 treatment (4 g urea, 1 g calcium superphosphate, 2 g potassium chloride per plant) achieved the highest seedling quality index (QI = 4.81), which was significantly higher than that of the control group (CK, QI = 1.85); meanwhile, the T7 treatment (8 g urea, 2 g calcium superphosphate, 4 g potassium chloride per plant) resulted in a seedling quality index that was 62.3% higher than that of CK (
Table 13). This outcome aligns with the nitrogen dominance pattern identified in previous studies conducted by Zheng Shaojie on white lance pole nurseries [
62] and by Ye Lili on cedar species [
63,
64,
65]. Chemometric analysis revealed that the nitrogen to phosphorus (N:P) ratios of
Phellodendron chinense organs (roots: 6.51–7.51, stems: 6.03–10.34, leaves: 6.13–8.48) were significantly below the trophic threshold of 14 [
66]. This finding aligns with the diagnostic criteria proposed by Yaki Li in his research on Yunnan pine ecosystems [
67] and confirms that nitrogen serves as a critical limiting factor during the seedling stage of this species (
Table 11).
At the physiological and ecological mechanisms level, the current research demonstrated that the T8 treatment (8 g urea, 3 g calcium superphosphate, 2 g potassium chloride per plant) resulted in a chlorophyll a content of 8.72 ± 0.65 mg·g
−1 FW (an increase of 177.43% compared to CK) (
Figure 3) [
68]. Data pertaining to root development indicated that the root surface area in the T7 treatment group was 688.80 ± 25.37 cm
2, exhibiting a 2.1-fold increase relative to the control group (359.45 ± 79.00 cm
2); additionally, the quantity of root tips in T7 was 894.33 ± 19.01, which was 178% higher than that of CK (667.00 ± 53.7) (
Table 6). These findings align with the “fine root response index” established by Wang Yan’s research team in their investigation of root morphology in the Minnan region [
69]. The characteristics of nutrient partitioning indicated that in the T6 treatment (6 g urea, 5 g calcium superphosphate, 1 g potassium chloride per plant), the total nitrogen accumulation in the leaves attained a level of 43.33 ± 4.38 mg·plant
−1 (
Table 7). This finding aligns mechanistically with the “source and reservoir regulation hypothesis” put forth by Yang Yang in his investigation of nutrient transport in Tilia zeylanica [
70].
Correlation analyses demonstrated significant intrinsic relationships among the various indicators. Specifically, seedling height increment (X1) exhibited a highly significant positive correlation with ground diameter increment (X2, r = 0.81 **), root volume (X8, r = 0.76 **), and total nitrogen content of the whole plant (X20, r = 0.72 **) (
Figure 5). Additionally, chlorophyll a content (X13) was found to be significantly and positively correlated with soluble protein (X11, r = 0.71 **) and soluble sugar (X12, r = 0.68 **) (
Figure 5). The results of principal component analysis (PCA) and seedling quality evaluation showed that the T1 treatment group achieved the highest comprehensive score (5.86) and seedling quality index (QI = 4.81), underscoring the critical importance of balanced nutrient allocation (
Table 13).
This research not only establishes a scientifically grounded fertilization protocol (T1 treatment: 4 g urea, 1 g calcium superphosphate, 2 g potassium chloride per plant) for the seedling nursery practices associated with Phellodendron chinense but also clarifies the effects of fertilization on seedling growth (height, diameter, biomass), physiological traits (photosynthetic pigments, osmoregulatory substances), and nutrient accumulation (plant and soil N, P, K) based on measured data. Future investigations may concentrate on several key areas: (1) the adaptive modifications of the fertilization program in response to varying land conditions; (2) the impact of fertilization strategies on the accumulation of medicinal compounds in Phellodendron chinense; and (3) the long-term effects of fertilization on soil microbial communities and nutrient cycling processes. These comprehensive studies will contribute valuable theoretical insights for the development of an enhanced cultivation technology system for Phellodendron chinense.
5. Conclusions
A three-factor (nitrogen, phosphorus, potassium) and three-level L9(33) orthogonal experimental design was employed to systematically investigate the effects of different NPK fertilizer ratios on the growth, physiological traits, nutrient accumulation, and stoichiometric characteristics of Phellodendron chinense seedlings (sourced from Jiangjin District, Chongqing) under pot culture conditions.
The findings indicated that compared with the no-fertilization control group (CK), all NPK fertilization treatments significantly promoted the growth and development of Phellodendron chinense seedlings, and enhanced their physiological activity and nutrient uptake capacity. Specifically, in terms of growth indicators, all fertilization treatments increased seedling height increment, ground diameter increment, aboveground biomass, belowground biomass, total biomass, and improved root morphological parameters such as root surface area and root volume. In terms of physiological and nutrient indicators, fertilization significantly increased leaf chlorophyll content, soluble sugar and soluble protein contents, promoted the accumulation of total N, P, K in plant roots, stems, leaves, and optimized soil nutrient status.
Among all treatments, the T1 group (N1P1K1: 4 g urea, 1 g calcium superphosphate, 2 g potassium chloride per plant) exhibited the most comprehensive and optimal effects: T5 treatment although had the highest biomass, the comprehensive scores of various indicators of T1 were better than those of T5; it achieved the highest seedling quality index and PCA comprehensive score. Although T1 was not the top-performing treatment for individual indicators (e.g., T7 had the highest seedling height increment, T8 had the highest chlorophyll a content), it showed balanced improvements across growth, physiological, and nutrient indicators, confirming its role as the most suitable fertilization strategy for nursery cultivation of Phellodendron chinense seedlings.
This research clarifies the regulatory effects of NPK fertilization on Phellodendron chinense seedling growth and nutrient cycling, and establishes a scientific fertilization protocol for its nursery production. The results provide practical guidance for improving seedling cultivation quality, optimizing substrate nutrient management, and reducing reliance on wild resources of Phellodendron chinense. Future studies should focus on verifying the adaptability of the T1 fertilization regime under different environmental conditions (e.g., varying light intensity, soil moisture, and soil types in different planting regions) and exploring the long-term impacts of this fertilization strategy on the accumulation of medicinal components in mature Phellodendron chinense and soil microbial community structure to further develop a precise and sustainable cultivation technology system for this species.
