Age-Dependent Differences in Leaf Sulfur Assimilation and Relationship with Resistance to Air Pollutant SO2
Abstract
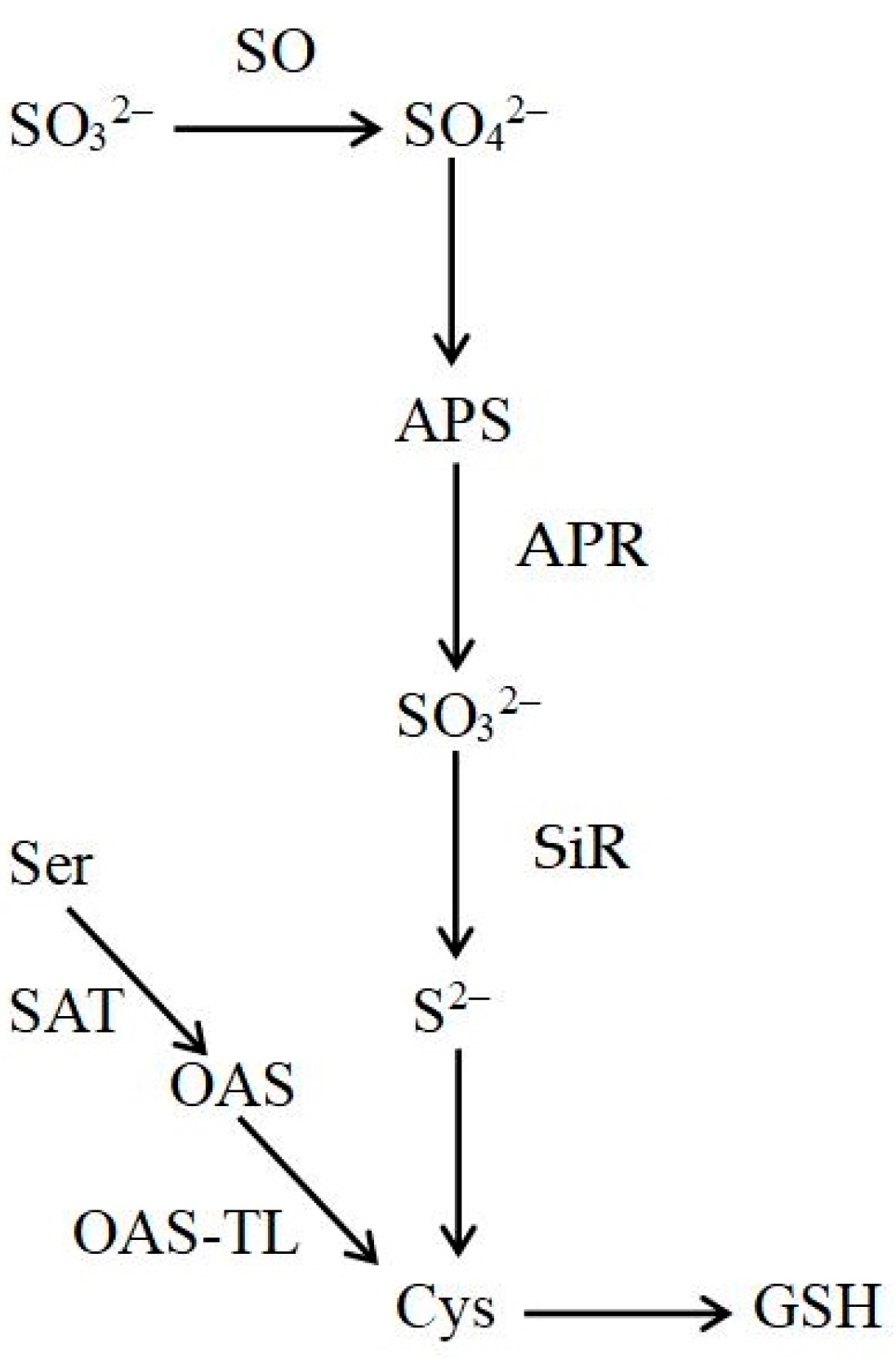
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Determination of Enzyme Activities
- (1)
- (2)
- (3)
- For adenosine 5′-phosphosulfate reductase (APR) activity measurements, frozen leaf material (100 mg) was ground in liquid nitrogen and homogenized in 3 mL extraction buffer [100 mM mono-/dipotassium phosphate buffer (pH 7.7) with 10 mM DTT, 10 mM Na2SO3, 5 mM sodium EDTA, 0.5 mM AMP, 1% Triton X-100, 10 mM L-Cys, and 2% polyvinylpyrrolidone (PVP40)]. The extract was centrifuged for 10 min at 12,000× g at 4 °C. The enzyme reaction solution contained 1 mL extract and 3 mL reaction buffer [50 mM Tris-HCl buffer (pH = 7.2) with 12 μmol/L Na2SO3, 1.5 μmol/L K3Fe(CN)6, 1.2 μmol/L AMP and 24 μmol/L EDTA] [27]. The light absorption was read at a wavelength of 420 nm.
