Functionalization of Wood for the Removal of Heavy Metal Ions from Waster Water: A Review
Abstract
1. Introduction
2. Structural and Functional Modification of Wood
3. Progress in Functionalized Wood for Absorbing Heavy Metal Ions
3.1. Lead Adsorption
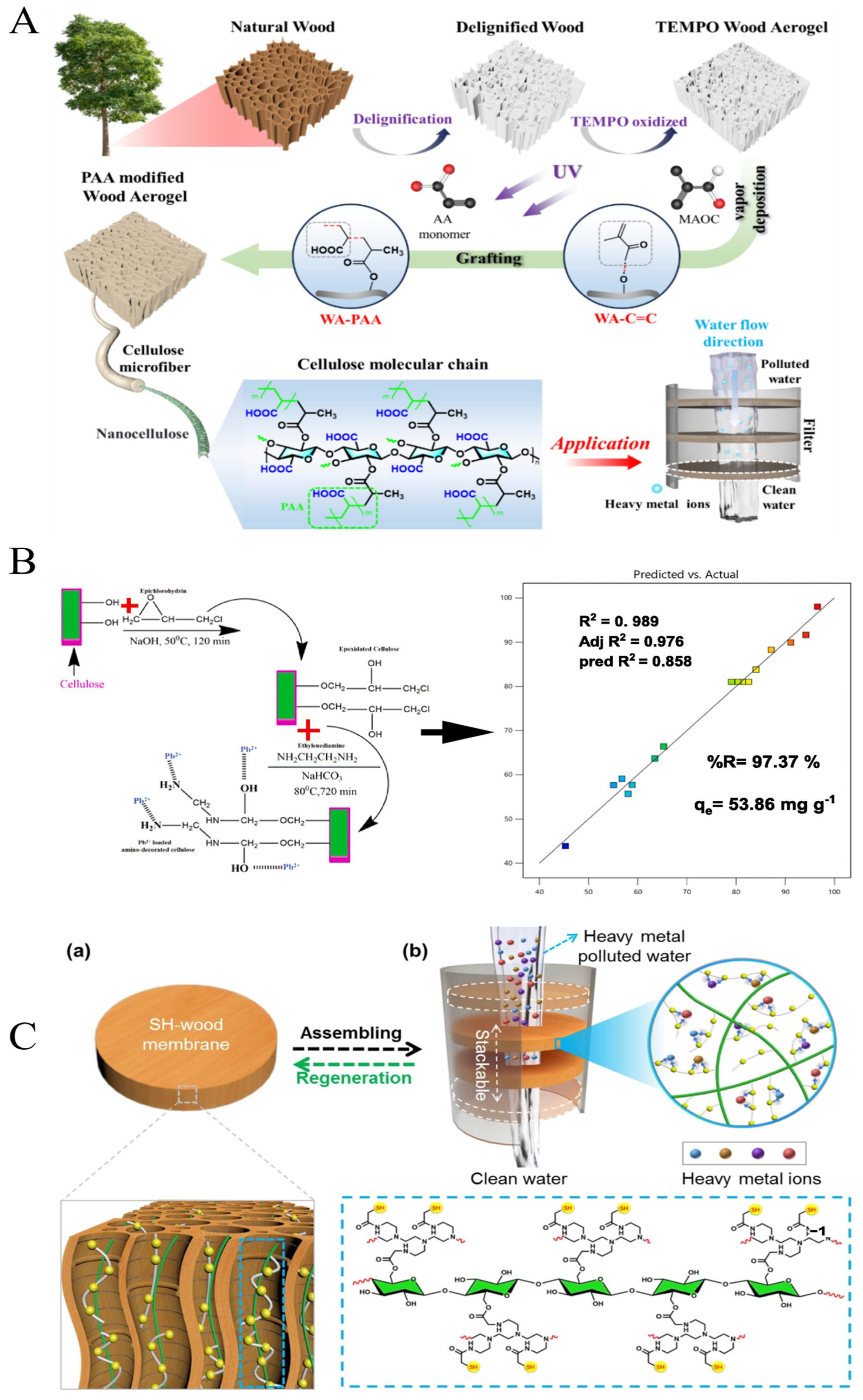
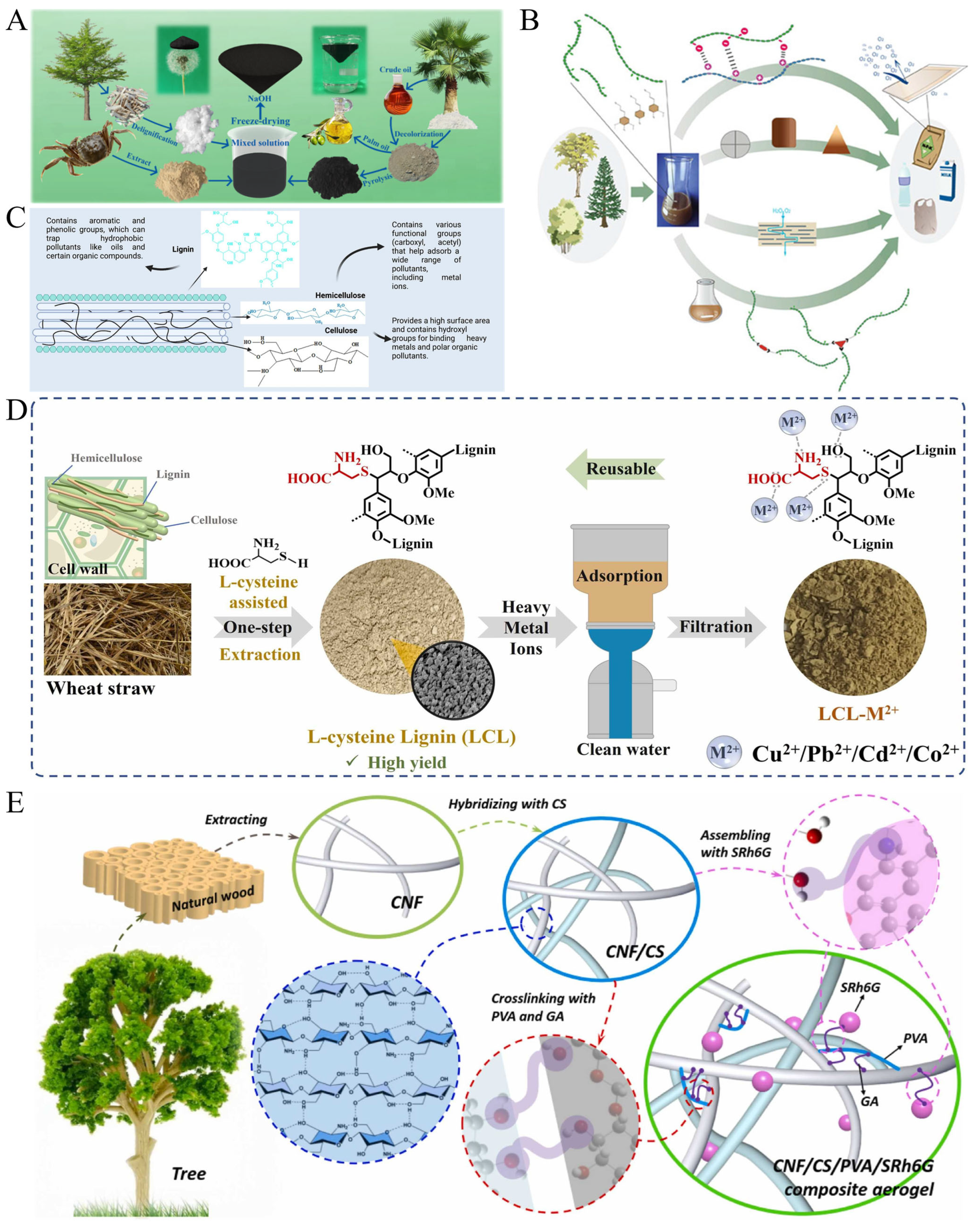
3.2. Cadmium Adsorption
3.3. Zinc Adsorption
3.4. Chromium Adsorption
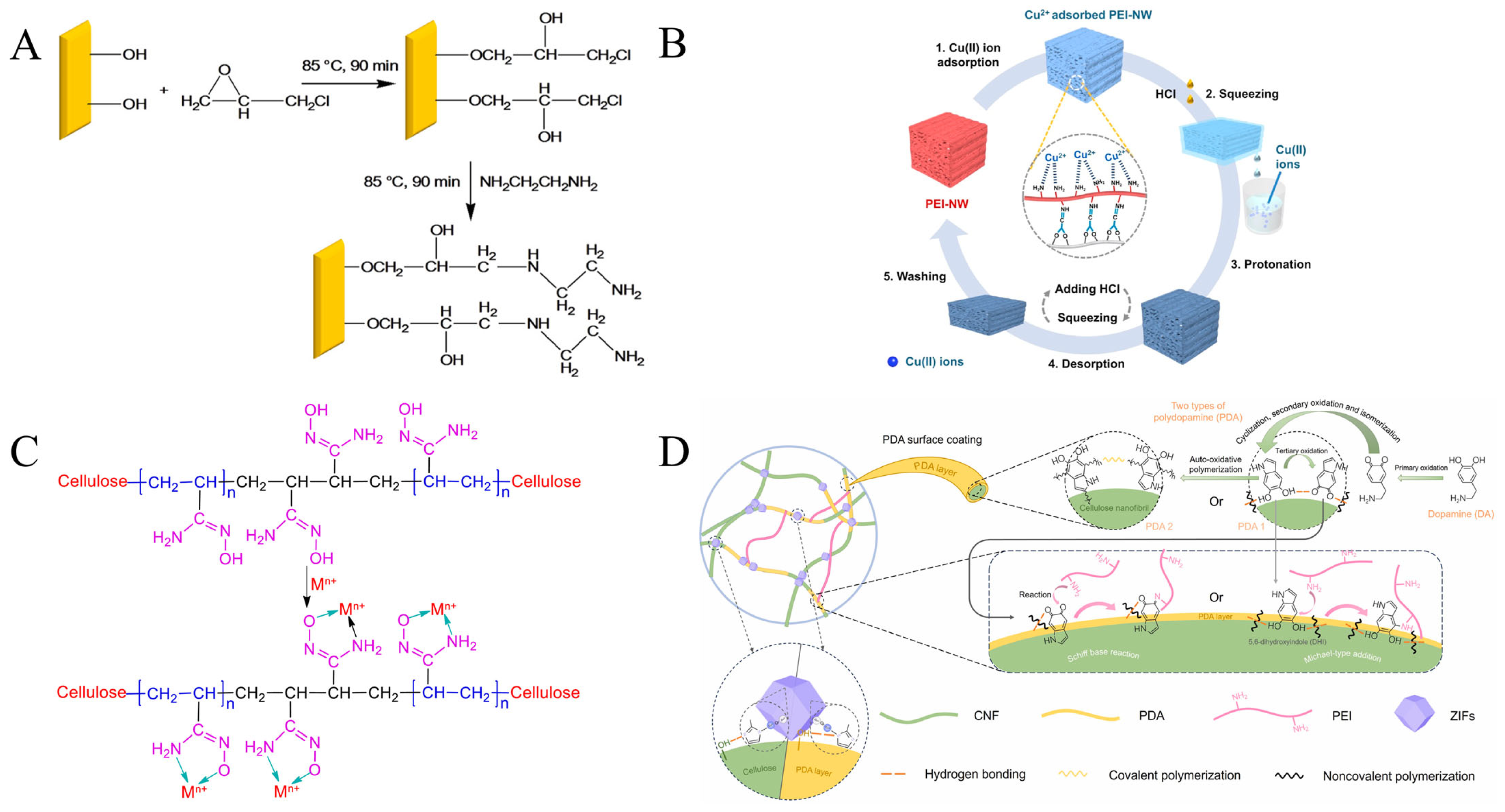
3.5. Copper Adsorption
4. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schubert, M.; Panzarasa, G.; Burgert, I. Sustainability in Wood Products: A New Perspective for Handling Natural Diversity. Chem. Rev. 2023, 123, 1889–1924. [Google Scholar] [CrossRef]
- Gao, S.; Wang, J.; Wang, K.; Cai, Y.; Li, C.; Dong, Y.; Li, J. Efficient Solar-Driven Interfacial Desalination of Wood through Surface Furfurylation. Colloids Surf. Physicochem. Eng. Asp. 2025, 709, 136161. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Tan, Y.; Dong, Y.; Wang, K.; Li, J. Lauric Acid Encapsulation for Continuous Evaporation of Wood-Based Solar Evaporator. Appl. Therm. Eng. 2025, 280, 128351. [Google Scholar] [CrossRef]
- He, Y.; Qiao, Z.; Fan, L.; Xia, Z.; Ma, J.; Zheng, X.; Deng, L.; Xu, X.; Liu, H. Lightweight, Ultra-Compressed, and Environmentally Friendly Wood/Tpu Aerogel Sensor Based on Optimized Performance of Dynamic 3D Pore Structure. J. Colloid Interface Sci. 2025, 678, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.; Niazi, M.B.K.; Miran, W.; Tawfeek, A.M.; Jahan, Z.; Kamel, E.M.; Ahmed, N.; Saeed Akhtar, M. Wood as a Green and Sustainable Alternative for Environmentally Friendly & Flexible Electronic Devices. Chemosphere 2023, 336, 139213. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, R.; Tardy, B.L.; Mattos, B.D.; Bai, L.; Rojas, O.J. Plant Nanomaterials and Inspiration from Nature: Water Interactions and Hierarchically Structured Hydrogels. Adv. Mater. 2021, 33, e2001085. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Liu, Y.; Han, J.; Duan, G.; Fu, Q.; Han, X.; Zhang, C.; He, S.; Jiang, S. Structure Modifications of Wood-Based Materials for Water Treatment Applications: A Review. Mater. Today 2025, 87, 252–286. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, Y.; Ma, N.L.; Ye, H.; Verma, M.; Ng, H.S.; Ge, S. Utilizing Adsorption of Wood and Its Derivatives as an Emerging Strategy for the Treatment of Heavy Metal-Contaminated Wastewater. Environ. Pollut. 2024, 340, 122830. [Google Scholar] [CrossRef]
- Huo, H.; Xu, J.; Hou, H.; Yu, Y.; Feng, W.; Lu, M.; Wang, S.; Min, D. Valorizing Wood to Toxic Heavy Metal Ions Biosorbent through in Situ Sulfation of Its Cellulose. Int. J. Biol. Macromol. 2025, 309, 142835. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, C.; Cui, J.; Dong, Y.; Cai, J.; Zhou, F.; Lyu, J.; Avramidis, S. Self-Diffusion Coefficient of Bound Water in Longleaf Pine Wood Investigated with Pulsed-Field-Gradient 1h-Nmr and Molecular Simulation. Cellulose 2025, 32, 3567–3581. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Qiu, Z.; Gong, W.; Wang, Y.; Xie, Y.; Xiao, Z. In Situ Growth of Zr-Based Metal-Organic Frameworks on Cellulose Sponges for Hg2+ and Methylene Blue Removal. Carbohydr. Polym. 2024, 328, 121750. [Google Scholar] [CrossRef]
