Comparative Analysis of Glutathione Metabolism in Pb-Tolerant and Pb-Sensitive Salix integra Genotypes Under Lead Stress
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Cysteine Content
2.3. Determination of GSH Content
2.4. Determination of PC Content
2.5. Determination of AsA Content
2.6. Analysis of γ-ECS Activity
2.7. Analysis of GR Activity
2.8. Analysis of APX Activity
2.9. Statistical Analysis
3. Results
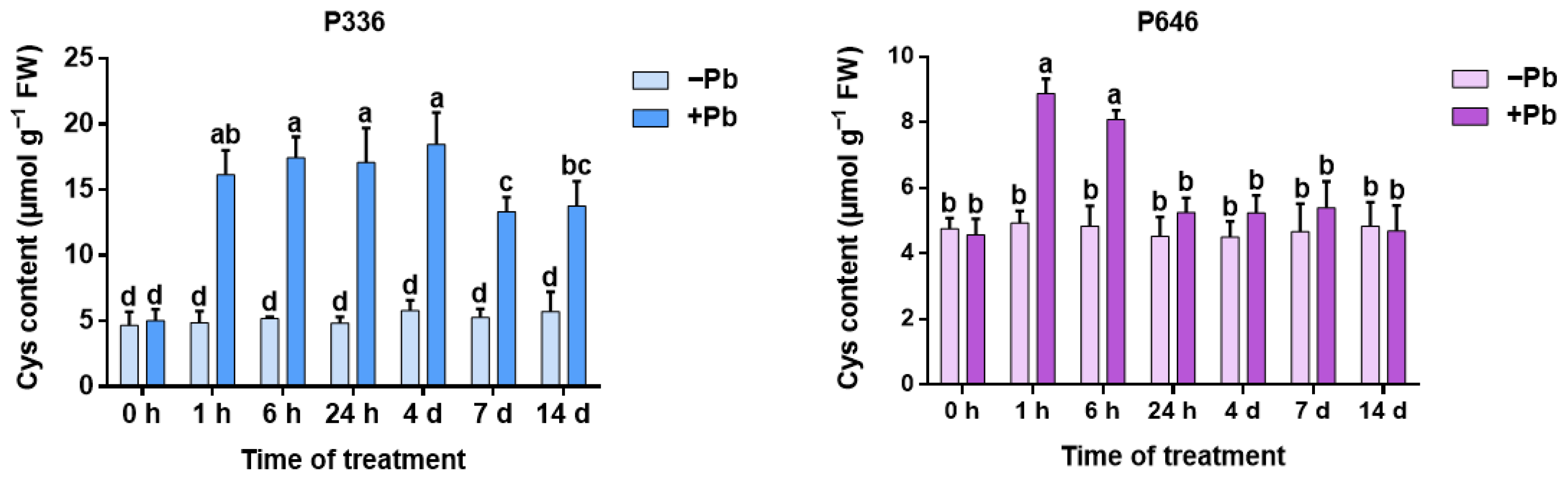
3.1. Analysis of Cys Content in Two Clones of Salix integra Exposed to Pb Stress
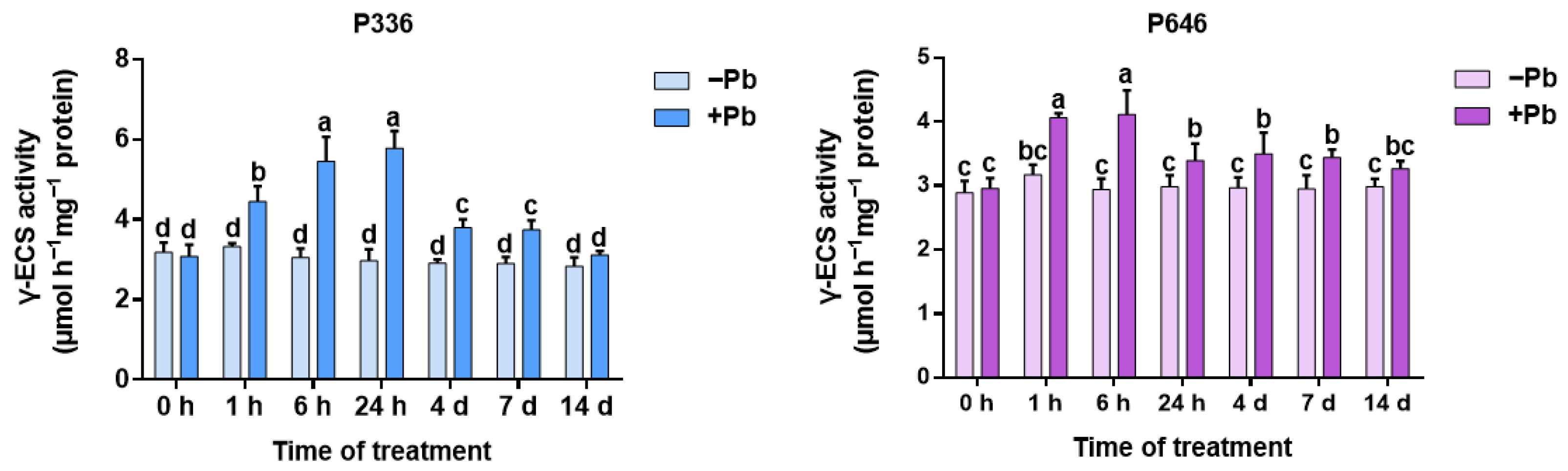
3.2. Analysis of γ-ECS Activity in Two Clones of Salix integra Exposed to Pb Stress
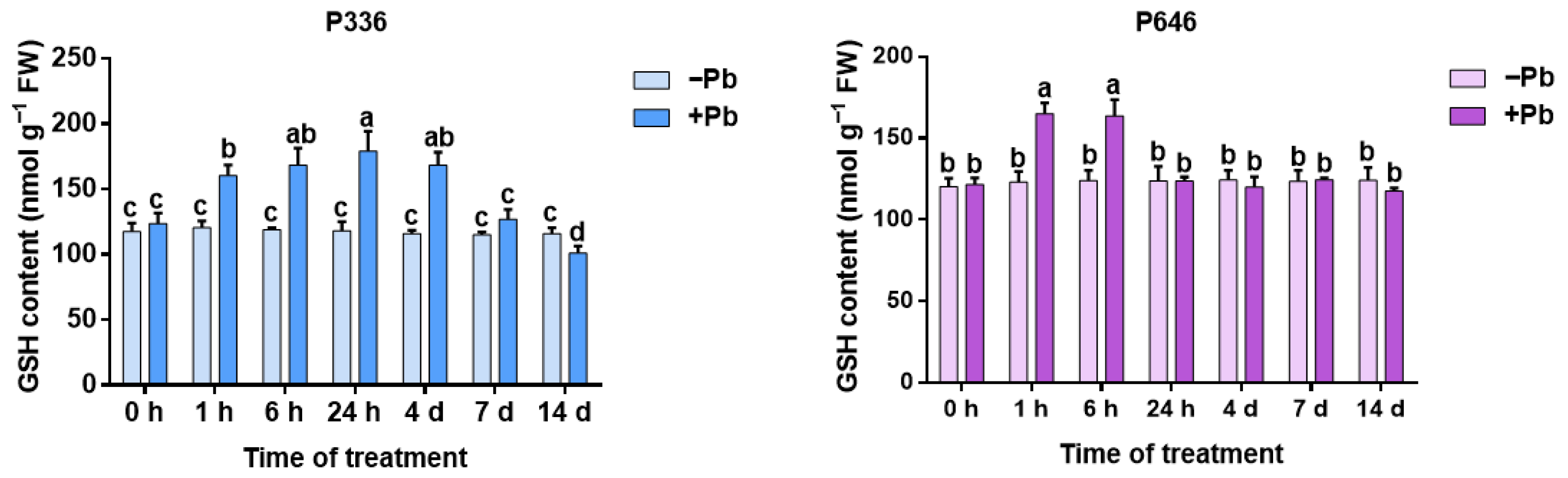
3.3. Analysis of GSH Content in Two Clones of Salix integra Exposed to Pb Stress
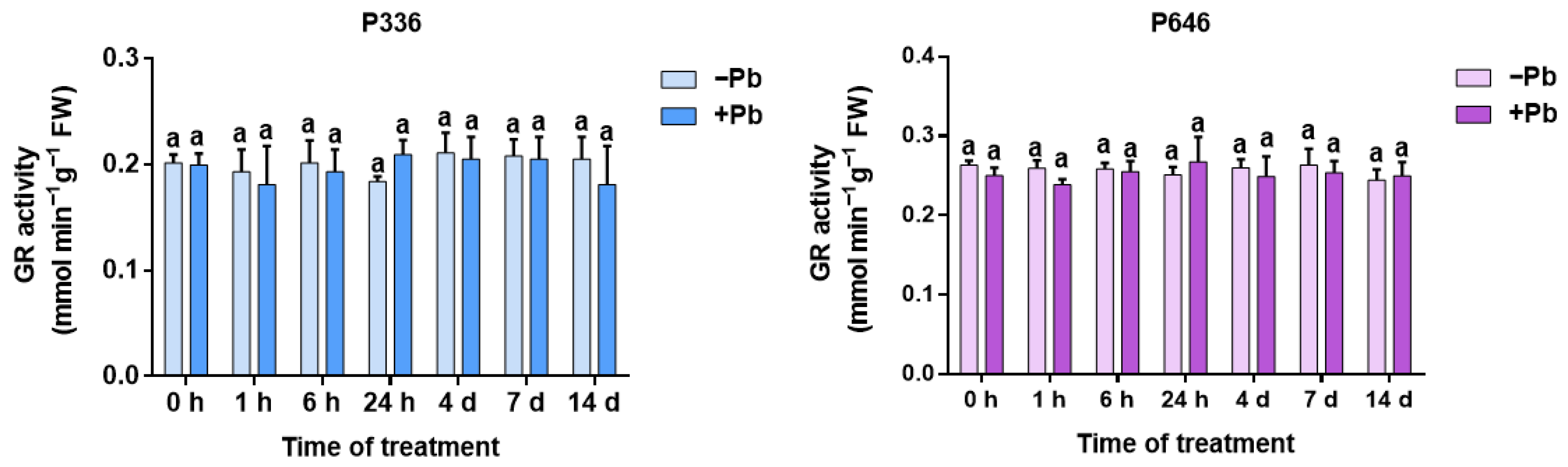
3.4. Analysis of GR Activity in Two Clones of Salix integra Exposed to Pb Stress
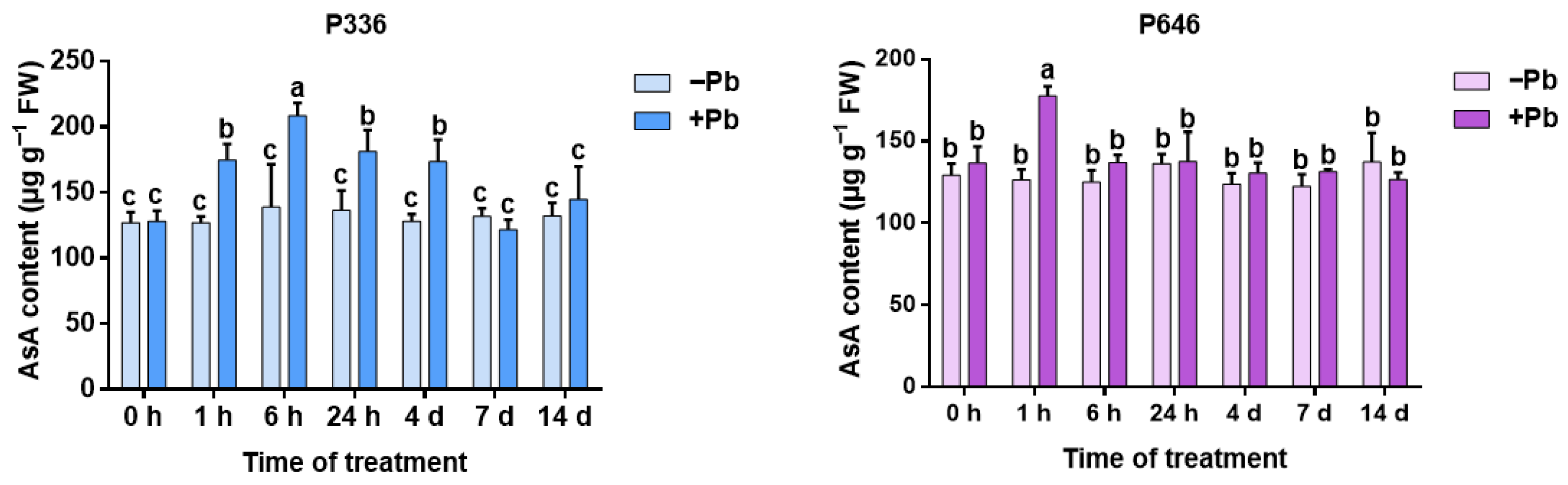
3.5. Analysis of AsA Content in Two Clones of Salix integra Exposed to Pb Stress
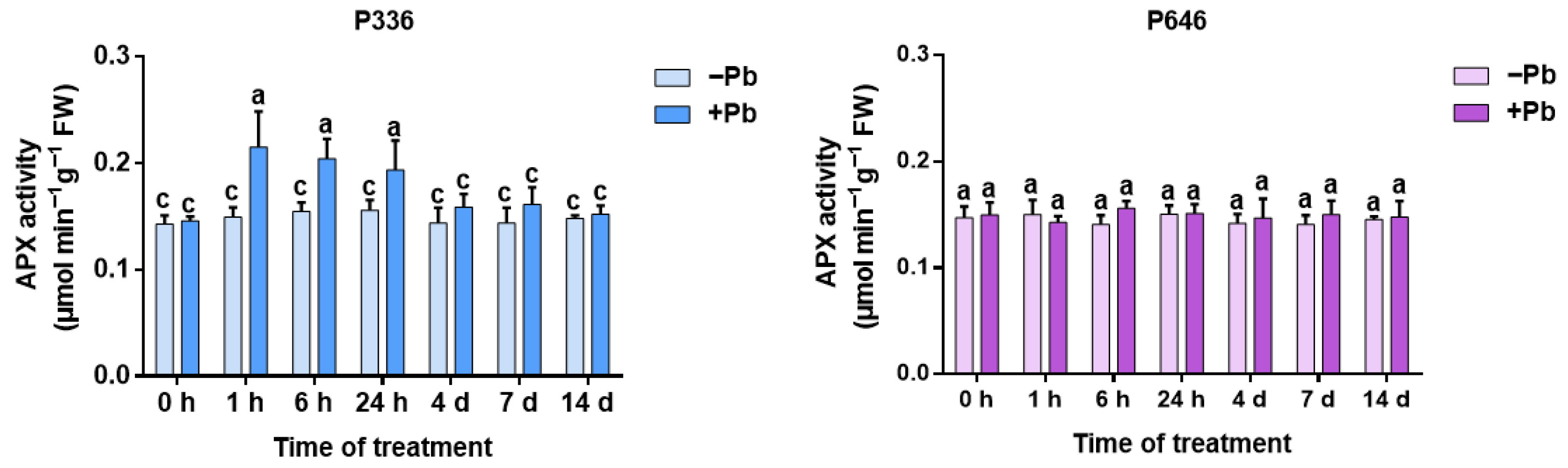
3.6. Analysis of APX Activity in Two Clones of Salix integra Exposed to Pb Stress
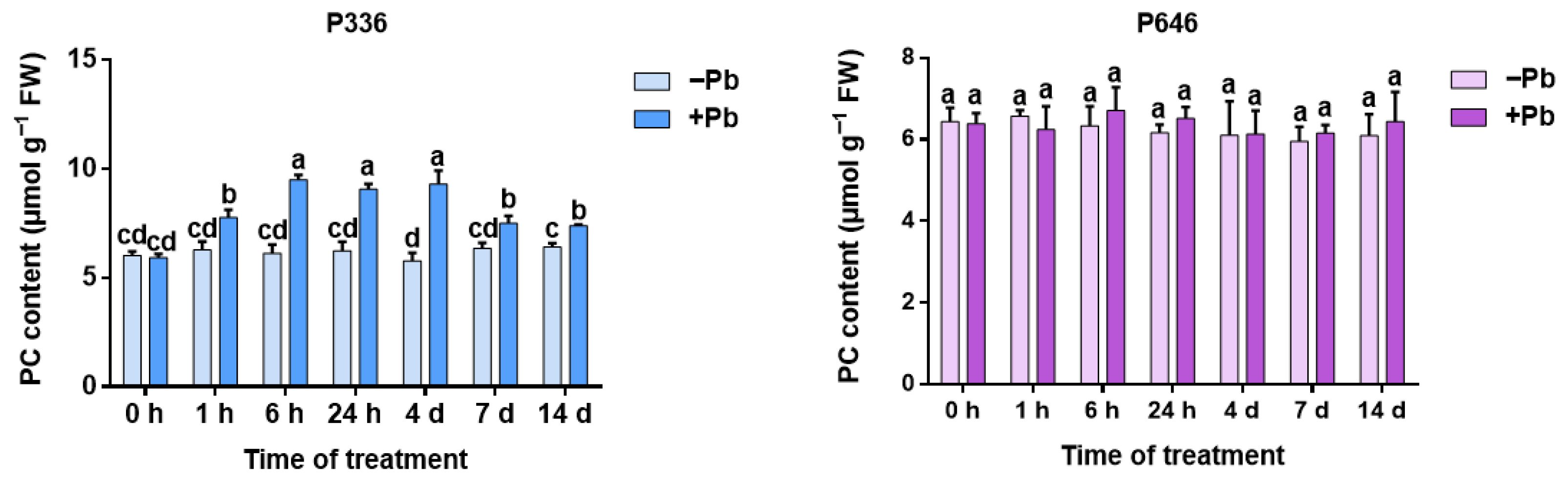