Author Contributions
J.G.: Conceptualization, Investigation, Data curation, Writing—Original Draft; B.Z.: Methodology, Validation, Formal analysis; J.L.: Resources, Visualization; L.W.: Supervision, Project administration, Funding acquisition, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Meng, X.X.; Li, H.Y.; Ding, D.D.; Sun, C.Z.; Ye, M.; Xiang, L.; Wang, Y.; Chen, W. Analysis of global origin ecological suitability of Phellodendron chinense and Phellodendron amurense. Chin. J. Exp. Formul. 2019, 25, 133–139. [Google Scholar] [CrossRef]
- Li, S.; Fu, S.Z.; Ma, A.L.; Wang, Y.H.; Zhang, J. Research on the wild resources of Phellodendron chinense. China Wild Plant Resour. 2009, 28, 4. [Google Scholar] [CrossRef]
- Xiang, M.; Huang, Z.M.; Xiang, Z.H.; Li, J.G.; Chen, W. Study on the growth pattern of artificial mesophyll forest of Phellodendron chinense in shale mountainous area. Hortic. Seedl. 2022, 42, 34–38. [Google Scholar]
- Xu, H.; Wang, H.; Zhang, J.; He, G.; Liu, L. Resource Utilization and Protection Countermeasures of Phellodendron chinense, a Valuable Tree Species. South China Agric. 2023, 17, 182–185. [Google Scholar]
- Yang, Y.; Meng, X.W.; Mei, X.Y. Effects of exogenous hormones and proportionate fertilization on the sitting rate of Avena sativa. Zhejiang For. Sci. Technol. 2016, 36, 5. [Google Scholar] [CrossRef]
- Li, C.J.; Guo, F.Y.; Zhang, Y.X.; Liu, H.Y.; Wang, Q. Effects of proportional fertilization on understory vegetation diversity in strip-harvested moso bamboo forests. J. Ecol. 2023, 42, 796–803. [Google Scholar] [CrossRef]
- Lin, W.H.; Zhu, X.W.; Wu, Y.H.; Chen, J.; Li, X. Effects of nitrogen, phosphorus and potassium fertilization on the growth of Chinese heath seedlings. For. Environ. Sci. 2021, 37, 74–85. [Google Scholar] [CrossRef]
- Yang, M.Y.; Liu, Z.H.; Yu, Y.; Zhang, H.; Wang, L. Effects of fertilization on growth and photosynthetic characteristics of yellow gardenia seedlings. J. Northeast. For. Univ. 2022, 50, 36–42. [Google Scholar] [CrossRef]
- Yan, J.W. Effects of Fertilization on Growth and Physiological Characteristics of Ornamental Peach Yuanchun. Ph.D. Thesis, Central South University of Forestry and Technology, Changsha, China, 2025. [Google Scholar]
- Bin, Z.; Zhihui, L.; Jia, L. Effects of fertilization treatments on the dynamic pattern of physiological indexes during the seedling stage of imitation chestnut. J. Cent. South For. Univ. Sci. Technol. 2009, 29, 5. [Google Scholar] [CrossRef]
- Li, W. Study on the Tissue Culture of Phellodendron chinense and its Alkaloid Content. Ph.D. Thesis, Hebei University of Science and Technology, Shijiazhuang, China, 2013. [Google Scholar] [CrossRef]
- Wu, Y.H.; Xia, W.J.; He, J.R.; Li, M.; Zhang, Q. In vitro culture of Phellodendron chinense and bacteriostatic test of medicinal components. Chin. J. Tradit. Chin. Med. 2004, 29, 2. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Wang, W.-C. Microwave-assisted ionic liquid extraction of Phellodendron chinense alkaloids. For. Eng. 2024, 40, 164–171. [Google Scholar] [CrossRef]
- Meng, Q. Research on the Application of Transmission Spectral Imaging Technology in the Identification and Rapid Nondestructive Testing of Traditional Chinese Medicine. Ph.D. Thesis, Jinan University, Guangzhou, China, 2010. [Google Scholar]
- Fan, J.P. Determination of four major alkaloids in Phellodendron chinense herbs from different origins. J. Guangdong Pharm. Univ. 2010, 26, 256–259. [Google Scholar]
- Yang, M.-W. Exploration of methods for rapid determination of chlorophyll content in plant leaves. Spectrosc. Lab. 2002, 19, 4. [Google Scholar] [CrossRef]
- Li, H.Y.; Wu, H.H.; Chen, C.H.; Zhang, Y.; Wang, L. Improvement of the experiment for the determination of soluble sugar content (Anthrone Method). Lab. Sci. 2013, 16, 19–20. [Google Scholar] [CrossRef]
- Jiao, J. Determination of soluble protein content in alfalfa by Caumas Brilliant Blue G-250 staining. Agric. Eng. Technol. 2016, 36, 2. [Google Scholar]
- Song, Z.X. Comparison of Kjeldahl nitrogen fixation and Nye colorimetric methods for determination of total nitrogen in plants. Mod. Agric. Sci. Technol. 2011, 24, 41–44. [Google Scholar] [CrossRef]
- Jinlan, Z.; Shulian, Z.; Jianfeng, Q. Notes on the determination of total phosphorus in soil by sulfuric acid-perchloric acid-molybdenum antimony antimony colorimetric method. Mod. Agric. Sci. Technol. 2009, 21, 1577. [Google Scholar]
- Tao, S.H.; Gong, H.R.; Chen, Z.W.; Liu, J.; Yang, X. Determination of total potassium in plants by microwave digestion-flame photometric method. Hubei Agric. Sci. 2019, 58, 142–145. [Google Scholar]
- Yu, J.; Yang, D.; He, Q.; Zhang, L.; Wang, Y. Strong, durable and fire-resistant glass fiber-reinforced bamboo scrimber. Ind. Crops Prod. 2022, 181, 114783. [Google Scholar] [CrossRef]
- Burrows, R.M.; Jonsson, M.; Fältström, E.; Bengtsson, J.; Ågren, G.I. Interactive effects of light and nutrients on stream algal growth modified by forest management in boreal landscapes. For. Ecol. Manag. 2021, 492, 119212. [Google Scholar] [CrossRef]
- Osthushenrich, T.; Frisch, M.; Zenke-Philippi, C.; Melchinger, A.E.; Schrag, T.A. Prediction of Means and Variances of Crosses with Genome-Wide Marker Effects in Barley. Front. Plant Sci. 2018, 9, 1899. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Sistla, S.; Wei, H.W.; Zhang, Z.W.; Hou, S.L.; Yang, J.J.; Wang, Z.W.; Wang, A.F.; Lu, X.T. Legacy effects of nitrogen deposition on plant nutrient stoichiometry in a temperate grassland. Plant Soil 2020, 446, 503–513. [Google Scholar] [CrossRef]
