Stem Photosynthesis in ‘Hybrid Poplar 275’ Remains Stable Following Defoliation Induced by Severe Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Water Potential
2.3. Gas Exchange
2.4. Fluorescence Measurement
2.5. Photosynthetic Pigments Content
2.6. Xylem Sap Composition
2.7. Stable Isotope Composition
2.8. Statistics
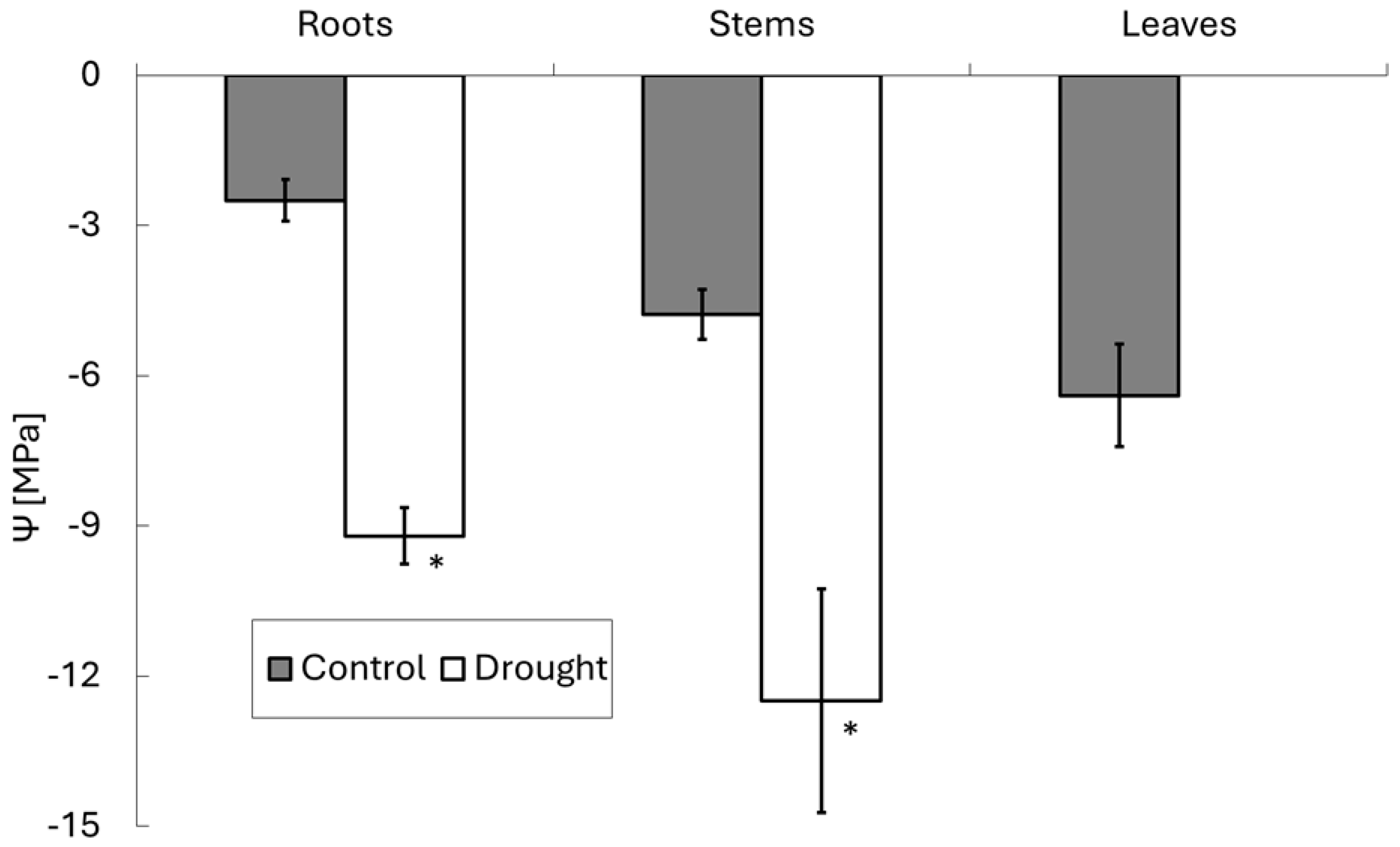
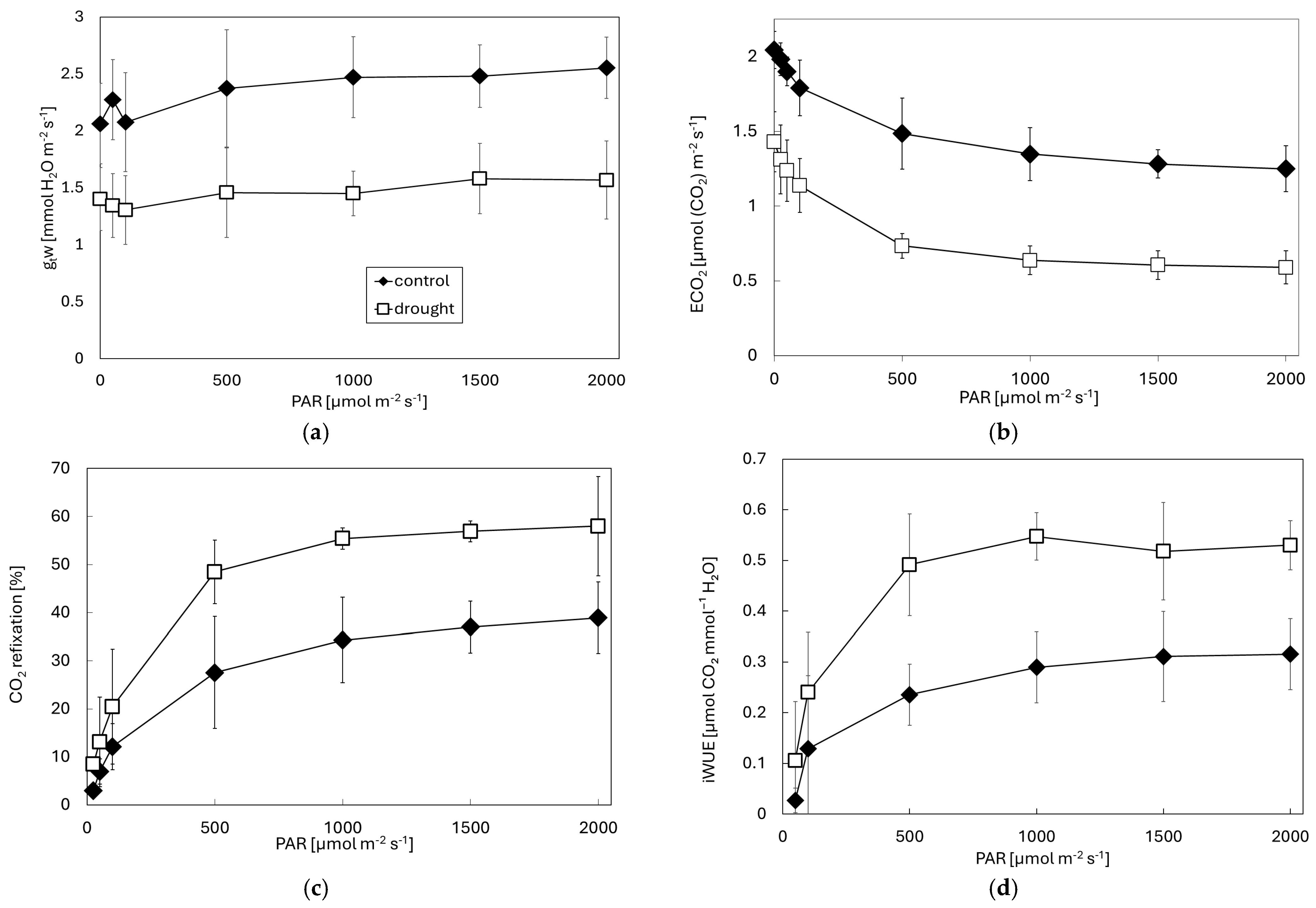
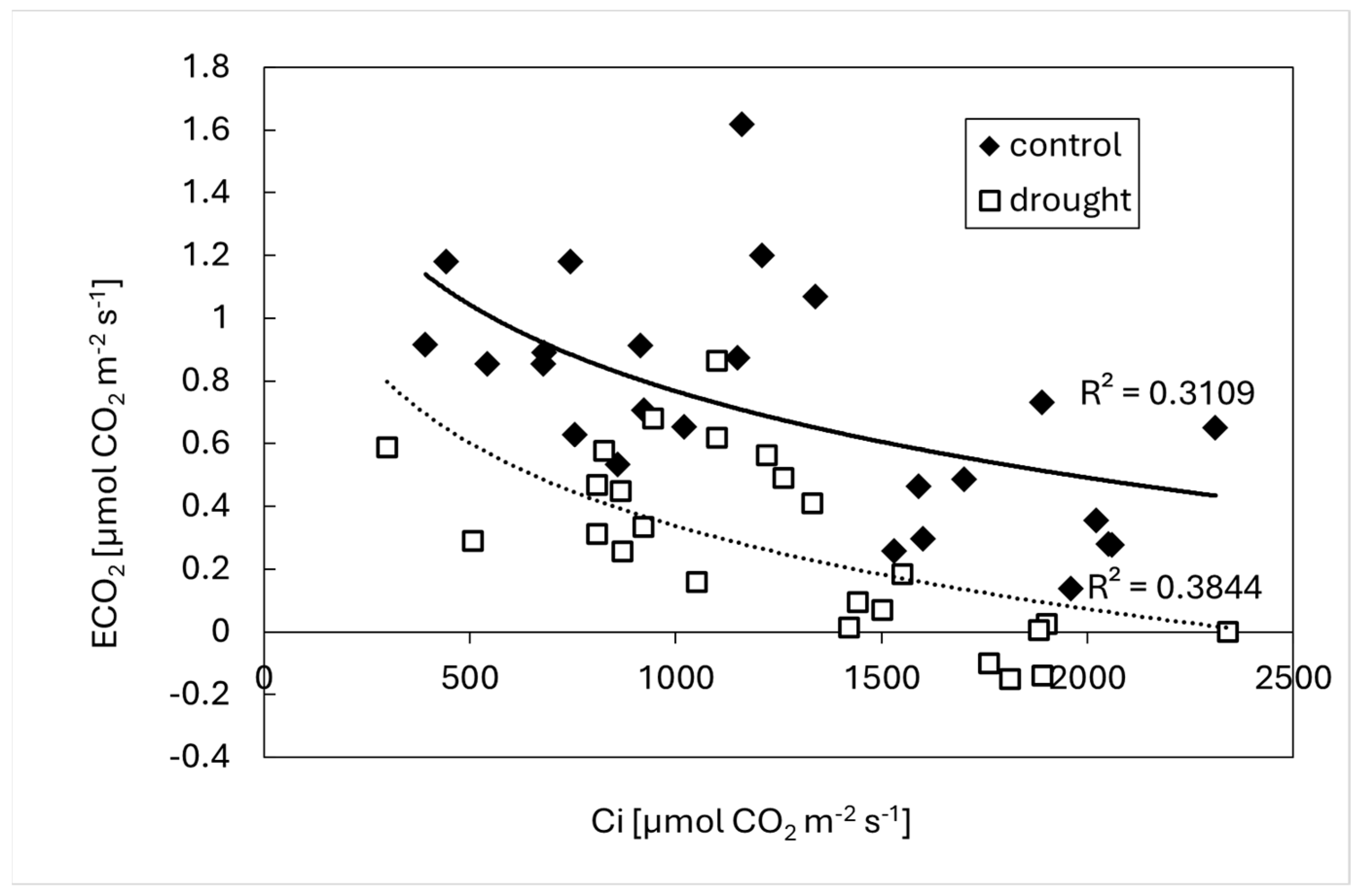
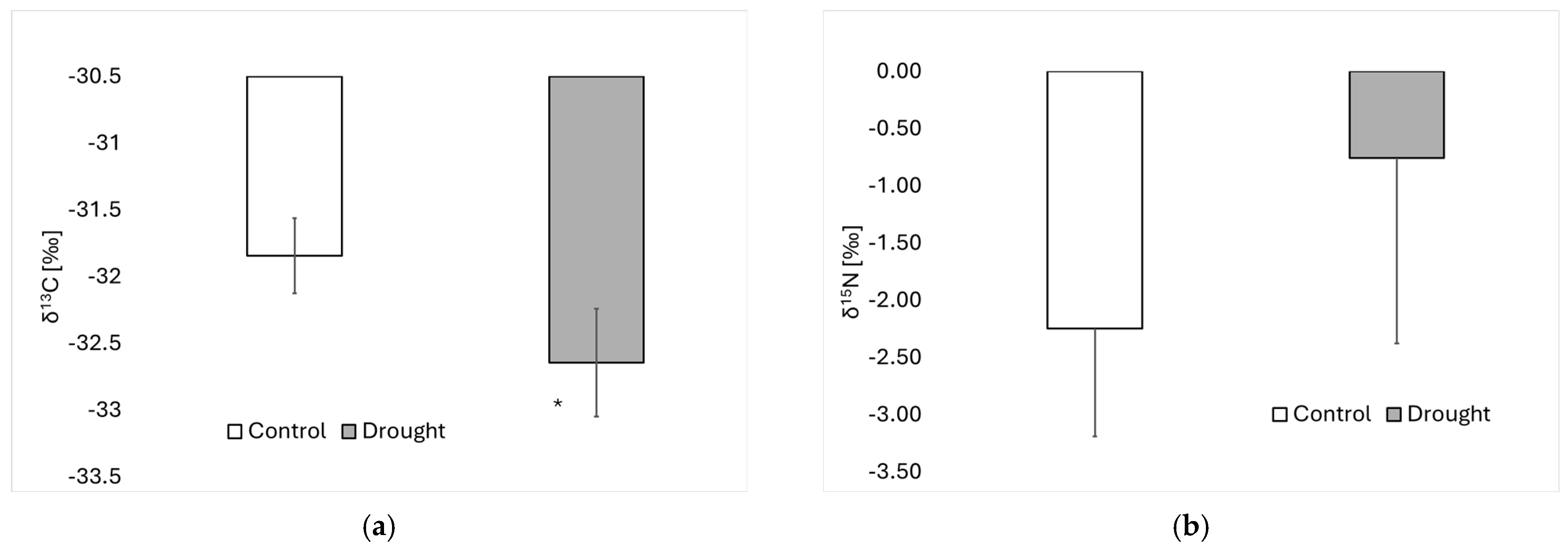
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| δ13C | carbon isotope composition |
| Chl a | Chlorophyll a |
| Chl b | chlorophyll b |
| Ci | corticular CO2 concentration |
| ECO2 | efflux of CO2 |
| ΦPSII | effective quantum yield of PSII photochemistry |
| iWUE | intrinsic water use efficiency |
| PEPC | phosphoenolpyruvate carboxylase |
| qP | photochemical quenching coefficient |
| PSII | photosystem II |
| Fv/Fm | maximum quantum efficiency of photosystem II |
| PAR | photosynthetically active radiation |
| δ15N | nitrogen isotope composition |
| NPQ | non-photochemical quenching |
| SNP | stem net photosynthesis |
| SRP | stem recycling photosynthesis |
| Ψ | water potential |
| gtw | water vapor conductance |
References
- Ávila-Lovera, E.; Tezara, W. Water-use efficiency is higher in green stems than in leaves of a tropical tree species. Trees 2018, 32, 1547–1558. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, E.T.; Thomas, B.R.; Campbell, M.M. Drought induces alterations in the stomatal development program in Populus. J. Exp. Bot. 2012, 63, 4959–4971. [Google Scholar] [CrossRef]
- Bloemen, J.; Vergeynst, L.L.; Overlaet-Michiels, L.; Steppe, K. How important is woody tissue photosynthesis in poplar during drought stress? Trees 2016, 30, 63–72. [Google Scholar] [CrossRef]
- De Roo, L.; Bloemen, J.; Dupon, Y.; Salomón, R.L.; Steppe, K. Axial Diffusion of Respired CO2 Confounds Stem Respiration Estimates during the Dormant Season. Ann. For. Sci. 2019, 76, 52. [Google Scholar] [CrossRef]