- Qi, X.; Chen, Y.; Liu, M.; Zhang, X.; Zuo, Y.; Ma, Q.; Xie, X.; Guo, X.; Wu, Y. Green and Cost-Effective: Bifunctional Wood for Efficient Adsorption and Sensitive Detection of Pb(II). Ind. Crops Prod. 2024, 210, 118162. [Google Scholar] [CrossRef]
- Chao, W.; Wang, S.; Li, Y.; Cao, G.; Zhao, Y.; Sun, X.; Wang, C.; Ho, S.-H. Natural Sponge-Like Wood-Derived Aerogel for Solar-Assisted Adsorption and Recovery of High-Viscous Crude Oil. Chem. Eng. J. 2020, 400, 125865. [Google Scholar] [CrossRef]
- Shang, Y.; Gao, R.; Wang, Y.; Dong, X.; Li, X.; Tang, J.; Wang, X.; Liu, J.; Xie, Y.; Li, J.; et al. Scalable Fabrication of Efficient and Recycling Wood Residue-Derived Sponge for Crude Oil Adsorption. J. Hazard. Mater. 2023, 441, 129979. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Guo, L.; Wu, M.; Wang, J.; Zhang, L.; Deng, S.; Xie, T.; Zhang, X.; Zhu, G. Scalable Synthesis of Hierarchical Porous Mof-199 Decorated Aminated Wood Sponge as Physisorbent Materials for Carbon Dioxide Capture from Wet Flue Gas. J. Environ. Chem. Eng. 2025, 13, 117128. [Google Scholar] [CrossRef]
- Marimuthu, T.; Chee, C.Y.; Sulaiman, N.M.N. A Review on the Use of Cellulose Nanomaterials for Wastewater Remediation of Heavy Metal Ions. Int. J. Environ. Sci. Technol. 2023, 20, 3421–3436. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-Functionalized Cellulose Nanofiber Membranes for the Effective Adsorption of Heavy Metal Ions in Water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Gu, Y.; Ye, M.; Wang, Y.; Li, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Lignosulfonate Functionalized G-C3n4/Carbonized Wood Sponge for Highly Efficient Heavy Metal Ion Scavenging. J. Mater. Chem. A 2020, 8, 12687–12698. [Google Scholar] [CrossRef]
- Páez-Hernández, M.E.; Galán-Vidal, C.A.; Ibarra, I.S.; Hernández, P.; Pérez-Silva, I. Recovery of Cd(II) and Zn(II) from Hcl Medium Using Cellulose Acetate Sponges Modified with Cyanex 923. Desalin. Water Treat. 2021, 221, 176–184. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.; Ouyang, S.; Liu, Y.; Hu, Z.; Wu, Y.; Qian, J.; Li, Z.; Wang, L.; Ma, S. A Comprehensive Review on Preparation and Functional Application of the Wood Aerogel with Natural Cellulose Framework. Int. J. Biol. Macromol. 2024, 275, 133340. [Google Scholar] [CrossRef]