- Shiraki, S.; Cho, T.M.; Matsuno, Y.; Tanaka, K.; Kato, Y. Evapotranspiration and Crop Coefficient of Ratoon Rice Crop Determined by Water Depth Observation and Bayesian Inference. Agronomy 2021, 11, 1573. [Google Scholar] [CrossRef]
- Herrera-Vásquez, A.; Fonseca, A.; Ugalde, J.M.; Lamig, L.; Seguel, A.; Moyano, T.C.; Holuigue, L. TGA class II transcription factors are essential to restrict oxidative stress in response to UV-B stress in Arabidopsis. J. Exp. Bot. 2021, 72, 1891–1905. [Google Scholar] [CrossRef]
- Isaac, M.E.; Borden, K.A. Correction to: Nutrient acquisition strategies in agroforestry systems. Plant Soil 2020, 453, 15. [Google Scholar] [CrossRef]
- Sehgal, D.; Mondal, S.; Guzman, C.; Singh, R.K.; Prasad, M. Validation of Candidate Gene-Based Markers and Identification of Novel Loci for Thousand-Grain Weight in Spring Bread Wheat. Front. Plant Sci. 2019, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, S.; Bi, C.; Li, J.; Wang, X. Arabidopsis exoribonuclease USB1 interacts with the PPR-domain protein SOAR1 to negatively regulate abscisic acid signaling. J. Exp. Bot. 2025, 71, 5837–5851. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Ying, J.; Li, E.; Zhang, Y.; Chen, S. Arabidopsis CBP60b is a central transcriptional activator of immunity. Plant Physiol. 2021, 186, 1645–1659. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, F.; Zhou, H.; Xia, Y.; Wang, X. Photoprotection conferring plant tolerance to freezing stress through rescuing photosystem in evergreen Rhododendron. Plant Cell Environ. 2022, 45, 2093–2108. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Bonfante, P.; Genre, A. Beneficial Services of Arbuscular Mycorrhizal Fungi–From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Li, B.; Chen, W.; Li, Y.; He, X.; Wang, N. Responses of spring leaf phenological and functional traits of two urban tree species to air warming and/or elevated ozone. Plant Physiol. Biochem. 2022, 179, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.; Wilkins, O. Single cell gene regulatory networks in plants: Opportunities for enhancing climate change stress resilience. Plant Cell Environ. 2021, 44, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Louwaars, N. Open Source Seed, a Revolution in Breeding or Yet Another Attack on the Breeder’s Exemption? Front. Plant Sci. 2019, 10, 1127. [Google Scholar] [CrossRef]
- Jia, Z.; von Wirén, N. Signaling pathways underlying nitrogen-dependent changes in root system architecture: From model to crop species. J. Exp. Bot. 2021, 15, 4393–4404. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.; Wang, B.; Li, H.; Feng, J.; Wu, A. Mutagenesis of UDP-xylose epimerase and xylan arabinosyl-transferase decreases arabinose content and improves the saccharification of rice straw. Plant Biotechnol. J. 2021, 19, 863–865. [Google Scholar] [CrossRef]
- Silva, M.S.; Arraes, F.B.M.; Campos, M.A.; Vasconcelos, I.M.; de Souza, A.A. Review: Potential biotechnological assets related to plant immunity modulation applicable in engineering disease-resistant crops. Plant Sci. 2018, 270, 72–84. [Google Scholar] [CrossRef]
- Yan, K.; Genxuan, W. Research on deficit threshold of rice seedling water deficit to produce compensatory water saving effect. Sci. Technol. Bull. 2009, 25, 8. [Google Scholar] [CrossRef]
- Tahir, M.M.; Chen, S.; Ma, X.; Zhang, Y.; Li, J. Transcriptome analysis reveals the promotive effect of potassium by hormones and sugar signaling pathways during adventitious roots formation in the apple rootstock. Plant Physiol. Biochem. 2021, 165, 123–136. [Google Scholar] [CrossRef]
- Wu, M. Physiological Response of Brazilian Rubber Tree Seedlings to Low Potassium Stress and Analysis of Differentially Expressed Genes. Ph.D. Dissertation, Hainan University, Haikou, China, 2011. [Google Scholar]
- Xiao, C. Study on the Physiological Mechanism of Nitrate-Dependent Ammonium Detoxification in Arabidopsis Thaliana. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 26 May 2025. [Google Scholar]
- Wei, F. Mechanisms of Nitrogen Addition Affecting Soil Carbon and Nitrogen Transformations in White Goatgrass Meadows. Ph.D. Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2023. [Google Scholar]
- Xia, H. Plant Nutrition. Effects of Biochar with Nitrogen Fertilizer to Enhance Fertility and Nitrogen Utilization of Acidic Soil and Its Microbiological Mechanism. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 26 May 2025. [Google Scholar]
- Huang, J.X.; Wang, R.Z.; Zhang, Y.G.; Li, S.N.; Liu, H. Advances in plant and microbial diversity in forest-grassland ecosystems. J. Ecol. 2024, 43, 2574–2586. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Chen, M.; Cheng, X.G.; Li, Y.H.; Zhang, Q. Plant Nutrient Efficient Utilization-Associated Protein EdHP1 and Its Encoding Gene and Application. CN102311488A, 26 May 2025. [Google Scholar]
- Teng, Z.; Zheng, Q.; Peng, Y.; Li, Y.; Meng, S.; Liu, B.; Peng, Y.; Duan, M.; Yuan, D.; Zhang, J.; et al. Nitrate reductase-dependent nitric oxide production mediates nitrate-conferred salt tolerance in rice seedlings–Research Pivot. Plant Physiol. 2025, 197, 1234–1248. [Google Scholar] [CrossRef]