- García Morote, F.A.; Andrés Abellán, M.; Rubio, E.; Pérez Anta, I.; García Saucedo, F.; López Serrano, F.R. Stem CO2 efflux as an indicator of forests’ productivity in relict juniper woodlands (Juniperus thurifera L.) of southern Spain. Forests 2021, 12, 1340. [Google Scholar] [CrossRef]
- Rentzou, A.; Psaras, G.K. Green plastids, maximal PSII photochemical efficiency and starch content of inner stem tissues of three Mediterranean woody species during the year. Flora 2008, 203, 350–357. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H. More than just CO2-recycling: Corticular photosynthesis as a mechanism to reduce the risk of an energy crisis induced by low oxygen. New Phytol. 2018, 219, 551–564. [Google Scholar] [CrossRef]
- Bužková, R.; Acosta, M.; Dařenová, E.; Pokorný, R.; Pavelka, M. Environmental factors influencing the relationship between stem CO2 efflux and sap flow. Trees 2015, 29, 333–343. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H. The optical, absorptive and chlorophyll fluorescence properties of young stems of five woody species. Environ. Exp. Bot. 2016, 121, 83–93. [Google Scholar] [CrossRef]
- Pilarski, J.; Tokarz, K. Chlorophyll distribution in the stems and trunk of beech trees. Acta Physiol. Plant. 2006, 28, 233–236. [Google Scholar] [CrossRef]
- Pilarski, J.; Tokarz, K.; Kocurek, M. Optical Properties of the Cork of Stems and Trunks of Beech (Fagus sylvatica L.). Pol. J. Environ. Stud. 2008, 17, 773–779. Available online: https://www.pjoes.com/Optical-Properties-of-the-Cork-of-Stems-r-nand-Trunks-of-Beech-Fagus-Sylvatica-L%2C88167%2C0%2C2.html (accessed on 25 September 2025).
- Kocurek, M.; Kornaś, A.; Wierzchnicki, R.; Miszalski, Z.; Lüttge, U. Importance of Stem Photosynthesis in Plant Carbon Allocation of Clusia minor. Trees 2020, 34, 1009–1020. [Google Scholar] [CrossRef]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, Fate and Significance of CO2 in Tree Stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kocurek, M.; Kornas, A.; Pilarski, J.; Tokarz, K.; Lüttge, U.; Miszalski, Z. Photosynthetic Activity of Stems in Two Clusia Species. Trees 2015, 29, 1029–1040. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H.; Loreto, F.; Centritto, M.; Pietrini, F.; Alessio, G. Stem CO2 Release under Illumination: Corticular Photosynthesis, Photorespiration or Inhibition of Mitochondrial Respiration? Plant Cell Environ. 2006, 29, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Egerton, J.J.G.; Lovelock, C.E.; Ball, M.C. Light-dependent maintenance of hydraulic function in mangrove branches: Do xylary chloroplasts play a role in embolism repair? New Phytol. 2012, 195, 40–46. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant–Environment Interaction Rather than a Simple Hydraulic Trait. Trends Plant Sci. 2017, 23, 112–120. [Google Scholar] [CrossRef]
- Wittmann, C.; Pfanz, H. Antitranspirant Functions of Stem Periderms and Their Influence on Corticular Photosynthesis under Drought Stress. Trees 2008, 22, 187–196. [Google Scholar] [CrossRef]
- Ávila, E.; Herrera, A.; Tezara, W. Contribution of Stem CO2 Fixation to Whole-Plant Carbon Balance in Nonsucculent Species. Photosynthetica 2014, 52, 3–15. [Google Scholar] [CrossRef]
- Ávila-Lovera, E.; Haro, R.; Choudhary, M.; Acosta-Rangel, A.; Pratt, R.B.; Santiago, L.S. The Benefits of Woody Plant Stem Photosynthesis Extend to Hydraulic Function and Drought Survival in Parkinsonia florida. Tree Physiol. 2024, 44, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Salomón, R.L.; Helm, J.; Gessler, A.; Grams, T.E.E.; Hilman, B.; Muhr, J.; Steppe, K.; Wittmann, C.; Hartmann, H. The Quandary of Sources and Sinks of CO2 Efflux in Tree Stems—New Insights and Future Directions. Tree Physiol. 2024, 44, tapd157. [Google Scholar] [CrossRef] [PubMed]
- Cerasoli, S.; McGuire, M.A.; Faria, J.; Mourato, M.; Schmidt, M.; Pereira, J.S.; Chaves, M.M.; Teskey, R.O. CO2 Efflux, CO2 Concentration and Photosynthetic Refixation in Stems of Eucalyptus globulus (Labill.). J. Exp. Bot. 2009, 60, 99–105. [Google Scholar] [CrossRef]