- (4)
- Sulfite oxidase (SO) activity was determined using the method by Randewig et al. [14,16]. Enzyme protein was extracted as described previously [20]. For SO activity measurement, 200 μL of the extract was mixed with 200 μL Tris acetate buffer, pH 7.25. Thereafter, 100 μL of 0.5 mM sulfite was added to start the reaction. A solution containing formaldehyde and acid fuchsin was used to stop the enzyme reaction. The color changes caused by SO-mediated sulfate formation were observed at a wavelength of 580 nm [18].
2.4. Metabolite Determination
- (1)
- Quantification of thiols
- (2)
- Quantification of sulfate
2.5. Principal Component Analysis (PCA)
2.6. Statistical Analyses
3. Results
3.1. Visible Symptoms of Injury in Response to SO2 Exposure
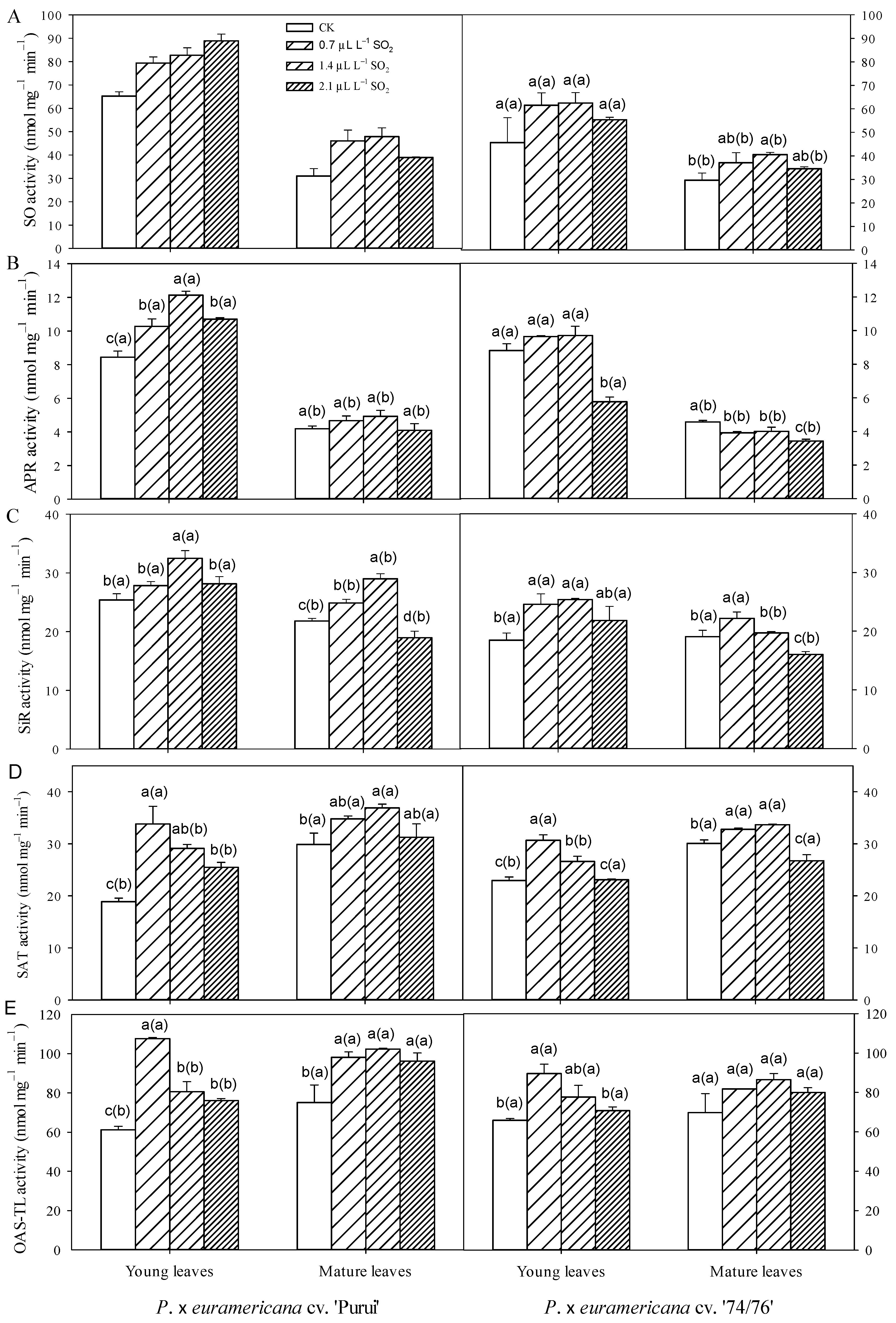
3.2. Variation in Enzyme Activities in Leaves
3.3. Variation in Sulfur Metabolites in Leaves
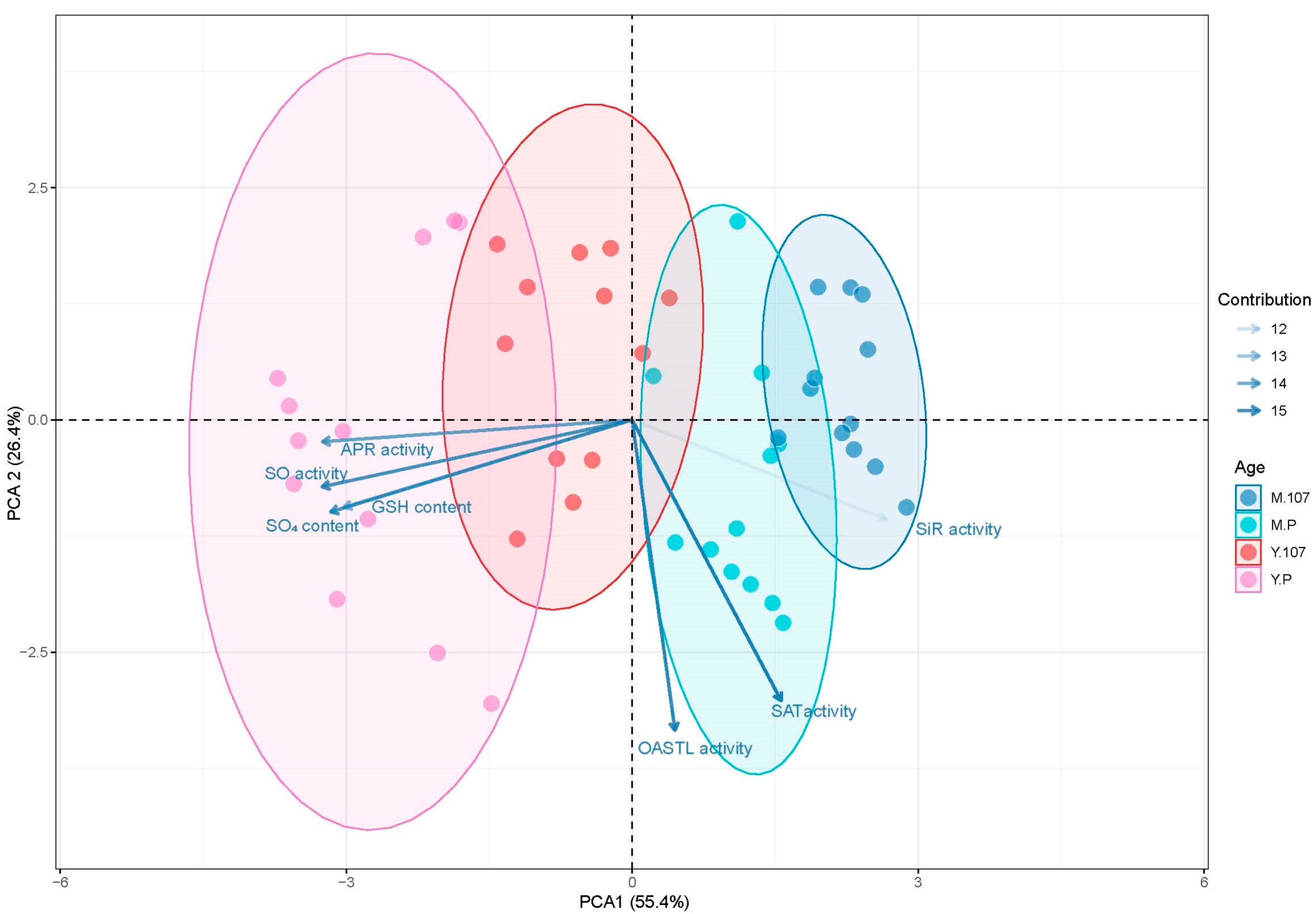
3.4. Major Variation Patterns of Enzymes Activities and Sulfur Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Purui | Populus × euramericana CV. ‘Purui’ |
| 74/76 | Populus × euramericana CV. ‘74/76’ |
| SO | sulfite oxidase |
| SiR | sulfite reductase |
| SAT | serine acetyltransferase |
| OAS | O-acetylserine |
| OASTL | O-acetylserine (thiol) lyase |
| APR | adenosine 5′-phosphosulfate reductase |
| GSH | glutathione |
| Cys | cysteine |
References
- Hänsch, R.; Mendel, R.R. Sulfite oxidation in plants. In Sulfur Metabolism in Phototrophic Organisms; Govindjee, Amesz, J., Barber, J., Blankenship, R.E., Murata, N., Ogren, W.L., Ort, D.R., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 27, pp. 223–230. [Google Scholar]
- Tanaka, K.; Otsubo, T.; Kondo, N. Participation of hydrogen peroxide in the inactivation of Calvin-cycle SH enzymes in SO2− fumigated spinach leaves. Plant Cell Physiol. 1982, 23, 1009–1018. [Google Scholar]
