High-Temperature Induction of 2n Female Gametes to Produce Triploid Birches: Timing, Parameters, and Growth Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Growth Conditions
2.2. Experimental Methods
2.2.1. Pistil Stigma Receptivity
2.2.2. Control Pollination and Morphological Observation of Ovary Development
2.2.3. Anatomical Observation
2.2.4. High-Temperature Treatment
2.2.5. Determination of Seed Germination Rate
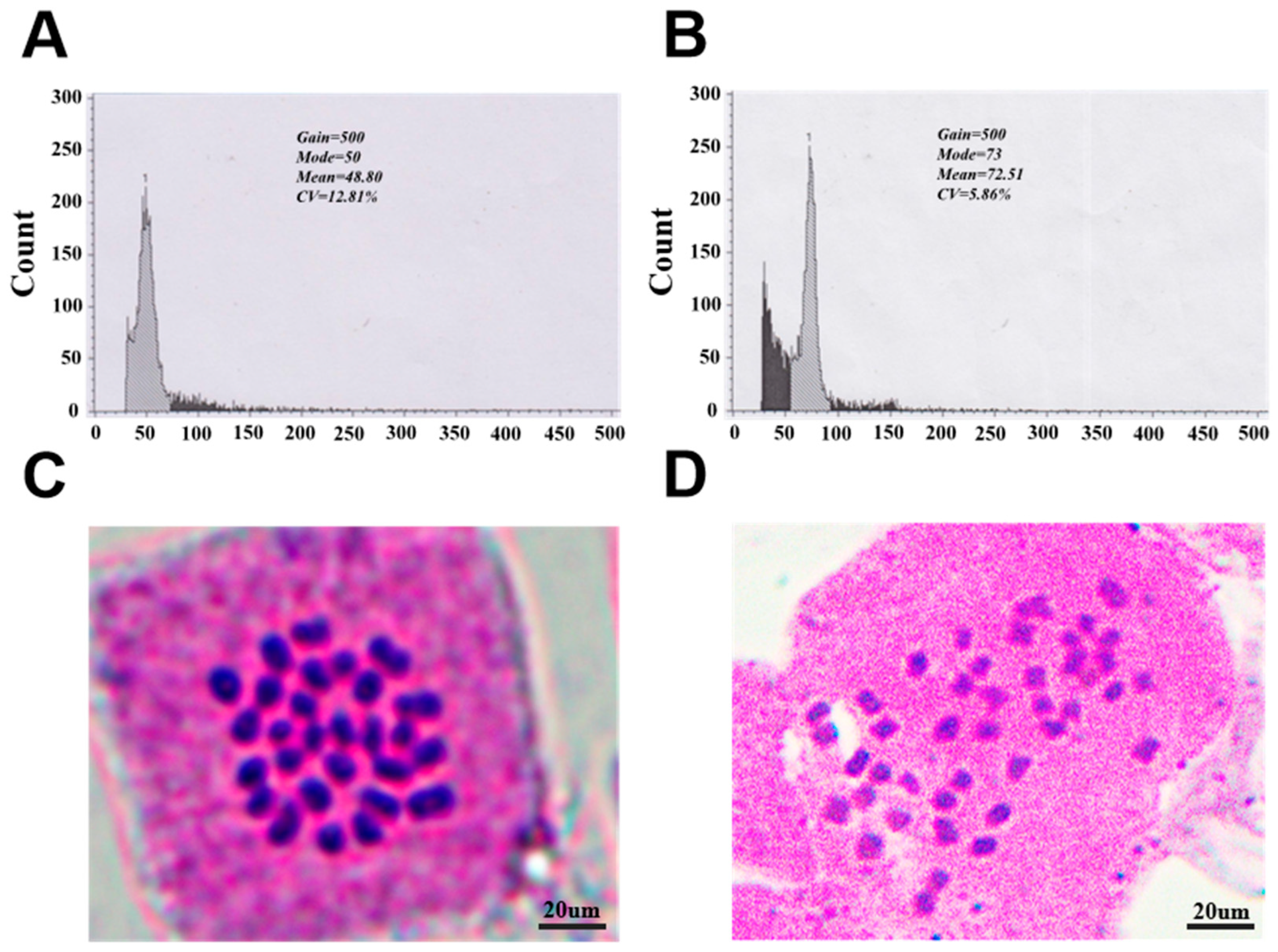
2.2.6. Determination of Ploidy Level
2.2.7. Growth Measurement of Triploid Birch
2.2.8. Calculation of Accumulated Growing Degree Hours and Model Construction
2.3. Statistical Analysis
3. Results
3.1. Dynamic Stigma Receptivity and Optimal Pollination Window
3.2. The Relationship Between the External Morphology of the Ovary, Megaspore and Embryo Sac Development
3.3. Inducing Female Megaspore Chromosome Doubling Through High-Temperature Treatment
3.4. Comparison of Growth Characteristics Between Triploid and Diploid Seedlings
4. Discussion
4.1. Optimal Pollination Window for Female Flower Stigmas
4.2. Effectiveness and Mechanism of the High-Temperature Treatment
4.3. Selection and Performance Differences in Hybrid Triploids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date | Days After Pollination (d) | Accumulated GDH (°C·h) | Observed Ratio | Predicted Ratio |
|---|---|---|---|---|
| 2023/4/21 | 3 | 301 | 0.546 | 0.417 |
| 2023/4/24 | 6 | 520 | 0.601 | 0.468 |
| 2023/4/27 | 9 | 901 | 0.619 | 0.569 |
| 2023/4/30 | 12 | 1202 | 0.758 | 0.658 |
| 2023/5/3 | 15 | 2044 | 0.827 | 0.947 |
| 2023/5/6 | 18 | 2660 | 0.915 | 1.181 |
| 2023/5/9 | 21 | 3231 | 1.202 | 1.395 |
| 2023/5/12 | 24 | 4043 | 1.764 | 1.672 |
| 2023/5/15 | 27 | 4807 | 2.110 | 1.880 |
| 2023/5/18 | 30 | 5529 | 2.125 | 2.026 |