3.7. Analysis of PC Content in Two Clones of Salix integra Exposed to Pb Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Yan, J.; Kong, N.; Liu, Q.; Wang, M.; Lv, K.; Zeng, H.; Chen, W.; Luo, J.; Lou, H.; Song, L.; et al. Ti3C2Tx MXene nanosheets enhance the tolerance of Torreya grandis to Pb stress. J. Hazard. Mater. 2022, 445, 130647. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; El Moneim, D.A.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, P.M.; Sagar, V.; Pandya, A.; Chinnappa, M.; Kumar, R.; Bahadur, A. Seed priming with ZnO and Fe3O4 nanoparticles alleviate the lead toxicity in Basella alba L. through reduced lead uptake and regulation of ROS. Plants 2022, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Hernández, M.; López-Delacalle, M.; Martí-Guillen, J.M.; Martínez-Lorente, S.E.; Rivero, R.M. ROS and NO phytomelatonin-induced signaling mechanisms under metal toxicity in plants: A review. Antioxidants 2021, 10, 775. [Google Scholar] [CrossRef]
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; Lobato, A.K.d.S. Exogenous 24-Epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: Central roles to minimize Pb content and oxidative stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef]
- Ito, T.; Ohkama-Ohtsu, N. Degradation of glutathione and glutathione conjugates in plants. J. Exp. Bot. 2023, 74, 3313–3327. [Google Scholar] [CrossRef]