- Thomas, M.D.; Hendricks, R.H.; Collier, T.R.; Hill, G.R. The utilization of sulfate and sulfur dioxide for the nutrition of alfalfa. Plant Physiol. 1943, 18, 345–371. [Google Scholar] [CrossRef]
- Kondo, N. Uptake, metabolism, and detoxification of sulfur dioxide. In Air Pollution and Plant Biotechnology; Omasa, K., Saji, H., Youssefian, S., Kondo, N., Eds.; Springer (India) Private Limited Publishing: Tokyo, Japan, 2005; pp. 179–199. [Google Scholar]
- Filner, P.; Rennenberg, H.; Sekiya, J.; Bressan, R.A.; Wilson, L.G.; LeCureux, L.; Shimei, T. Biosynthesis and emission of hydrogen sulfide by higher plants. In Gaseous Air Pollutants and Plant Metabolism; Koziol, M.J., Whatley, F.R., Eds.; Butterworth: London, UK, 1984; pp. 291–312. [Google Scholar]
- Heber, U.; Hüve, K. Action of SO2 on plants and metabolic detoxification of SO2. Int. Rev. Cytol. A Surv. Cell Biol. 1998, 177, 255–286. [Google Scholar]
- Scheerer, U.; Hänsch, R.; Mendel, R.R.; Kopriva, S.; Rennenberg, H.; Herschbach, C. Sulfur flux through the sulfate assimilation pathway is differently controlled by adenosine 5′-phosphosulfate reductase under stress and in transgenic poplar plants overexpressing gamma-ECS, SO, or APR. J. Exp. Bot. 2010, 61, 609–622. [Google Scholar] [CrossRef]
- Pfanz, H.; Dietz, K.-J.; Weinerth, I.; Oppmann, B. Detoxification of sulfur dioxide by apoplastic peroxidases. In Sulfur Nutrition and Sulfur Assimilation in Higher Plants; Rennenberg, H., Brunold, C., De Kok, L.J., Stulen, I., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 229–233. [Google Scholar]
- Eilers, T.; Schwarz, G.; Brinkmann, H.; Witt, C.; Richter, T.; Nieder, J.; Koch, B.; Hille, R.; Hänsch, R.; Mendel, R.R. Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase. A new player in plant sulfur metabolism. J. Biol. Chem. 2001, 276, 46989–46994. [Google Scholar] [CrossRef]