| 2023/5/21 | 33 | 6307 | 2.321 | 2.135 |
| 2023/5/24 | 36 | 7488 | 2.158 | 2.232 |
| 2023/5/27 | 39 | 8515 | 2.088 | 2.275 |
| 2023/5/30 | 42 | 9439 | 2.250 | 2.296 |
| Days After Pollination | Germination Rate % (40 °C) | Germination Rate % (42 °C) | ||
|---|---|---|---|---|
| 1 h | 2 h | 1 h | 2 h | |
| CK | 66.00 ± 1.67 f | 66.00 ± 1.67 e | 66.00 ± 1.67 f | 66 ± 1.67 e |
| 3 | 64.00 ± 2.77 ef | — | — | — |
| 6 | — | — | — | — |
| 9 | 68.00 ± 2.69 fg | 46.30 ± 2.88 bcd | — | — |
| 12 | 77.20 ± 2.16 g | 48.50 ± 2.67 cd | 25.10 ± 2.25 a | 8.50 ± 1.18 a |
| 15 | 63.00 ± 2.5 ef | 35.30 ± 2.47 ab | 32.00 ± 2.40 ab | 13.10 ± 1.51 a |
| 18 | 59.20 ± 2.46 def | 45.80 ± 2.50 bcd | 47.80 ± 2.51 de | 34.80 ± 2.36 b |
| 21 | 55.20 ± 2.52 de | 45.20 ± 2.52 bcd | 36.50 ± 2.41 bcd | 27.80 ± 2.19 b |
| 24 | 36.70 ± 2.4 b | 32.90 ± 2.32 a | 56.30 ± 2.5 ef | 52.10 ± 2.52 d |
| 27 | 42.60 ± 2.52 bc | 25.70 ± 2.13 a | 44.70 ± 2.54 cde | 27.30 ± 2.19 b |
| 30 | 42.70 ± 2.47 bc | 51.30 ± 2.50 d | 50.00 ± 2.51 e | 58.70 ± 2.46 de |
| 33 | 49.20 ± 2.52 cd | 54.10 ± 2.51 d | 33.10 ± 2.32 abc | 37.60 ± 2.42 bc |
| 36 | 36.70 ± 2.39 b | 36.30 ± 2.39 abc | 50.30 ± 2.52 e | 50.00 ± 2.52 d |
| 39 | 23.50 ± 2.08 a | 26.50 ± 2.19 a | 31.50 ± 2.34 ab | 34.90 ± 2.43 b |
| 42 | 32.90 ± 2.33 ab | 30.40 ± 2.26 a | 49.80 ± 2.53 e | 46.90 ± 2.52 cd |

References
- Chen, S.; Wang, Y.; Yu, L.; Zheng, T.; Wang, S.; Yue, Z.; Jiang, J.; Kumari, S.; Zheng, C.; Tang, I.; et al. Genome Sequence and Evolution of Betula platyphylla. Hortic. Res. 2021, 8, 37. [Google Scholar] [CrossRef]
- Mu, H.-Z.; Liu, Z.-J.; Lin, L.; Li, H.-Y.; Jiang, J.; Liu, G.-F. Transcriptomic Analysis of Phenotypic Changes in Birch (Betula Platyphylla) Autotetraploids. Int. J. Mol. Sci. 2012, 13, 13012–13029. [Google Scholar] [CrossRef]