- Huang, W.; Xu, Y.; Chen, N.; Cheng, G.; Ke, H. Amino-Modified Hemp Stem for High-Capacity Adsorption of Cr(VI) from Aqueous Solution. J. Environ. Chem. Eng. 2023, 11, 111441. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel Based on Modified Chitosan for Oil Spill Cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of Palladium(II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Lu, Y.; Wu, G.; Bai, L.; Barker-Rothschild, D.; Lyu, J.; Liu, S.; Rojas, O.J. Wood Elasticity and Compressible Wood-Based Materials: Functional Design and Applications. Prog. Mater. Sci. 2025, 147, 101354. [Google Scholar] [CrossRef]
- Yu, M.; Du, S.; Zhu, B.; Zhu, L.; Yang, J. All-Natural Photothermal Hydrogel for Efficient Desalination and Heavy Metal Enrichment. Langmuir 2025, 41, 5664–5675. [Google Scholar] [CrossRef] [PubMed]
- Ibn Yaich, A.; Edlund, U.; Albertsson, A.-C. Transfer of Biomatrix/Wood Cell Interactions to Hemicellulose-Based Materials to Control Water Interaction. Chem. Rev. 2017, 117, 8177–8207. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Bansal, M.; Garima; Wilson, K.; Gupta, S.; Dhanawat, M. Lignocellulose Biosorbents: Unlocking the Potential for Sustainable Environmental Cleanup. Int. J. Biol. Macromol. 2025, 294, 139497. [Google Scholar] [CrossRef]
- Li, S.; Duan, Y.; Fang, J.; Chen, S.; Wang, J.; Jiang, H.; Xiao, R.; Ni, C.; Wu, S.; Deng, Q.; et al. Soybean Hull Hemicellulose–Soybean Protein Isolate Composite Aerogel: Adsorption Material for Remediation of Heavy Metal-Polluted Water. Int. J. Biol. Macromol. 2025, 310, 143298. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Hu, S.; Wu, H.; Ren, J.; Yue, F. One-Step Isolation of L-Cysteine Functionalized Lignin with High Adsorption Capacity for Heavy Metal Ions. Ind. Crops Prod. 2024, 222, 120026. [Google Scholar] [CrossRef]
- An, C.; Zhang, M.; Xiao, Z.; Yang, Q.; Feng, L.; Li, S.; Shi, M. Lignocellulose/Chitosan Hybrid Aerogel Composited with Fluorescence Molecular Probe for Simultaneous Adsorption and Detection of Heavy Metal Pollutants. J. Environ. Chem. Eng. 2023, 11, 111205. [Google Scholar] [CrossRef]
- Anang, S.; Ibrahim, M.G.; Nasr, M. Industrial Wastewater Treatment by Downflow Hanging Sponge System: Techno-Economic Analysis, Life Cycle Assessment, and Sustainable Development Goals Fulfillment. J. Environ. Chem. Eng. 2025, 13, 115944. [Google Scholar] [CrossRef]
- Babar, Z.B.; Iftikhar, R.; Rizwan, K.; Munir, S.; Urfi, M.; Ashraf, F.; Inam, M.A.; Iqbal, S.; Saad, M.; Mahmood, S.; et al. Biomitigation of Noxious Metal Ions Using Engineered Lignin-Based Nanocomposites for Sustainable Environment. J. Water Process Eng. 2025, 70, 106938. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, Y.; Jiang, Y.; Yu, W.; Ma, D.; Bai, L.; Huo, P.; Li, Z.; Liu, Y. Polydopamine/Fe3o4 Modified Wood-Based Evaporator for Efficient and Continuous Water Purification. J. Colloid Interface Sci. 2023, 652, 1271–1281. [Google Scholar] [CrossRef]