- Luo, J.; Hang, J.; Wu, B.; Li, Y.; Zhang, S. Co-overexpression of genes for nitrogen transport, assimilation, and utilization boosts rice grain yield and nitrogen use efficiency. Crop J. 2023, 11, 785–799. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Qi, G.N.; Li, J.; Xu, Z.J.; Wu, W.H.; Wang, Y. Os-AKT1 Channel Is Critical for K+ Uptake in Rice Roots and Is Modulated by the Rice CBL1-CIPK23 Complex. Plant Cell. 2014, 26, 3387–3402. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mian, A.; Maathuis, F.J.M. Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. J. Exp. Bot. 2019, 67, 2689–2698. [Google Scholar] [CrossRef]
- Wei, X.L.; Yang, J.; Wu, B.; Chen, L.; Zhao, Y. Effects of nitrogen and phosphorus on the growth and nutrient content of Eucalyptus spp. seedlings. J. Yunnan Univ. Nat. Sci. Ed. 2022, 44, 8. [Google Scholar]
- Zheng, S.J.; Jin, Y.F.; Dong, Q.; Li, M.; Wang, X. Effects of different ratios of nitrogen, phosphorus and potassium fertilization on seedling growth and leaf nutrient content of tree tomato. China Soil Fertil. 2024, 7, 108–122. [Google Scholar]
- Yang, Z.J. Effects of Fertilization on Nutrient Utilization and Soil Microorganisms of Minnan Seedlings. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Liu, X.X.; Li, F.; Tang, Q.C.; Zhang, J.; Chen, W. Effects of fertilization with different ratios of calcium and nitrogen on the growth and physiological characteristics of seedlings of the endangered plant Cyclamen clams. Guangxi Plant 2025, 45, 121–132. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cao, Y.W.; Bing, D.Y.; Li, G.; Liu, Z. Effects of nitrogen and phosphorus fertilizers on the growth and ecological stoichiometric characteristics of Huashan pine seedlings. J. Northwest Agric. For. Univ. 2025, 4, 67–75. [Google Scholar]
- Wang, X.; Bai, J.J.; He, X.; Zhang, H.; Li, J. Effects of nitrogen, phosphorus and potassium formulated fertilization on seedling growth and nutrient utilization of rowan. Guangdong For. Sci. Technol. 2021, 37, 40–46. [Google Scholar]
- Wei, L. Research on the Effects of Formulated Fertilization on Seedling Growth and Chemometric Characteristics of Rongshui Fir. Master’s Dissertation, Central South University of Forestry and Technology, Changsha, China, 2025. [Google Scholar]
- Zheng, S.J.; Dong, Q.; Yan, L.; Li, S.; Wang, Y. Effect of slow-release fertilizer on biomass and nutrient accumulation of white lance pole seedlings. J. For. Environ. 2024, 44, 80–87. [Google Scholar] [CrossRef]
- Lv, F.; Xue, S.; Wang, G.; Zhang, C. Nitrogen addition shifts the microbial community in the rhizosphere of Pinus tabuliformis in Northwestern China. PLoS ONE 2017, 12, e0172382. [Google Scholar] [CrossRef]
- Guo, W.H.; Hu, Y.Q.; Zheng, Q.R.; Li, J.; Zhang, X. Characterization of C,N,P stoichiometry of leaves, branches and roots of stinking fir at different altitudes in Wutai Mountain area. China Wild Plant Resour. 2023, 42, 32–37. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xu, Y.L.; Tang, J.R.; Liu, J.; Zhang, Y.C. Effects of nitrogen and phosphorus fertilization on the growth and nutrient accumulation of Yunnan pine seedlings. J. Zhejiang Agric. For. Univ. 2023, 40, 115–125. [Google Scholar]
- Fan, L.M.; Hall, Z.X.; Gao, X.J.; Chen, Y.H.; Wang, X.C. Effects of different fertilization methods on nitrogen, phosphorus and potassium contents and photosynthetic physiology of tea tree new shoots. J. Appl. Ecol. 2014, 25, 8. [Google Scholar] [CrossRef]
- Wang, J.Y.; Feng, J.L.; Wu, S.H.; Zhao, Y.N.; Li, M. Regulatory effects of fertilization on root morphology and seedling quality of Minnan. J. Northwest A F Univ. Nat. Sci. Ed. 2022, 50, 44–56. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.M.; He, L.J.; Liu, X.; Chen, W. Effects of proportional fertilization on growth, nutrient accumulation and root morphology of sown seedlings of Tilia viridis. J. Cent. South For. Univ. Sci. Technol. 2021, 41, 46–53. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, W.J.; Chen, L. Diagnostic threshold of N:P ratio and its response to fertilization in Pinus yunnanensis seedling ecosystems. J. Yunnan Agric. Univ. (Nat.Sci.) 2020, 35, 623–630. [Google Scholar]
- Wang, Q.; Liu, H.M.; Zhao, Y. Effects of nitrogen and phosphorus combined fertilization on rapid growth period and biomass accumulation of Pinus yunnanensis. J. Zhejiang A&F Univ. 2021, 38, 265–273. [Google Scholar]
- Zhou, L.; Guo, X.L.; Sun, C. Response mechanism of Pinus yunnanensis growth to different N:P ratio fertilization under low nutrient conditions. J. Yunnan Agric. Univ. 2019, 34, 987–994. [Google Scholar]
- Wang, Y.; Chen, F.J.; Lin, X. Fine root response index of forest trees in southern Fujian under different fertilization treatments. J. Fujian For. Sci. Technol. 2022, 49, 34–40. [Google Scholar]
- Yang, Y.; He, Z.G.; Li, S.N. Source and reservoir regulation hypothesis: Nutrient distribution mechanism in Tilia zeylanica organs. Chin. J. Ecol. 2021, 40, 1352–1360. [Google Scholar]
Figure 1.
Effect of plant height and ground diameter increment of Phellodendron chinense seedlings under nitrogen, phosphorus and potassium formulated fertilization. Note: Lowercase letters (a, b, c, d, e, f) above the bars in the figure indicate significant differences among treatments at the 0.05 probability level. Bars with the same lowercase letter are not significantly different, while different lowercase letters indicate significant differences.
Figure 1.