- Mu, H.; Jiang, J.; Li, H.; Liu, G. Seed Vigor, Photosynthesis and Early Growth of Saplings of Different Triploid Betula Families. Dendrobiology 2012, 68, 11–20. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Jiang, J.; Liu, G.; Zhao, X. Progeny Test of Tetraploid Betula Platyphylla and Preliminary Selection of Hybrid Parents. J. For. Res. 2016, 27, 665–674. [Google Scholar] [CrossRef]
- Lin, L.; Yao, Q.; Xu, H.; Mu, H.; Jiang, J. Characteristics of the Staminate Flower and Pollen from Autotetraploid Betula platyphylla. Dendrobiology 2013, 69, 3–11. [Google Scholar] [CrossRef]
- Guo, L.; Xu, W.; Zhang, Y.; Zhang, J.; Wei, Z. Inducing Triploids and Tetraploids with High Temperatures in Populus Sect. Tacamahaca. Plant Cell Rep. 2017, 36, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhu, Z. A study on the 2n pollen vitality and germinant characteristics of white poplars. Acta Bot. Yunnanica 1997, 19, 402–406. [Google Scholar]
- Kang, X.; Zhu, Z.; Lin, H. Radiosensitivity of different ploidy pollen in poplar and its application. Acta Genet. Sin. 2000, 27, 78–82. [Google Scholar]
- Kang, N.; Bai, F.; Zhang, P.; Kang, X. Inducing chromosome doubling of embryo sac in Populus tomentosa with high temperature exposure for hybrid triploids. J. Beijing For. Univ. 2015, 37, 79–86. [Google Scholar] [CrossRef]
- Tian, M.; Li, Y.; Zhang, P.; Wang, J.; Hao, J. Pollen Chromosome Doubling Induced by High Temperature Exposure to Produce Hybrid Triploids in Populus canescens. Sci. Silvae Sin. 2018, 54, 39–47. Available online: https://kns.cnki.net/kcms2/article/abstract?v=IPzr95zWmwROtlBV1PGn_MD4Cbj5ZSsv3bTypS5VWr95QfEWWbidzeTm5eC1JopGNPH5oaSi5cbezN1uHHNF78UvHwa5Ohh_aBztLMjfzx1GL7ILwOimq8DRQybcJPbleSCMBTMlDcNjTgxrtFAqSjBP_DV29QSQypLbeEhH-JU=&uniplatform=NZKPT&language=CHS (accessed on 15 August 2025).