- Lin, X.; Jin, J.; Guo, X.; Jia, X. All-Carboxymethyl Cellulose Sponges for Removal of Heavy Metal Ions. Cellulose 2021, 28, 3113–3122. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from Aqueous Single Metal Solutions by Guanyl-Modified Cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, L.; Wei, J.; Zhao, Z.; Wang, J.; Gao, H.; Nie, Y. Wood Microchannels with Multi-Carboxyl Groups for Selective Adsorption of Fe3+ from Ionic Liquids Aqueous Solution: Experimental and DFT Study. J. Environ. Chem. Eng. 2023, 11, 110348. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, S. Bioinspired Highly Anisotropic, Robust and Environmental Resistant Wood Aerogel Composite with Semi-Interpenetrating Polymer Networks for Cu(II) Ion Removal. Cellulose 2022, 29, 8353–8370. [Google Scholar] [CrossRef]
- Meng, J.; Guan, H.; Dai, X.; Wang, X. Amino-Functionalized Wood Aerogel for Efficient Removal of Copper Ions from Water. Int. J. Polym. Sci. 2021, 2021, 4913226. [Google Scholar] [CrossRef]
- Chen, F.; Gong, A.S.; Zhu, M.; Chen, G.; Lacey, S.D.; Jiang, F.; Li, Y.; Wang, Y.; Dai, J.; Yao, Y.; et al. Mesoporous, Three-Dimensional Wood Membrane Decorated with Nanoparticles for Highly Efficient Water Treatment. ACS Nano 2017, 11, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, M.; Zhu, H.; Huang, Y.; Song, B.; Wang, P.; Jiang, S.; Wang, B. Zif-8-Decorated Wood Sponge Derived from Biomimetic Mineralization of Polydopamine. Green Mater. 2024, 12, 253–260. [Google Scholar] [CrossRef]
- Esteves, B.; Cruz-Lopes, L.; Figueirinha, A.; de Lemos, L.T.; Ferreira, J.; Pereira, H.; Domingos, I. Heat-Treated Wood as Chromium Adsorption Material. Eur. J. Wood Wood Prod. 2017, 75, 903–909. [Google Scholar] [CrossRef]
- Li, H.; Yang, K.; Huang, J.; Chen, F. Design and Construction of UiO-66-NH2/Wood Composite for Efficient Removal of Trace Heavy Metal Ions from Water. Chem. J. Chin. Univ. 2022, 43, 32–39. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of Heavy Metals and Soil Properties at a Nigerian E-Waste Site on Soil Microbial Community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly Isolated Bacillus Sp. Ps-6 Assisted Phytoremediation of Heavy Metals Using Phragmites Communis: Potential Application in Wastewater Treatment. Bioresour. Technol. 2021, 320, 124353. [Google Scholar] [CrossRef]
- Abdulla, M. Chapter 13—Lead. In Essential and Toxic Trace Elements and Vitamins in Human Health; Prasad, A.S., Brewer, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 181–191. [Google Scholar] [CrossRef]
- Das, S.; Nath, T.C.; Rahman, M.; Uddin, J.; Naher, N.; Akter, M.; Rahman, M.; Adhikari, A. Occupational Hazards in Lead-Acid Battery Factories in Bangladesh: Assessing Excess Heat, Noise, Chemical Exposures, and Health Impacts on Workers. Saf. Health Work 2025, 16, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chetyrkina, M.R.; Kameneva, L.; Mishchenko, D.V.; Klimanova, E.N.; Sashenkova, T.E.; Allayarova, U.Y.; Kostyuk, S.V.; Frolova, L.A.; Aldoshin, S.M.; Troshin, P.A. Lead, Tin, Bismuth or Organics: Assessment of Potential Environmental and Human Health Hazards Originating from Mature Perovskite PV Technology. Sol. Energy Mater. Sol. Cells 2023, 252, 112177. [Google Scholar] [CrossRef]