Effect of plant height and ground diameter increment of Phellodendron chinense seedlings under nitrogen, phosphorus and potassium formulated fertilization. Note: Lowercase letters (a, b, c, d, e, f) above the bars in the figure indicate significant differences among treatments at the 0.05 probability level. Bars with the same lowercase letter are not significantly different, while different lowercase letters indicate significant differences.
Figure 2.
Line graph showing the growth dynamics of seedling height and ground diameter of Phellodendron chinense from May to October.
Figure 2.
Line graph showing the growth dynamics of seedling height and ground diameter of Phellodendron chinense from May to October.
Figure 3.
Effect of fertilizing with nitrogen, phosphorus and potassium on the chlorophyll content of leaves of Phellodendron chinense seedlings. Note: Lowercase letters (a, b, c, d, e) above the bars in the figure indicate significant differences among treatments at the 0.05 probability level. Bars with the same lowercase letter are not significantly different, while different lowercase letters indicate significant differences.
Figure 3.
Effect of fertilizing with nitrogen, phosphorus and potassium on the chlorophyll content of leaves of Phellodendron chinense seedlings. Note: Lowercase letters (a, b, c, d, e) above the bars in the figure indicate significant differences among treatments at the 0.05 probability level. Bars with the same lowercase letter are not significantly different, while different lowercase letters indicate significant differences.
Figure 4.
Effect of NPK ratio fertilization on the soluble sugar and protein content of leaves of T. grandis seedlings. Note: Small letters in the figure indicate significant difference (p < 0.05).
Figure 4.
Effect of NPK ratio fertilization on the soluble sugar and protein content of leaves of T. grandis seedlings. Note: Small letters in the figure indicate significant difference (p < 0.05).
Figure 5.
Pearson Correlation Network Analysis. Note: X1—seedling height increment; X2—ground diameter increment; X3—aboveground biomass; X4—underground biomass; X5—total biomass; X6—root surface area; X7—total root length; X8—root volume; X9—mean diameter of the root system; X10—number of root tips; X11—soluble proteins; X12—soluble sugars; X13—chlorophyll A; X14—chlorophyll B; and X15—carotenoids; X16—total chlorophyll; X17—whole plant whole N accumulation; X18—whole plant whole P accumulation; X19—whole plant whole K accumulation; X20—whole plant whole N content; X21—whole plant whole P content; X22—whole plant whole K content; X23—soil whole N; X24—soil whole P; X25—soil whole K. (highly significant correlation).
Figure 5.
Pearson Correlation Network Analysis. Note: X1—seedling height increment; X2—ground diameter increment; X3—aboveground biomass; X4—underground biomass; X5—total biomass; X6—root surface area; X7—total root length; X8—root volume; X9—mean diameter of the root system; X10—number of root tips; X11—soluble proteins; X12—soluble sugars; X13—chlorophyll A; X14—chlorophyll B; and X15—carotenoids; X16—total chlorophyll; X17—whole plant whole N accumulation; X18—whole plant whole P accumulation; X19—whole plant whole K accumulation; X20—whole plant whole N content; X21—whole plant whole P content; X22—whole plant whole K content; X23—soil whole N; X24—soil whole P; X25—soil whole K. (highly significant correlation).
Table 1.
Main nutrient status of the growth substrate.
Table 1.
Main nutrient status of the growth substrate.
| PH | Substrate | Total/(mg/kg) |
|---|
| 6.65 | mg/kg | Total Nitrogen | Total Phosphorus | Total Potassium |
| 113.42 | 2.29 | 1.05 | 1.63 |
Table 2.
Initial nutrient status of Phellodendron chinense seedlings.
Table 2.
Initial nutrient status of Phellodendron chinense seedlings.
| Organs | Root | Stem | Leaf |
|---|
| Norm | TN | TP | TK | TN | TP | TK | TN | TP | TK |
| | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg |
| Result | 2.33 | 1.14 | 1.49 | 2.39 | 1.22 | 1.95 | 2.66 | 1.38 | 1.74 |
Table 3.
Fertilizer application test factor-level table.
Table 3.
Fertilizer application test factor-level table.
| | Factors |
|---|
| Level | Urea (g/plant) | Calcium Superphosphate (g/plant) | Potassium Chloride (g/plant) |
|---|
| 1 | 4 | 1 | 2 |
| 2 | 6 | 3 | 3 |
| 3 | 8 | 5 | 4 |
| CK | 0 | 0 | 0 |
Table 4.
Four-factor three-level orthogonal experimental design table.
Table 4.
Four-factor three-level orthogonal experimental design table.
| NO. | Treatment Combination | Urea (g/plant) | Calcium Phosphate (g/plant) | Potassium Chloride (g/plant) |
|---|
| 1 | T1(N1P1K1) | 4 | 1 | 2 |
| 2 | T2(N1P2K2) | 4 | 3 | 3 |
| 3 | T3(N1P3K3) | 4 | 5 | 4 |
| 4 | T4(N2P1K2) | 6 | 1 | 3 |
| 5 | T5(N2P2K3) | 6 | 3 | 4 |
| 6 | T6(N2P3K1) | 6 | 5 | 2 |
| 7 | T7(N3P1K3) | 8 | 1 | 4 |
| 8 | T8(N3P2K1) | 8 | 3 | 2 |
| 9 | T9(N3P3K2) | 8 | 5 | 3 |
| CK | CK(N0P0K0) | 0 | 0 | 0 |
Table 5.
The impact of balanced fertilization on the biomass of Phellodendron amurense seedlings.
Table 5.
The impact of balanced fertilization on the biomass of Phellodendron amurense seedlings.
| Treatment | Below-Ground Biomass | Above-Ground Biomass | Total Biomass |
|---|
| T1(N1P1K1) | 15.13 ± 0.9 a | 21.70 ± 0.70 e | 33.63 ± 1.38 d |
| T2(N1P2K2) | 11.93 ± 0.68 b | 21.63 ± 0.92 e | 33.30 ± 0.95 d |
| T3(N1P3K3) | 11.67 ± 0.57 b | 26.47 ± 1.50 c | 38.23 ± 1.57 c |
| T4(N2P1K2) | 11.77 ± 0.45 b | 23.67 ± 0.76 d | 31.57 ± 1.16 e |
| T5(N2P2K3) | 7.90 ± 0.40 c | 36.23 ± 0.58 a | 47.67 ± 0.23 a |
| T6(N2P3K1) | 11.43 ± 0.50 b | 23.27 ± 0.06 d | 31.50 ± 0.66 e |
| T7(N3P1K3) | 8.23 ± 0.60 c | 16.93 ± 0.40 f | 25.57 ± 0.29 g |
| T8(N3P2K1) | 8.63 ± 0.25 c | 18.17 ± 0.46 f | 29.83 ± 1.07 f |
| T9(N3P3K2) | 11.67 ± 0.65 b | 27.97 ± 1.22 b | 43.10 ± 0.87 b |
| CK(N0P0K0) | 7.87 ± 0.38 c | 8.63 ± 0.38 g | 16.50 ± 0.10 h |
Table 6.