- Rennenberg, H. The fate of excess sulfur in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 121–153. [Google Scholar] [CrossRef]
- Hänsch, R.; Lang, C.; Riebeseel, E.; Lindigkeit, R.; Gessler, A.; Rennenberg, H.; Mendel, R.R. Plant sulfite oxidase as novel producer of H2O2: Combination of enzyme catalysis with a subsequent non-enzymatic reaction step. J. Biol. Chem. 2006, 281, 6884–6888. [Google Scholar] [CrossRef]
- Hamisch, D.; Randewig, D.; Schliesky, S.; Bräutigam, A.; Weber, A.P.M.; Geffers, R.; Herschbach, C.; Rennenberg, H.; Mendel, R.R.; Hänsch, R. Impact of SO2 on Arabidopsis thaliana transcriptome in wildtype and sulfite oxidase knockout plants analyzed by RNA deep sequencing. New Phytol. 2012, 196, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.N.; Tarczynski, M.C.; Shen, B.; Leustek, T. The role of 5′-adenylylsulfate reductase in controlling sulfate reduction in plants. Photosynth. Res. 2005, 86, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Randewig, D.; Hamisch, D.; Herschbach, C.; Eiblmeier, M.; Gehl, C.; Jurgeleit, J.; Skerra, J.; Mendel, R.R.; Rennenberg, H.; Hänsch, R. Sulfite oxidase controls sulfur metabolism under SO2 exposure in Arabidopsis thaliana. Plant Cell Environ. 2012, 35, 100–115. [Google Scholar] [CrossRef]
- Rennenberg, H.; Herschbach, C. A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J. Exp. Bot. 2014, 65, 5711–5724. [Google Scholar] [CrossRef]
- Randewig, D.; Hamisch, D.; Eiblmeier, K.; Boedecker, C.; Kreuzwieser, J.; Mendel, R.R.; Hänsch, R.; Herschbach, C.; Rennenberg, H. Oxidation and reduction of sulfite contribute to susceptibility and detoxification of SO2 in Populus × canescens leaves. Trees 2014, 28, 399–411. [Google Scholar] [CrossRef]