- Pecrix, Y.; Rallo, G.; Folzer, H.; Cigna, M.; Gudin, S.; Le Bris, M. Polyploidization Mechanisms: Temperature Environment Can Induce Diploid Gamete Formation in Rosa sp. J. Exp. Bot. 2011, 62, 3587–3597. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Shang, F.; Kang, X. High Temperature-Induced Production of Unreduced Pollen and Its Cytological Effects in Populus. Sci. Rep. 2017, 7, 5281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, X.; Liu, L.; Jiang, P.; Fu, G. Stigma receptivity and the optimal pollination period of Populus spp. (Section Tacamahaca). J. Beijing For. Univ. 2009, 31, 30–35. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X. Stigma Receptivity and Its Detection Methods of White Poplars. Northwest Bot. J. 2007, 27, 864–870. Available online: https://kns.cnki.net/kcms2/article/abstract?v=IPzr95zWmwQLl7Nyh_YeN7HiSYNeiPJBTjfAfAUz16Mn9MD9GW2TKvo8s13XC-LLFrEyZy6XZBwuBNE3aHHoGCB-egcmlA5_yvakgKgbsC1aOhftNJjS2gYNM4ybWOQN3KmCjmPHsSXB58qWqFPrp02U99iBKHhfjdnDdRL1vnQ=&uniplatform=NZKPT&language=CHS (accessed on 15 August 2025).
- Kang, X. Trees Non-Isolated Branch and Bud Heating Treatment Device. Patent CN101057555A, 24 October 2007. [Google Scholar]
- Geng, X.; Ren, Y.; Han, Z.; Du, K.; Kang, X. Production of hybrid triploids via inducing chromosome doubling of megaspore with high temperature treatment in Leuce poplar. J. Beijing For. Univ. 2018, 40, 12–18. [Google Scholar] [CrossRef]
- Bedinger, P.A.; Broz, A.K.; Tovar-Mendez, A.; McClure, B. Pollen-Pistil Interactions and Their Role in Mate Selection. Plant Physiol. 2017, 173, 79–90. [Google Scholar] [CrossRef]
- Liu, Y.; Joly, V.; Dorion, S.; Rivoal, J.; Matton, D.P. The Plant Ovule Secretome: A Different View toward Pollen-Pistil Interactions. J. Proteome Res. 2015, 14, 4763–4775. [Google Scholar] [CrossRef]
- Maity, A.; Chakarbarty, S.K.; Pramanik, P.; Gupta, R.; Parmar, S.S.; Sharma, D.K. Response of Stigma Receptivity in CMS and Male Fertile Line of Indian Mustard (B. Juncea) under Variable Thermal Conditions. Int. J. Biometeorol. 2019, 63, 143–152. [Google Scholar] [CrossRef]
- Ramos Abril, L.N.; Pineda, L.M.; Wasek, I.; Wedzony, M.; Ceballos, H. Reproductive Biology in Cassava: Stigma Receptivity and Pollen Tube Growth. Commun. Integr. Biol. 2019, 12, 96–111. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Li, Z.; Bai, H. Study on Stigma Receptivity and Effects of Female Flowering Branches Quality on Flower Bud Development of Populus davidiana. For. Eng. 2021, 37, 11–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, X. Cytological Characteristics of Numerically Unreduced Pollen Production in Populus Tomentosa Carr. Euphytica 2010, 173, 151–159. [Google Scholar] [CrossRef]