Effects of fertilizer rationing on the root system of Phellodendron chinense seedlings.
Table 6.
Effects of fertilizer rationing on the root system of Phellodendron chinense seedlings.
| Treatment | Root Surface Area (cm2) | Total Root Length (cm) | Root Volume (cm3) | Mean Root Diameter (mm) | Number of Root Tips |
|---|
| T1(N1P1K1) | 717.96 ± 112.48 a | 1241.53 ± 137.31 c | 17.90 ± 2.59 bc | 1.47 ± 0.12 ab | 1076.00 ± 133.43 a |
| T2(N1P2K2) | 567.57 ± 61.21 abc | 1434.56 ± 133.00 bc | 13.13 ± 1.5 cd | 1.26 ± 0.07 c | 938.33 ± 87.55 abc |
| T3(N1P3K3) | 404.68 ± 54.99 de | 1208.92 ± 145.32 c | 20.85 ± 4.58 ab | 1.16 ± 0.05 cd | 743.00 ± 9.64 de |
| T4(N2P1K2) | 629.29 ± 51.8 abc | 1508.27 ± 160.18 b | 18.63 ± 3.22 abc | 1.49 ± 0.03 ab | 969.67 ± 164.29 ab |
| T5(N2P2K3) | 555.09 ± 127.53 bc | 1525.68 ± 140.65 b | 22.01 ± 2.62 ab | 1.36 ± 0.16 bc | 765.33 ± 116 cde |
| T6(N2P3K1) | 669.61 ± 107.64 ab | 1462.89 ± 92.73 bc | 24.85 ± 3.37 a | 1.32 ± 0.11 bc | 892.67 ± 48.09 bcd |
| T7(N3P1K3) | 688.80 ± 25.37 ab | 1851.53 ± 117.63 a | 17.44 ± 3.99 bc | 1.52 ± 0.09 ab | 894.33 ± 19.01 bcd |
| T8(N3P2K1) | 512.72 ± 26.09 cd | 952.85 ± 105.61 d | 22.63 ± 5.6 ab | 1.64 ± 0.06 a | 779.67 ± 62.5 cde |
| T9(N3P3K2) | 621.89 ± 92.45 abc | 1299.78 ± 186.16 bc | 19.46 ± 1.62 ab | 1.47 ± 0.12 ab | 929.00 ± 90.07 abc |
| CK(N0P0K0) | 359.45 ± 79.00 e | 870.29 ± 125.82 d | 9.21 ± 1.39 d | 1.01 ± 0.17 d | 667.00 ± 53.7 e |
Table 7.
Effects of fertilizer rationing on nutrient accumulation in root, stem and leaf of Phellodendron chinense seedlings.
Table 7.
Effects of fertilizer rationing on nutrient accumulation in root, stem and leaf of Phellodendron chinense seedlings.
| Treatment | Nutrient Accumulation (mg/plant) |
|---|
| N | P | K |
|---|
| Root | Stem | Leaf | Total | Root | Stem | Leaf | Total | Root | Stem | Leaf | Total |
|---|
| T1(N1P1K1) | 20.07 ± 1.22 | 12.97 ± 0.62 | 40.31 ± 1.58 | 73.35 ± 1.79 | 3.08 ± 0.22 | 1.45 ± 0.05 | 5.08 ± 0.13 | 9.60 ± 0.20 | 18.58 ± 1.53 | 7.49 ± 0.41 | 32.37 ± 1.57 | 58.44 ± 2.65 |
| T2(N1P2K2) | 15.61 ± 0.93 | 10.88 ± 0.31 | 26.76 ± 1.42 | 53.24 ± 2.58 | 2.33 ± 0.15 | 1.18 ± 0.04 | 3.28 ± 0.17 | 6.79 ± 0.35 | 16.38 ± 1.40 | 7.24 ± 0.41 | 24.23 ± 1.85 | 47.85 ± 3.62 |
| T3(N1P3K3) | 15.68 ± 0.80 | 9.65 ± 0.33 | 33.78 ± 1.85 | 59.11 ± 2.23 | 2.39 ± 0.13 | 1.07 ± 0.02 | 4.22 ± 0.17 | 7.67 ± 0.19 | 13.83 ± 1.07 | 5.28 ± 0.17 | 25.87 ± 1.45 B | 44.97 ± 2.24 |
| T4(N2P1K2) | 16.35 ± 0.59 | 14.15 ± 1.18 | 34.36 ± 0.96 | 64.85 ± 1.99 | 2.31 ± 0.09 | 1.45 ± 0.10 | 3.97 ± 0.11 | 7.73 ± 0.18 | 14.99 ± 0.21 | 8.16 ± 0.69 | 27.24 ± 0.18 | 50.39 ± 0.67 |
| T5(N2P2K3) | 11.05 ± 0.55 | 12.67 ± 0.46 | 31.20 ± 0.70 | 54.92 ± 1.70 | 1.52 ± 0.10 | 1.26 ± 0.06 | 3.51 ± 0.13 | 6.29 ± 0.28 | 10.80 ± 0.57 | 7.87 ± 0.29 | 26.28 ± 0.83 | 44.95 ± 1.66 |
| T6(N2P3K1) | 15.33 ± 0.67 | 19.19 ± 0.63 | 43.33 ± 4.38 | 77.86 ± 3.14 | 2.04 ± 0.12 | 1.86 ± 0.08 | 4.73 ± 0.42 | 8.63 ± 0.23 | 16.49 ± 0.83 | 13.19 ± 0.41 | 40.11 ± 4.30 | 69.79 ± 3.69 |
| T7(N3P1K3) | 11.35 ± 0.81 | 10.54 ± 0.3 | 37.80 ± 1.23 | 59.7 ± 0.82 | 1.67 ± 0.10 | 1.13 ± 0.02 | 4.57 ± 0.21 | 7.37 ± 0.17 | 11.68 ± 0.63 | 6.93 ± 0.28 | 33.48 ± 1.34 | 52.08 ± 0.72 |
| T8(N3P2K1) | 12.09 ± 0.34 | 7.10 ± 0.15 | 30.89 ± 1.03 | 50.09 ± 0.94 | 1.78 ± 0.07 | 0.76 ± 0.02 | 3.73 ± 0.11 | 6.28 ± 0.13 | 10.86 ± 0.36 | 4.00 ± 0.20 | 24.01 ± 1.30 | 38.87 ± 1.65 |
| T9(N3P3K2) | 15.76 ± 0.90 | 9.54 ± 0.45 | 22.53 ± 0.83 | 47.83 ± 1.03 | 2.22 ± 0.10 | 0.98 ± 0.03 | 2.61 ± 0.11 | 5.81 ± 0.05 | 14.13 ± 0.94 | 5.33 ± 0.32 | 17.51 ± 0.34 | 36.97 ± 0.93 |
| CK(N0P0K0) | 9.96 ± 0.50 | 4.53 ± 0.11 | 8.84 ± 0.85 | 23.33 ± 0.41 | 1.39 ± 0.08 | 0.46 ± 0.01 | 1.02 ± 0.10 | 2.87 ± 0.04 | 9.30 ± 0.72 | 2.62 ± 0.07 | 7.18 ± 0.52 | 19.10 ± 0.24 |
Table 8.