- He, W.; Wei, B.; Liang, S.; Wang, R.; Ji, Q.; Hu, G.; Li, W.; He, L.; Yu, J.; Zhu, H.; et al. Highly Nanostructured and Carboxylated Wood Aerogel-Based Adsorption Membrane Reconstructed by Grafting of Polyacrylic Acid for Efficient Removal of Heavy-Metal Ions. Chem. Eng. J. 2024, 493, 152411. [Google Scholar] [CrossRef]
- El Hajam, M.; Kandri, N.I.; Plavan, G.-I.; Harrath, A.H.; Mansour, L.; Boufahja, F.; Zerouale, A. Pb2+ Ions Adsorption onto Raw and Chemically Activated Dibetou Sawdust: Application of Experimental Designs. J. King Saud Univ. Sci. 2020, 32, 2176–2189. [Google Scholar] [CrossRef]
- Obsa, A.L.; Shibeshi, N.T.; Workeneh, G.A.; Mulugeta, E. Enhanced Lead (II) Adsorption from Aqueous Solution Using Sawdust-Derived Amino-Decorated Cellulose: Optimization, Isotherm, Kinetics, and Reusability Studies. Results Chem. 2024, 7, 101506. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, K.; Yan, Q.; Zhang, S.; Li, J.; Ji, Y. Synthesis of Amino-Functionalized Waste Wood Flour Adsorbent for High-Capacity Pb(II) Adsorption. ACS Omega 2019, 4, 10475–10484. [Google Scholar] [CrossRef]
- Chen, Z.; Su, X.; Li, K.; Niu, S.; Shen, Z.; Li, X.; Chen, S.; Wu, W. A Thiolated TiO2-Based Degradable Superhydrophobic Wood for Oil–Water Separation and Heavy Metal Treatment. Sep. Purif. Technol. 2025, 354, 128949. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.; Li, J.; Yang, K.; Zhang, Z.; Chen, F.; Wang, B. High-Throughput Metal Trap: Sulfhydryl-Functionalized Wood Membrane Stacks for Rapid and Highly Efficient Heavy Metal Ion Removal. ACS Appl. Mater. Interfaces 2020, 12, 15002–15011. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Nogawa, K.; Nordberg, M. Chapter 32—Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 667–716. [Google Scholar] [CrossRef]
- Sattar, S.; Yahya, M.; Aslam, S.; Hussain, R.; Shah, S.M.M.; Rauf, Z.; Zamir, A.; Ullah, R.; Shahzad, A. Environmental Occurrence, Hazards, and Remediation Strategies for the Removal of Cadmium from the Polluted Environment. Results Eng. 2025, 25, 104322. [Google Scholar] [CrossRef]
- Chen, Y.; Zhen, C.; Zeng, L.; Feng, H.; Wang, J.; Ai, Q.Y.H.; Ai, S.; Zhang, J.; Liang, Y.Y.; Xue, H.; et al. Association of Blood Cadmium and Physical Activity with Mortality: A Prospective Cohort Study. Ecotoxicol. Environ. Saf. 2025, 290, 117541. [Google Scholar] [CrossRef]
- Baldev; Kumar, G.; Sharma, V.; Nemiwal, M. Biomass-Derived Zirconium Composite: An Adsorbent for Preferential Removal of Heavy Metals and Contaminants in Wastewater. J. Water Process Eng. 2025, 69, 106778. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Removal of Cadmium (II) from Aqueous Solutions by Adsorption Using Meranti Wood. Wood Sci. Technol. 2012, 46, 221–241. [Google Scholar] [CrossRef]
- Jia, Z.; Liang, F.; Xu, X.; Zhou, H.; Zhang, Y.; Liang, P. One-Pot Amination and Carboxylation Functionalization of Lignin for Efficient Adsorption of Cr(VI) and Cd(II): Influence of Functional Groups on Adsorption Equilibrium and Mechanism. Colloids Surf. Physicochem. Eng. Asp. 2024, 703, 135278. [Google Scholar] [CrossRef]