- Zhang, P.; Kang, X. Occurrence and Cytological Mechanism of Numerically Unreduced Pollen in Diploid Populus euphratica. Silvae Genet. 2013, 62, 285–291. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, P.; Kang, X. Induction of 2n Female Gametes in Populus Adenopoda Maxim by High Temperature Exposure during Female Gametophyte Development. Breed. Sci. 2013, 63, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, X.Y.; Li, D.L. High Temperature-Induced Triploid Production during Embryo Sac Development in Populus. Silvae Genet. 2012, 61, 85–93. [Google Scholar] [CrossRef]
- Li, Y.; Tian, M.; Zhang, P. Embryo Sac Chromosome Doubling in Populus alba × P. Glandulosa Induced by High Temperature Exposure to Produce Triploids. Breed. Sci. 2017, 67, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, X.Y.; Li, D.L.; Chen, H.W.; Zhang, P.D. Induction of Diploid Eggs with Colchicine During Embryo Sac Development in Populus. Silvae Genet. 2010, 59, 40-U1. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X. Triploid induction in Populus alba × P. glandulosa by chromosome doubling of female gametes. J. Beijing For. Univ. 2007, 29, 22–25. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, P.; Yang, J.; Kang, X. Induction of Unreduced Megaspores in Eucommia Ulmoides by High Temperature Treatment during Megasporogenesis. Euphytica 2016, 212, 515–524. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Qiu, B.; Ma, Z.; Lu, T.; Kang, X.; Yang, J. Induction and Characterization of Tetraploid Through Zygotic Chromosome Doubling in Eucalyptus Urophylla. Front. Plant Sci. 2022, 13, 870698. [Google Scholar] [CrossRef]
- Liao, T.; Cheng, S.; Zhu, X.; Min, Y.; Kang, X. Effects of Triploid Status on Growth, Photosynthesis, and Leaf Area in Populus. Trees-Struct. Funct. 2016, 30, 1137–1147. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, P.; Gao, P.; Zhao, F. Discovery of a new way of poplar triploids induced with colchicine after pollination. J. Beijing For. Univ. 2004, 26, 1–4. [Google Scholar] [CrossRef]