The impact of balanced fertilization on soil nutrient content for Phellodendron amurense seedlings.
Table 8.
The impact of balanced fertilization on soil nutrient content for Phellodendron amurense seedlings.
| Treatment | N (mg/kg)
Below-Ground Biomass | P (mg/kg)
Above-Ground Biomass | K (mg/kg)
Total Biomass |
|---|
| T1(N1P1K1) | 0.6896 ± 0.0015 f | 0.0148 ± 0.0001 a | 0.8526 ± 0.0139 cd |
| T2(N1P2K2) | 0.6804 ± 0.0016 g | 0.0142 ± 0.0001 bc | 0.9571 ± 0.0144 b |
| T3(N1P3K3) | 0.6985 ± 0.0016 e | 0.0149 ± 0.0001 a | 0.8214 ± 0.0160 d |
| T4(N2P1K2) | 0.7216 ± 0.0016 b | 0.0142 ± 0.0001 b | 0.8871 ± 0.0138 c |
| T5(N2P2K3) | 0.7262 ± 0.0016 a | 0.0138 ± 0.0001 c | 0.9544 ± 0.0149 b |
| T6(N2P3K1) | 0.6972 ± 0.0017 e | 0.0129 ± 0.0001 d | 1.0085 ± 0.0159 a |
| T7(N3P1K3) | 0.7167 ± 0.0017 c | 0.0148 ± 0.0001 a | 0.9925 ± 0.0166 ab |
| T8(N3P2K1) | 0.7274 ± 0.0017 a | 0.015 ± 0.0001 a | 0.8745 ± 0.0164 c |
| T9(N3P3K2) | 0.7022 ± 0.0016 d | 0.0139 ± 0.0001 bc | 0.8409 ± 0.0146 cd |
| CK(N0P0K0) | 0.6592 ± 0.0016 h | 0.0128 ± 0.0001 d | 0.8197 ± 0.0142 d |
Table 9.
Effects of fertilizer ratios on nutrient accumulation in roots, stems and leaves of Phellodendron chinense seedlings.
Table 9.
Effects of fertilizer ratios on nutrient accumulation in roots, stems and leaves of Phellodendron chinense seedlings.
| Treatment | Nutrient Accumulation (mg/kg) |
|---|
| N | P | K |
|---|
| Root | Stem | Leaf | Total | Root | Stem | Leaf | Total | Root | Stem | Leaf | Total |
|---|
| T1(N1P1K1) | 1.326 | 0.799 | 3.436 | 5.560 | 0.204 | 0.089 | 0.433 | 0.726 | 1.227 | 0.461 | 2.759 | 4.447 |
| T2(N1P2K2) | 1.308 | 0.788 | 3.387 | 5.483 | 0.195 | 0.085 | 0.415 | 0.696 | 1.371 | 0.524 | 3.065 | 4.961 |
| T3(N1P3K3) | 1.344 | 0.809 | 3.482 | 5.635 | 0.205 | 0.090 | 0.435 | 0.729 | 1.184 | 0.442 | 2.667 | 4.294 |
| T4(N2P1K2) | 1.389 | 0.836 | 3.604 | 5.829 | 0.196 | 0.086 | 0.417 | 0.698 | 1.274 | 0.482 | 2.859 | 4.616 |
| T5(N2P2K3) | 1.398 | 0.841 | 3.628 | 5.868 | 0.192 | 0.084 | 0.408 | 0.684 | 1.367 | 0.522 | 3.057 | 4.946 |
| T6(N2P3K1) | 1.341 | 0.808 | 3.476 | 5.625 | 0.179 | 0.078 | 0.380 | 0.636 | 1.442 | 0.555 | 3.216 | 5.214 |
| T7(N3P1K3) | 1.379 | 0.830 | 3.578 | 5.787 | 0.203 | 0.089 | 0.432 | 0.724 | 1.420 | 0.545 | 3.168 | 5.133 |
| T8(N3P2K1) | 1.401 | 0.842 | 3.634 | 5.877 | 0.207 | 0.090 | 0.439 | 0.736 | 1.258 | 0.475 | 2.824 | 4.556 |
| T9(N3P3K2) | 1.351 | 0.813 | 3.502 | 5.666 | 0.191 | 0.083 | 0.405 | 0.679 | 1.211 | 0.454 | 2.724 | 4.389 |
| CK(N0P0K0) | 1.266 | 0.764 | 3.276 | 5.305 | 0.177 | 0.078 | 0.377 | 0.631 | 1.182 | 0.442 | 2.662 | 4.285 |
Table 10.
Effect of fertilization on stoichiometric ratios of various organs of Phellodendron chinense seedlings.
Table 10.