- Faizan, M.; Alam, P.; Hussain, A.; Karabulut, F.; Tonny, S.H.; Cheng, S.H.; Yusuf, M.; Adil, M.F.; Sehar, S.; Alomrani, S.O.; et al. Phytochelatins: Key regulator against heavy metal toxicity in plants. Plant Stress 2024, 11, 100355. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Al Mahmud, J.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Farooq, S.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Combined application of asparagine and thiourea improves tolerance to lead stress in wheat by modulating AsA-GSH cycle, lead detoxification and nitrogen metabolism. Plant Physiol. Biochem. 2022, 190, 119–132. [Google Scholar] [CrossRef]
- Hauser, F.; Li, Z.; Waadt, R.; Schroeder, J.I. SnapShot: Abscisic acid signaling. Cell 2017, 171, 1708–1708.e1. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Chen, Z.; Gao, Q.; Xu, Q.; Cao, H. A role for APX1 gene in lead tolerance in Arabidopsis thaliana. Plant Sci. 2017, 256, 94–102. [Google Scholar] [CrossRef]
- Navabpour, S.; Yamchi, A.; Bagherikia, S.; Kafi, H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2020, 26, 793–802. [Google Scholar] [CrossRef]
- Fujii, K. Plant strategy of root system architecture and exudates for acquiring soil nutrients. Ecol. Res. 2024, 39, 623–633. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, J.; Han, X.; Wang, H.; Yang, Q.; Pan, H.; Lou, Y.; Zhuge, Y. A novel phytoremediation technology for polluted cadmium soil: Salix integra treated with spermidine and activated carbon. Chemosphere 2022, 306, 135582. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Chen, Y.; Wang, S.; Mu, C.; Shi, X. The phytoremediation potential of 14 Salix clones grown in Pb/Zn and Cu mine tailings. Forests 2024, 15, 257. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Chen, H.; Zhang, J.; White, J.C.; Chen, G.; Xing, B. Xylem-based long-distance transport and phloem remobilization of copper in Salix integra Thunb. J. Hazard. Mater. 2020, 392, 122428. [Google Scholar] [CrossRef]
- Niu, X.; Zhou, Y.; Zhou, J.; Wang, X.; Gao, Z.; Huang, D. The effects of different lead pollution levels on soil microbial quantities and metabolic function with/without Salix integra Thunb. planting. Forests 2019, 10, 77. [Google Scholar] [CrossRef]
- He, X.; Sui, D.; Wang, H.; Huang, R.; Zheng, J.; Wang, B. Research progresses of willow genetic breeding in China. J. Nanjing For. Univ. 2022, 46, 51. [Google Scholar]
- Chen, J.; Liu, Z.; Yan, J. BPC1 and BPC2 positively regulates the waterlogging stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2025, 747, 151296. [Google Scholar] [CrossRef]