- Saeed, A.; Akhter, M.W.; Iqbal, M. Removal and Recovery of Heavy Metals from Aqueous Solution Using Papaya Wood as a New Biosorbent. Sep. Purif. Technol. 2005, 45, 25–31. [Google Scholar] [CrossRef]
- He, L.-T.; Zhao, L.-D.; Sun, W.; Fang, J.; Liu, X.-W.; Qi, J.-J.; Qian, Y.; Li, H. 3d Wood Microfilter for Fast and Efficient Removal of Heavy Metal Ions from Water. Langmuir 2023, 39, 15319–15327. [Google Scholar] [CrossRef] [PubMed]
- El-Sorogy, A.S.; Al-kahtany, K.; Alharbi, T.; Alarifi, S.S. Distribution Patterns, Health Hazards, and Multivariate Assessment of Contamination Sources of as, Pb, Ni, Zn, and Fe in Agricultural Soils. J. King Saud Univ. Sci. 2024, 36, 103489. [Google Scholar] [CrossRef]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, S3, 0495. [Google Scholar] [CrossRef]
- Kovacova, Z.; Demcak, S.; Balintova, M.; Pla, C.; Zinicovscaia, I. Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water. Materials 2020, 13, 3575. [Google Scholar] [CrossRef]
- Scholz, P.; Vogel, C.; Schuck, G.; Simon, F.-G. Speciation of Copper and Zinc Compounds Relevant for the Hazard Property (Hp) 14 Classification of Municipal Solid Waste Incineration Bottom and Fly Ashes. Waste Manag. 2024, 189, 421–426. [Google Scholar] [CrossRef]
- Pereira, F.V.; Gurgel, L.V.A.; Gil, L.F. Removal of Zn2+ from Aqueous Single Metal Solutions and Electroplating Wastewater with Wood Sawdust and Sugarcane Bagasse Modified with Edta Dianhydride (Edtad). J. Hazard. Mater. 2010, 176, 856–863. [Google Scholar] [CrossRef]
- Syeda, H.I.; Muthukumaran, S.; Baskaran, K. Shape-Memory Cellulose Nanofiber/Polyglutamic Acid-Based Aerogels as Novel Adsorbents for the Removal of Heavy Metals from Aqueous Solutions. J. Water Process Eng. 2024, 58, 104780. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Braghiroli, F.L.; Neculita, C.M.; Koubaa, A. Advances in Modified Wood-Based Adsorbents for Contaminant Removal: Valorization Methods, Modification Mechanisms, and Environmental Applications. Curr. For. Rep. 2023, 9, 444–460. [Google Scholar] [CrossRef]
- Shao, T.; Yin, Q.; Bai, J.; Zhu, J.; Gan, M. Adsorption and Catalytic Reduction of Hexavalent Chromium Based on Nanomaterials: A Review on Metal, Metallic Oxide, Metallic Sulfide and Carbon-Based Catalyst. Environ. Res. 2025, 266, 120449. [Google Scholar] [CrossRef] [PubMed]
- den Braver-Sewradj, S.P.; van Benthem, J.; Staal, Y.C.M.; Ezendam, J.; Piersma, A.H.; Hessel, E.V.S. Occupational Exposure to Hexavalent Chromium. Part II. Hazard Assessment of Carcinogenic Effects. Regul. Toxicol. Pharmacol. 2021, 126, 105045. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Anand, U.; Thiruvenkataswamy, S.; Babu, H.W.S.; Narayanasamy, A.; Prajapati, V.K.; Tiwari, C.K.; Gopalakrishnan, A.V.; Bontempi, E.; Sonne, C.; et al. A Review of Chromium (Cr) Epigenetic Toxicity and Health Hazards. Sci. Total Environ. 2023, 882, 163483. [Google Scholar] [CrossRef]