- Zhang, Z.; Gou, X.; Xun, H.; Bian, Y.; Ma, X.; Li, J.; Li, N.; Gong, L.; Feldman, M.; Liu, B.; et al. Homoeologous Exchanges Occur through Intragenic Recombination Generating Novel Transcripts and Proteins in Wheat and Other Polyploids. Proc. Natl. Acad. Sci. USA 2020, 117, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Macaya-Sanz, D.; Suter, L.; Joseph, J.; Barbara, T.; Alba, N.; Gonzalez-Martinez, S.C.; Widmer, A.; Lexer, C. Genetic Analysis of Post-Mating Reproductive Barriers in Hybridizing European Populus Species. Heredity 2011, 107, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liao, T.; Du, K.; Wei, H.; Kang, X. Transcriptome Comparison of Different Ploidy Reveals the Mechanism of Photosynthetic Efficiency Superiority of Triploid Poplar. Genomics 2021, 113, 2211–2220. [Google Scholar] [CrossRef]
- Wang, L.; Tu, M.; Li, J.; Sun, S.; Song, H.; Xu, Z.; Chen, D.; Liang, G. Photosynthetic Efficiency and Glyco-Metabolism Changes in Artificial Triploid Loquats Contribute to Heterosis Manifestation. Int. J. Mol. Sci. 2022, 23, 11337. [Google Scholar] [CrossRef]

| Stigma Emerges from the Bracts (Days) | Stigma Morphological Characteristics | Angles Between the Bracts and the Female Flowers (°) | Benzidine-Hydrogen Peroxide Staining Reaction |
|---|---|---|---|
| 1 | No mucus is seen | <30 | - |
| 2~3 | No mucus is seen | 30 | - |
| 4 | Secretes a small amount of mucus | 30~45 | + |
| 5 | Secretion of mucus, stigma elongated | 45~60 | +++ |
| 6 | Secretes a large amount of mucus, the stigma is elongated | 60~90 | +++ |
| 7 | Secretion of mucus | 60~90 | ++ |
| 8 | The columella is wilted | 45~60 | - |
| Phase | Morphological Characteristics | Operational Recommendation |
|---|---|---|
| Initial Phase | Bract-inflorescence axis angle < 30°, no mucus on stigma surface | Pollination not feasible |
| Initial Receptive Phase | Bract-inflorescence axis angle ~30°–45°, stigma begins secreting a small amount of mucus | Pollination can be initiated |
| Optimal Receptive Phase | Bract-inflorescence axis angle > 45° (ideally 60°–90°), stigma secretes copious mucus and elongates significantly | Pollinate immediately |
| Late Receptive Phase | Bract-inflorescence axis angle decreases to 45°–60°, mucus secretion decreases, stigma begins to wilt | Complete pollination as soon as possible |
| Terminal Phase | Bract-inflorescence axis angle < 45°, stigma withered | Cease pollination |
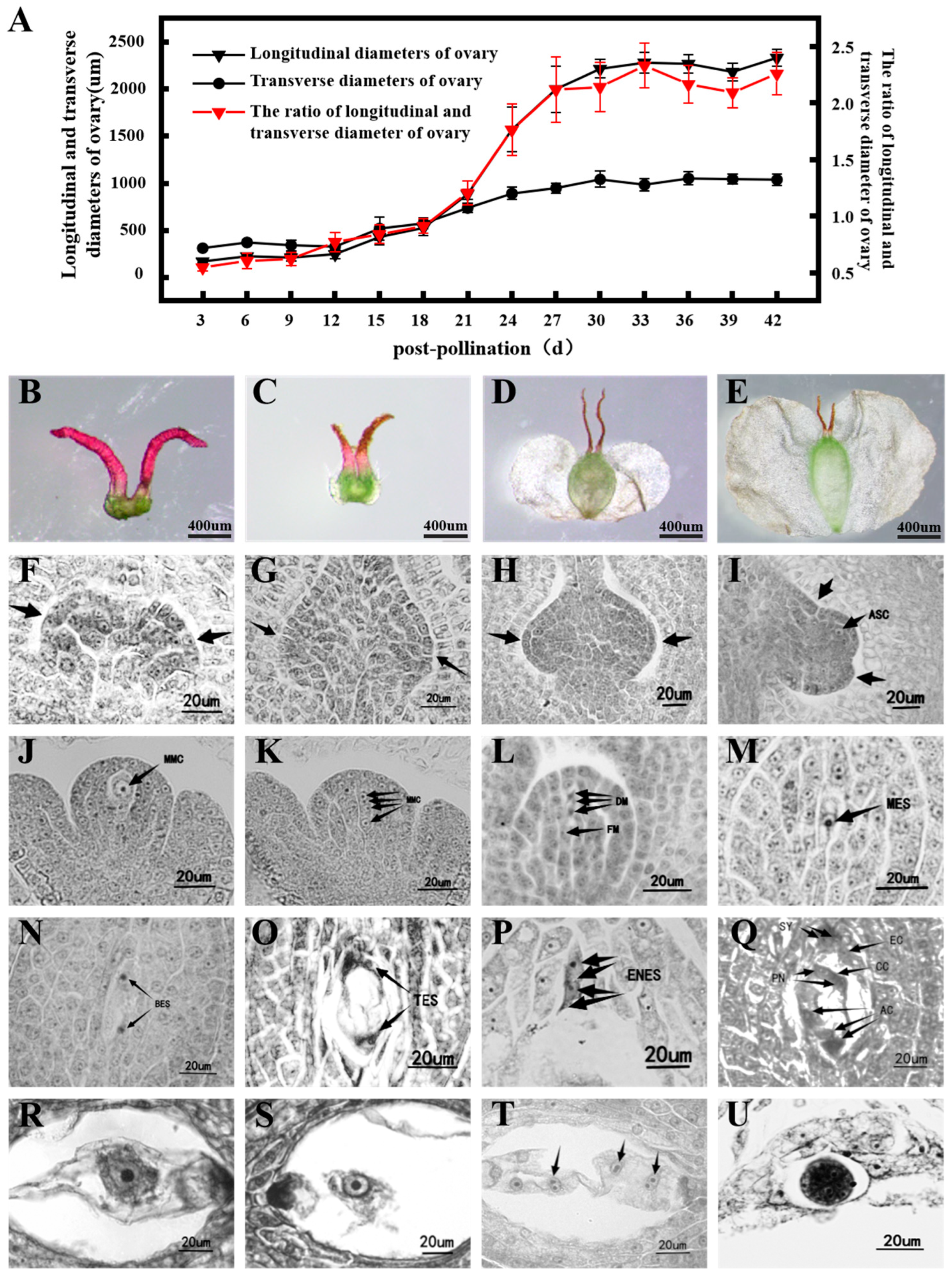
| Days After Pollination (Days) | Longitudinal: Transverse Diameter Ratio | External Morphology of the Ovary | Female Megaspore and Embryo Sac Development | |
|---|---|---|---|---|
| 3 | 0.55 ± 0.03 g | Ovule primordium | ||
| 6 | 0.60 ± 0.07 fg | |||
| 9 | 0.62 ± 0.06 fg | |||
| 12 | 0.76 ± 0.07 ef | Seed wings begin to form | Beads have been differentiated from primordium | |