- Halabicky, O.; Giang, C.; Miller, A.; Peterson, K. Lead exposure, glucocorticoids, and physiological stress across the life course: A systematic review. Environ. Pollut. 2024, 345, 123329. [Google Scholar] [CrossRef]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead toxicity in cereals: Mechanistic insight into toxicity, mode of action, and management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Chen, L.; Lu, Y.; An, Y. Ectopic expression γ-glutamylcysteine synthetase of Vicia sativa increased cadmium tolerance in Arabidopsis. Gene 2022, 823, 146358. [Google Scholar] [CrossRef]
- Chhikara, S.; Singh, Y.; Long, S.; Minocha, R.; Musante, C.; White, J.C.; Dhankher, O.P. Overexpression of bacterial γ-glutamylcysteine synthetase increases toxic metal (loid) s tolerance and accumulation in Crambe abyssinica. Plant Cell Rep. 2024, 43, 270. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione metabolism and the novel role of mitochondrial GSH in retinal degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Boutin, C.; Clément, C.; Rivoal, J. Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction. Int. J. Mol. Sci. 2024, 25, 9845. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kitaiwa, T.; Nishizono, K.; Umahashi, M.; Miyaji, S.; Agake, S.; Kuwahara, K.; Yokoyama, T.; Fushinobu, S.; Maruyama-Nakashita, A.; et al. Glutathione degradation activity of γ-glutamyl peptidase 1 manifests its dual roles in primary and secondary sulfur metabolism in Arabidopsis. Plant J. 2022, 111, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; He, X.; Wang, H.; Shi, S.; Wang, B. Comparative Analysis of Glutathione Metabolism in Pb-Tolerant and Pb-Sensitive Salix integra Genotypes Under Lead Stress. Forests 2025, 16, 1592. https://doi.org/10.3390/f16101592
Huang R, He X, Wang H, Shi S, Wang B. Comparative Analysis of Glutathione Metabolism in Pb-Tolerant and Pb-Sensitive Salix integra Genotypes Under Lead Stress. Forests. 2025; 16(10):1592. https://doi.org/10.3390/f16101592
Chicago/Turabian StyleHuang, Ruifang, Xudong He, Hongling Wang, Shizheng Shi, and Baosong Wang. 2025. "Comparative Analysis of Glutathione Metabolism in Pb-Tolerant and Pb-Sensitive Salix integra Genotypes Under Lead Stress" Forests 16, no. 10: 1592. https://doi.org/10.3390/f16101592
APA StyleHuang, R., He, X., Wang, H., Shi, S., & Wang, B. (2025). Comparative Analysis of Glutathione Metabolism in Pb-Tolerant and Pb-Sensitive Salix integra Genotypes Under Lead Stress. Forests, 16(10), 1592. https://doi.org/10.3390/f16101592