- Luo, M.; Duan, W.; Li, S.W.; Yang, Y.; Yang, G.; Li, H.; Yu, X. Remediation of Hexavalent Chromium in Water and Soil by Pristine and Chemically Modified Pine Barks: Effects and Mechanisms. Environ. Technol. Innov. 2024, 36, 103876. [Google Scholar] [CrossRef]
- Xie, T.; Wang, Y.; Zhang, Q.; Shen, S.; Guo, W.; Chen, X.; Wang, Q.; Qu, L.; Li, C. Wood Aerogels Decorated Amino-Functionalized Mil-101(Cr) as Efficient Filter for Multistage Purification of Wastewater. Chemosphere 2024, 350, 141052. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, D.; Tang, Y.; Guo, Y.; Li, P.; Qv, W.; Deng, F.; Lu, F. Adsorption of Hexavalent Chromium from an Aqueous Phase by Hydroxypropyl Methylcellulose Modified with Diethylenetriamine. J. Chem. Eng. Data 2019, 64, 98–106. [Google Scholar] [CrossRef]
- Yang, R.; Aubrecht, K.B.; Ma, H.; Wang, R.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-Modified Cellulose Nanofibrous Composite Membranes for Chromium (VI) and Lead (II) Adsorption. Polymer 2014, 55, 1167–1176. [Google Scholar] [CrossRef]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.-J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent Chromium Removal by Various Adsorbents: Powdered Activated Carbon, Chitosan, and Single/Multi-Walled Carbon Nanotubes. Sep. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-Based Electrospun Nanofiber Membrane with Core-Sheath Structure and Robust Photocatalytic Activity for Simultaneous and Efficient Oil Emulsions Separation, Dye Degradation and Cr(VI) Reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, D.G.; Møller, L.B.; Aaseth, J. Chapter 35—Copper. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, MA, USA, 2015; pp. 765–786. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The Potential Exposure and Hazards of Copper Nanoparticles: A Review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Ugwu, C.E.; Igbokwe, A.M.; Suru, S.M.; Dike, C.C.; Mbachu, A.N.; Maduka, H.C.C. Evaluating the Human Health Risks of Heavy Metal Contamination in Copper and Steel Factory Effluents in Nnewi, Anambra State, Nigeria. Toxicol. Rep. 2024, 12, 614–621. [Google Scholar] [CrossRef]
- Nagarajan, D.; Venkatanarasimhan, S. Kinetics and Mechanism of Efficient Removal of Cu(II) Ions from Aqueous Solutions Using Ethylenediamine Functionalized Cellulose Sponge. Int. J. Biol. Macromol. 2020, 148, 988–998. [Google Scholar] [CrossRef]
- Chu, Z.; Zheng, P.; Yang, Y.; Wang, C.; Yang, Z. Compressible Nanowood/Polymer Composite Adsorbents for Wastewater Purification Applications. Compos. Sci. Technol. 2020, 198, 108320. [Google Scholar] [CrossRef]