- Dukat, P.; Hölttä, T.; Oren, R.; Salmon, Y.; Urbaniak, M.; Vesala, T.; Aalto, J.; Lintunen, A. Partitioning Seasonal Stem Carbon Dioxide Efflux into Stem Respiration, Bark Photosynthesis, and Transport-Related Flux in Scots Pine. J. Exp. Bot. 2024, 75, 4944–4959. [Google Scholar] [CrossRef]
- De Roo, L.; Salomón, R.L.; Steppe, K. Woody Tissue Photosynthesis Reduces Stem CO2 Efflux by Half and Remains Unaffected by Drought Stress in Young Populus tremula Trees. Plant Cell Environ. 2020, 43, 981–991. [Google Scholar] [CrossRef]
- De Roo, L.; Salomón, R.L.; Oleksyn, J.; Steppe, K. Woody Tissue Photosynthesis Delays Drought Stress in Populus tremula Trees and Maintains Starch Reserves in Branch Xylem Tissues. New Phytol. 2020, 228, 70–81. [Google Scholar] [CrossRef]
- Lauriks, F.; Salomón, R.L.; De Roo, L.; Sobrino-Plata, J.; Rodríguez-García, A.; Steppe, K. Limited Mitigating Effects of Elevated CO2 in Young Aspen Trees to Face Drought Stress. Environ. Exp. Bot. 2022, 201, 104942. [Google Scholar] [CrossRef]
- Trifilò, P.; Natale, S.; Gargiulo, S.; Abate, E.; Casolo, V.; Nardini, A. Stem Photosynthesis Affects Hydraulic Resilience in the Deciduous Populus alba but Not in the Evergreen Laurus nobilis. Water 2021, 13, 2911. [Google Scholar] [CrossRef]
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Gennaro, M.; Nervo, G.; Secchi, F.; Carra, A. Responses to Drought Stress in Poplar: What Do We Know and What Can We Learn? Life 2023, 13, 533. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Marshall, J.D. Photosynthetic Refixation in Branches of Western White Pine. Funct. Ecol. 2001, 15, 300–306. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V. Regulation of Photosystem II. Photosynth. Res. 1992, 34, 375–385. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Geiser, L.; Varesio, E.; Veuthey, J.-L. Simultaneous Analysis of Metabisulfite and Sulfate by CE with Indirect UV Detection: Application to and Validation for a Pharmaceutical Formulation. J. Pharm. Biomed. Anal. 2003, 31, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Bossard, C.C.; Rejmánek, M. Why Have Green Stems? Funct. Ecol. 1992, 6, 197–205. [Google Scholar] [CrossRef]