| 15 | 0.83 ± 0.08 e | Megaspore mother cells are produced | ||
| 18 | 0.91 ± 0.07 e | Longitudinal diameter: transverse diameter ≈ 1 | Functional megaspore | |
| 21 | 1.20 ± 0.11 d | The longitudinal diameter grows rapidly | Development progress of embryo sac | |
| 24 | 1.76 ± 0.23 c | Mature embryo sac | ||
| 27 | 2.12 ± 0.30 b | Longitudinal diameter: transverse diameter ≈ 1 | The polar nucleus is combined with the sperm nucleus | |
| 30 | 2.14 ± 0.22 ab | |||
| 33 | 2.33 ± 0.20 a | The primary endosperm nucleus is formed | ||
| 36 | 2.16 ± 0.17 ab | The disintegration of the bead heart tissue produces a free endosperm nucleus | ||
| 39 | 2.09 ± 0.13 b | Proembryo | ||
| 42 | 2.26 ± 0.18 ab | Globular embryo and Cardioid embryo | ||
| Days After Pollination (Days) | Induction Rate % (40 °C) | Induction Rate % (42 °C) | ||
|---|---|---|---|---|
| 1 h | 2 h | 1 h | 2 h | |
| CK | 0 | 0 | 0 | 0 |
| 3 | 0.52 | — | — | — |
| 6 | — | — | — | — |
| 9 | 0 | 0 | — | — |
| 12 | 0.49 | 0 | 0.99 | — |
| 15 | 1.38 | 32.05 | 33.82 | 5.97 |
| 18 | 4.05 | 22.54 | 4.05 | 1.00 |
| 21 | 2.44 | 0 | 0 | 1.22 |
| 24 | 2.86 | 2.88 | 1.72 | 1.32 |
| 27 | 0.88 | 10.99 | 0.68 | 2.94 |
| 30 | 2.13 | 0 | 3.26 | 1.41 |
| 33 | 1.09 | 1.59 | 7.94 | 1.34 |
| 36 | 0 | 2.35 | 0.79 | 0 |
| 39 | 0.88 | 3.51 | 0 | 2.72 |
| 42 | 1.54 | 1.67 | 0 | 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hu, Y.; Zhang, J.; Duan, S.; Feng, J.; Cui, Y.; Liu, G.; Jiang, J.; Li, H. High-Temperature Induction of 2n Female Gametes to Produce Triploid Birches: Timing, Parameters, and Growth Outcomes. Forests 2025, 16, 1667. https://doi.org/10.3390/f16111667
Li J, Hu Y, Zhang J, Duan S, Feng J, Cui Y, Liu G, Jiang J, Li H. High-Temperature Induction of 2n Female Gametes to Produce Triploid Birches: Timing, Parameters, and Growth Outcomes. Forests. 2025; 16(11):1667. https://doi.org/10.3390/f16111667
Chicago/Turabian StyleLi, Jingnan, Yijie Hu, Jie Zhang, Shaoqing Duan, Jie Feng, Ying Cui, Guifeng Liu, Jing Jiang, and Huiyu Li. 2025. "High-Temperature Induction of 2n Female Gametes to Produce Triploid Birches: Timing, Parameters, and Growth Outcomes" Forests 16, no. 11: 1667. https://doi.org/10.3390/f16111667
APA StyleLi, J., Hu, Y., Zhang, J., Duan, S., Feng, J., Cui, Y., Liu, G., Jiang, J., & Li, H. (2025). High-Temperature Induction of 2n Female Gametes to Produce Triploid Birches: Timing, Parameters, and Growth Outcomes. Forests, 16(11), 1667. https://doi.org/10.3390/f16111667