Effect of fertilization on stoichiometric ratios of various organs of Phellodendron chinense seedlings.
| | Root | Stem | Leaf |
|---|
| Treatment | N:P | N:K | K:P | N:P | N:K | K:P | N:P | N:K | K:P |
|---|
| CK(N0P0K0) | 7.15 | 1.07 | 6.67 | 9.85 | 1.73 | 5.70 | 8.70 | 1.23 | 7.07 |
| T1(N1P1K1) | 6.51 | 1.08 | 6.03 | 8.96 | 1.73 | 5.18 | 7.94 | 1.25 | 6.37 |
| T2(N1P2K2) | 6.70 | 0.95 | 7.02 | 9.23 | 1.50 | 6.14 | 8.16 | 1.11 | 7.38 |
| T3(N1P3K3) | 6.57 | 1.13 | 5.79 | 9.04 | 1.83 | 4.94 | 8.01 | 1.31 | 6.13 |
| T4(N2P1K2) | 7.09 | 1.09 | 6.50 | 9.75 | 1.73 | 5.62 | 8.65 | 1.26 | 6.86 |
| T5(N2P2K3) | 7.29 | 1.02 | 7.12 | 10.02 | 1.61 | 6.23 | 8.90 | 1.19 | 7.49 |
| T6(N2P3K1) | 7.51 | 0.93 | 8.08 | 10.34 | 1.45 | 7.11 | 9.16 | 1.08 | 8.48 |
| T7(N3P1K3) | 6.78 | 0.97 | 6.98 | 9.34 | 1.52 | 6.13 | 8.28 | 1.13 | 7.33 |
| T8(N3P2K1) | 6.78 | 1.11 | 6.09 | 9.33 | 1.77 | 5.25 | 8.28 | 1.29 | 6.43 |
| T9(N3P3K2) | 7.09 | 1.12 | 6.35 | 9.76 | 1.79 | 5.45 | 8.64 | 1.29 | 6.72 |
Table 11.
Effect of fertilization on soil stoichiometric ratios of Kawartha seedlings.
Table 11.
Effect of fertilization on soil stoichiometric ratios of Kawartha seedlings.
| Treatment | N:P | N:K | K:P |
|---|
| CK(N0P0K0) | 51.50 | 0.80 | 64.04 |
| T1(N1P1K1) | 46.59 | 0.81 | 57.61 |
| T2(N1P2K2) | 47.92 | 0.71 | 67.40 |
| T3(N1P3K3) | 46.88 | 0.85 | 55.13 |
| T4(N2P1K2) | 50.82 | 0.81 | 62.47 |
| T5(N2P2K3) | 52.62 | 0.76 | 69.16 |
| T6(N2P3K1) | 54.05 | 0.69 | 78.18 |
| T7(N3P1K3) | 48.43 | 0.72 | 67.06 |
| T8(N3P2K1) | 48.49 | 0.83 | 58.30 |
| T9(N3P3K2) | 50.52 | 0.84 | 60.50 |
Table 12.
Principal Component Analysis Component Score Table.
Table 12.
Principal Component Analysis Component Score Table.
| Treatment | Total Dry Weight (g) | Height-Diameter Ratio | Stem-Root Ratio | QI | Sort |
|---|
| T1(N1P1K1) | 43.10 | 6.56 | 2.40 | 4.81 | 1 |
| T2(N1P2K2) | 33.63 | 6.48 | 1.43 | 4.25 | 3 |
| T3(N1P3K3) | 33.30 | 6.26 | 1.81 | 4.13 | 4 |
| T4(N2P1K2) | 38.23 | 6.28 | 2.27 | 4.47 | 2 |
| T5(N2P2K3) | 31.57 | 6.91 | 2.01 | 3.54 | 8 |
| T6(N2P3K1) | 47.67 | 7.21 | 4.59 | 4.04 | 5 |
| T7(N3P1K3) | 31.50 | 6.07 | 2.03 | 3.89 | 6 |
| T8(N3P2K1) | 25.57 | 7.76 | 2.06 | 2.61 | 9 |
| T9(N3P3K2) | 29.83 | 6.28 | 2.10 | 3.56 | 7 |
| CK(N0P0K0) | 16.50 | 7.80 | 1.10 | 1.85 | 10 |
Table 13.
Evaluation of seedling quality of Phellodendron chinense seedlings by proportionate fertilization.
Table 13.
Evaluation of seedling quality of Phellodendron chinense seedlings by proportionate fertilization.
|
Treatment
|
Component Score
|
Comprehensive Score
|
Ranking
|
|---|
|
Principal Component 1
|
Principal Component 2
|
Principal Component 3
|
Principal Component 4
|
Principal Component 5
|
|---|
| T1(N1P1K1) | 0.877434312 | 4.214809158 | 1.99872016 | 0.037384563 | −0.755722187 | 5.859288193 | 1 |
| T2(N1P2K2) | −0.477701669 | 1.363153091 | −0.250292308 | 1.088603422 | 2.466173544 | 3.278651881 | 4 |
| T3(N1P3K3) | −0.725157954 | −0.388659371 | 1.81528827 | −1.868275241 | 0.89128498 | 0.254945856 | 5 |
| T4(N2P1K2) | 1.418100005 | 0.287227576 | 0.483264164 | −0.184934183 | −0.626420911 | 4.943545777 | 2 |
| T5(N2P2K3) | 1.777621142 | −2.569888619 | −1.919614708 | −0.6371241 | 0.705985603 | −1.856869618 | 8 |
| T6(N2P3K1) | 3.778359196 | 1.381991415 | −3.413558268 | −1.254580429 | −0.931029085 | −1.84058805 | 7 |
| T7(N3P1K3) | 2.441902098 | −0.499681423 | −0.427207323 | 3.215852129 | −0.28928752 | 4.909920609 | 3 |
| T8(N3P2K1) | 1.127123961 | −3.304778292 | 2.325119373 | 0.315838551 | −1.032676332 | −2.551823115 | 9 |
| T9(N3P3K2) | −0.18225549 | −0.436751391 | 0.746092534 | −1.024459734 | 0.325858689 | −1.090266337 | 6 |
| CK(N0P0K0) | −10.0354256 | −0.047422146 | −1.357811894 | 0.311695023 | −0.754166782 | −11.9068052 | 10 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).