- Hukin, D.; Cochard, H.; Dreyer, E.; Le Thiec, D.; Bogeat-Triboulot, M.B. Cavitation Vulnerability in Roots and Shoots: Does Populus euphratica Oliv., a Poplar from Arid Areas of Central Asia, Differ from Other Poplar Species? J. Exp. Bot. 2005, 56, 2003–2010. [Google Scholar] [CrossRef]
- Eyles, A.; Pinkard, E.A.; O’Grady, A.P.; Worledge, D.; Warren, C.R. Role of Corticular Photosynthesis Following Defoliation in Eucalyptus globulus. Plant Cell Environ. 2009, 32, 1004–1014. [Google Scholar] [CrossRef]
- Trifilò, P.; Kiorapostolou, N.; Petruzzellis, F.; Vitti, S.; Petit, G.; Lo Gullo, M.A.; Nardini, A.; Casolo, V. Hydraulic Recovery from Xylem Embolism in Excised Branches of Twelve Woody Species: Relationships with Parenchyma Cells and Non-Structural Carbohydrates. Plant Physiol. Biochem. 2019, 139, 513–520. [Google Scholar] [CrossRef]
- Natale, S.; Tomasella, M.; Gargiulo, S.; Petruzzellis, F.; Tromba, G.; Boccato, E.; Casolo, V.; Nardini, A. Stem Photosynthesis Contributes to Non-Structural Carbohydrate Pool and Modulates Xylem Vulnerability to Embolism in Fraxinus ornus L. Environ. Exp. Bot. 2023, 210, 105315. [Google Scholar] [CrossRef]
- Raveh, E.; Levy, Y. Analysis of xylem water as an indicator of current chloride uptake status in citrus trees. Sci. Hortic. 2005, 103, 265–276. [Google Scholar] [CrossRef]
- Trifilò, P.; Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Salleo, S. Ion-mediated compensation for drought-induced loss of xylem hydraulic conductivity in field-growing plants of Laurus nobilis. Funct. Plant Biol. 2011, 38, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Beikircher, B.; Mayr, S. Winter Peridermal Conductance of Apple Trees: Lammas Shoots and Spring Shoots Compared. Trees 2013, 27, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Salomón, R.L.; De Schepper, V.; Valbuena-Carabaña, M.; Gil, L.; Steppe, K. Daytime Depression in Temperature-Normalised Stem CO2 Efflux in Young Poplar Trees Is Dominated by Low Turgor Pressure Rather than by Internal Transport of Respired CO2. New Phytol. 2018, 217, 586–598. [Google Scholar] [CrossRef]