- Rahman, M.L.; Shamrih, S.A.; Azlyzan, N.A.; Sarjadi, M.S.; Arsad, S.E.; Sarkar, S.M.; Kumar, S. Removal of Heavy Metal Ions from Wastewater Using Modified Cornstalk Cellulose-Derived Poly(Amidoxime) Ligand. Carbohydr. Polym. Technol. Appl. 2025, 9, 100633. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Q.; Han, Y.; Hu, S.; Xu, S. Efficient Adsorption of Cu2+ Using Znco Bimetallic Organic Frameworks Loaded Cellulose-Based Modified Aerogel: Adsorption Behavior and Mechanism. Environ. Res. 2025, 269, 120877. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, Y.; Yang, L.; Yang, F.; Wang, Y.; Cheng, T. A Wood Sponge Sensor for Heavy Metal Ion Detection and Adsorption. Wood Sci. Technol. 2022, 56, 1175–1190. [Google Scholar] [CrossRef]
- Mautner, A.; Kwaw, Y.; Weiland, K.; Mvubu, M.; Botha, A.; John, M.J.; Mtibe, A.; Siqueira, G.; Bismarck, A. Natural Fibre-Nanocellulose Composite Filters for the Removal of Heavy Metal Ions from Water. Ind. Crops Prod. 2019, 133, 325–332. [Google Scholar] [CrossRef]
| Material | Lead Adsorption (mg/g) | Cadmium Adsorption (mg/g) | Zinc Adsorption (mg/g) | Chromium Adsorption (mg/g) | Copper Adsorption (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Highly nanostructured and carboxylated wood aerogel-based adsorption film | 268.00 | 255.00 | 248.00 | [46] | ||
| activated Dibetou sawdust | 61.73 | [47] | ||||
| amino-decorated cellulose | 53.86 | [48] | ||||
| Sulfhydryl—Functionalized Wood Membrane Stacks | 384.10 | 593.90 | 169.50 | [49] | ||
| Meranti wood | 175.43 | [56] | ||||
| One-pot amination and carboxylation functionalization of lignin | 103.10 | 1298.60 | [58] | |||
| Cellulose grafted with carboxyl and thiol groups | 173.63 | 153.30 | [60] | |||
| wood sawdust, Manilkara sp. | 80.00 | [63] | ||||
| Cellulose nanofiber/polyglutamic acid-based aerogels | 101.81 | 59.26 | 100.59 | [64] | ||
| wood aerogel | 6.46 | [71] | ||||
| Amine modified cellulose sponge | 596.96 | [79] | ||||
| nanowood with polyethyleneimine | 93.06 | [80] | ||||
| cellulose nanofibril composite | 274.73 | [81] | ||||
| wood sponge | 60.00 | [82] | ||||
| poly(amidoxime) ligand | 220.00 | 310.00 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Du, Y.; Du, X.; Zhang, Y.; Deng, L.; Li, C.; Guo, J. Functionalization of Wood for the Removal of Heavy Metal Ions from Waster Water: A Review. Forests 2025, 16, 1684. https://doi.org/10.3390/f16111684
Liu Y, Zhang X, Du Y, Du X, Zhang Y, Deng L, Li C, Guo J. Functionalization of Wood for the Removal of Heavy Metal Ions from Waster Water: A Review. Forests. 2025; 16(11):1684. https://doi.org/10.3390/f16111684
Chicago/Turabian StyleLiu, Yang, Xiaolin Zhang, Yanzhuo Du, Xuebin Du, Yi Zhang, Layun Deng, Cheng Li, and Jianhui Guo. 2025. "Functionalization of Wood for the Removal of Heavy Metal Ions from Waster Water: A Review" Forests 16, no. 11: 1684. https://doi.org/10.3390/f16111684
APA StyleLiu, Y., Zhang, X., Du, Y., Du, X., Zhang, Y., Deng, L., Li, C., & Guo, J. (2025). Functionalization of Wood for the Removal of Heavy Metal Ions from Waster Water: A Review. Forests, 16(11), 1684. https://doi.org/10.3390/f16111684