- Atkin, O.K.; Macherel, D. The Crucial Role of Plant Mitochondria in Orchestrating Drought Tolerance. Ann. Bot. 2009, 103, 581–597. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.L.; Oliveira, A.A.R.; Oliveira, R.S.; Bittencourt, P.L.; Costa, P.B.; Giles, A.L.; Sosa, A.I.; Coughlin, I.; Godlee, J.L.; et al. Drought Stress and Tree Size Determine Stem CO2 Efflux in a Tropical Forest. New Phytol. 2018, 218, 1393–1405. [Google Scholar] [CrossRef]
- Dannoura, M.; Kominami, Y.; Tamai, K.; Jomura, M.; Miyama, T.; Goto, Y.; Kanazawa, Y. Development of an Automatic Chamber System for Long-Term Measurements of CO2 Flux from Roots. Tellus B Chem. Phys. Meteorol. 2006, 58, 502–512. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragão, L.E.O.C.; Lobo-do-Vale, R.; Galbraith, D.; Fisher, R.A.; Chaves, M.M.; Maroco, J.P.; da Costa, A.C.L.; de Almeida, S.; et al. Shifts in Plant Respiration and Carbon Use Efficiency at a Large-Scale Drought Experiment in the Eastern Amazon. New Phytol. 2010, 187, 608–621. [Google Scholar] [CrossRef]
- Steppe, K.; Saveyn, A.; McGuire, M.A.; Lemeur, R.; Teskey, R.O. Resistance to Radial CO2 Diffusion Contributes to Between-Tree Variation in CO2 Efflux of Populus deltoides Stems. Funct. Plant Biol. 2007, 34, 785–792. [Google Scholar] [CrossRef]
- Salomón, R.L.; Valbuena-Carabaña, M.; Gil, L.; McGuire, M.A.; Teskey, R.O.; Aubrey, D.P.; González-Doncel, I.; Rodríguez-Calcerrada, J. Temporal and Spatial Patterns of Internal and External Stem CO2 Fluxes in a Sub-Mediterranean Oak. Tree Physiol. 2016, 36, 1409–1421. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Quick, W.P. Characteristics of C4 Photosynthesis in Stems and Petioles of C3 Flowering Plants. Nature 2002, 415, 451–454. [Google Scholar] [CrossRef]
- Helm, J.; Salomón, R.L.; Hilman, B.; Muhr, J.; Knohl, A.; Steppe, K.; Gibon, Y.; Cassan, C.; Hartmann, H. Differences between Tree Stem CO2 Efflux and O2 Influx Rates Cannot Be Explained by Internal CO2 Transport or Storage in Large Beech Trees. Plant Cell Environ. 2023, 46, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel Growth Dynamics in Tree Stems: Linking Anatomy and Ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gu, J.; Yu, Y.; Ju, G.; Sun, Z. Stem Photosynthesis of Twig and Its Contribution to New Organ Development in Cutting Seedlings of Salix matsudana Koidz. Forests 2018, 9, 207. [Google Scholar] [CrossRef]
- Natale, S.; Peralta Ogorek, L.L.; Caracciolo, L.; Morosinotto, T.; van Amerongen, H.; Casolo, V.; Pedersen, O.; Nardini, A. Net O2 Exchange Rates under Dark and Light Conditions across Different Stem Compartments. New Phytol. 2024, 243, 72–81. [Google Scholar] [CrossRef]
- Rustioni, L.; Bianchi, D. Drought Increases Chlorophyll Content in Stems of Vitis Interspecific Hybrids. Theor. Exp. Plant Physiol. 2021, 33, 69–78. [Google Scholar] [CrossRef]
- Valverdi, N.A.; Guzmán-Delgado, P.; Goldsmith, G.R.; Ávila-Lovera, E. Does Green Stem Photosynthesis Affect Plant Drought Tolerance and Recovery in Avocado? AoB Plants 2025, 17, plaf044. [Google Scholar] [CrossRef]
- Kharouk, V.I.; Middleton, E.M.; Spencer, S.L.; Rock, B.N. Aspen Bark Photosynthesis and Its Significance to Remote Sensing and Carbon Budget Estimates in the Boreal Ecosystem. Water Air Soil Pollut. 1995, 82, 483–497. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Hutley, L.B. Stable Isotopes Reveal the Contribution of Corticular Photosynthesis to Growth in Branches of Eucalyptus miniata. Plant Physiol. 2011, 155, 515–523. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Tcherkez, G.; Keitel, C.; Cornwell, W.K.; Santiago, L.S.; Knohl, A.; Barbour, M.M.; Williams, D.G.; Reich, P.B.; Ellsworth, D.S. Why Are Non-Photosynthetic Tissues Generally 13C Enriched Compared with Leaves in C3 Plants? Review and Synthesis of Current Hypotheses. Funct. Plant Biol. 2009, 36, 199–213. [Google Scholar] [CrossRef]
- Saveyn, A.; Steppe, K.; Ubierna, N.; Dawson, T.E. Woody Tissue Photosynthesis and Its Contribution to Trunk Growth and Bud Development in Young Plants. Plant Cell Environ. 2010, 33, 1949–1958. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Marshall, J.D.; Comstock, J.P.; Balster, N.J. Carbon Isotope Discrimination in Photosynthetic Bark. Oecologia 2001, 128, 24–35. [Google Scholar] [CrossRef]
- Ghashghaie, J.; Badeck, F.W. Opposite Carbon Isotope Discrimination during Dark Respiration in Leaves versus Roots—A Review. New Phytol. 2014, 201, 751–769. [Google Scholar] [CrossRef]

| Treatment | ||
|---|---|---|
| Control | Drought | |
| Parameter | ||
| Chl a [mg m−2] | 128.50 ± 28.94 | 118.49 ± 17.43 |
| Chl b [mg m−2] | 63.60 ± 17.81 | 63.07 ± 13.98 |
| Chl a/b | 2.10 ± 0.53 | 1.93 ± 0.37 |
| Chl a + b [mg m−2] | 192.10 ± 43.31 | 181.55 ± 26.58 |
| Carotenoids [mg m−2] | 31.41 ± 20.14 | 30.20 ± 16.63 |
| Cl− [mg ml−1] | 0.287 ± 0.328 | 0.123 ± 0.073 |
| Malate [mg ml−1] | 0.370 ± 0.167 | 0.513 ± 0.400 |
| Citrate [mg ml−1] | 0.017 ± 0.063 | 0.003 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocurek, M.; Gieniec, M.; Waligórski, P.; Miszalski, Z. Stem Photosynthesis in ‘Hybrid Poplar 275’ Remains Stable Following Defoliation Induced by Severe Drought. Forests 2025, 16, 1682. https://doi.org/10.3390/f16111682
Kocurek M, Gieniec M, Waligórski P, Miszalski Z. Stem Photosynthesis in ‘Hybrid Poplar 275’ Remains Stable Following Defoliation Induced by Severe Drought. Forests. 2025; 16(11):1682. https://doi.org/10.3390/f16111682
Chicago/Turabian StyleKocurek, Maciej, Miron Gieniec, Piotr Waligórski, and Zbigniew Miszalski. 2025. "Stem Photosynthesis in ‘Hybrid Poplar 275’ Remains Stable Following Defoliation Induced by Severe Drought" Forests 16, no. 11: 1682. https://doi.org/10.3390/f16111682
APA StyleKocurek, M., Gieniec, M., Waligórski, P., & Miszalski, Z. (2025). Stem Photosynthesis in ‘Hybrid Poplar 275’ Remains Stable Following Defoliation Induced by Severe Drought. Forests, 16(11), 1682. https://doi.org/10.3390/f16111682