- Brychkova, G.; Xia, Z.; Yang, G.; Yesbergenova, Z.; Zhang, Z.; Davydov, O.; Fluhr, R.; Sagi, M. Sulfite oxidase protects plants against sulfur dioxide toxicity. Plant J. 2007, 50, 696–709. [Google Scholar] [CrossRef]
- Lang, C.; Popko, J.; Wirtz, M.; Hell, R.; Herschbach, C.; Kreuzwieser, J.; Rennenberg, H.; Mendel, R.R.; Hänsch, R. Sulfite oxidase as key enzyme for protecting plants against sulfur dioxide. Plant Cell Environ. 2007, 30, 447–455. [Google Scholar] [CrossRef]
- Yarmolinsky, D.; Brychkova, G.; Fluhr, R.; Sagi, M. Sulfite reductase protects plants against sulfite toxicity. Plant Physiol. 2013, 161, 725–743. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Liu, W.; Wan, X.; Chen, Z.; Zhao, J. Differences in detoxification mechanism and gene expression changes of sulfur metabolism in coping with the air pollutant SO2 between the resistant and ordinary poplar variety. Acta Physiol. Plant 2022, 44, 124. [Google Scholar] [CrossRef]
- Bressan, R.A.; Wilson, L.G.; Filner, W.P. Mechanisms of resistance to sulfur dioxide in the Curcurbitaceae. Plant Physiol. 1978, 61, 761–767. [Google Scholar] [CrossRef]
- Olszyk, D.M.; Tibbitts, T.W. Stomatal response and leaf injury of Pisum sativum L. with SO2 and O3 exposures. I. influence of pollutant level and leaf maturity. Plant Physiol. 1981, 67, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, J.; Filner, W.P. Resistance to injury by sulfur dioxide: Correlation with its reduction to, and emission of, hydrogen sulfide in cucurbitaceae. Plant Physiol. 1982, 70, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bai, K.D.; Wan, X.C.; Cheng, G.H.; Zhang, C.Y.; Zhang, Z.X. Responses of poplar (Populus × euramericana cv. “74/76”) SO2-resistant clone to SO2 fumigation and the variation in antioxidant systems. Sci. Silvae Sin. 2011, 47, 66–71. [Google Scholar]
- Hartmann, T.; Mult, S.; Suter, M.; Rennenberg, H.; Herschbach, C. Leaf age-dependent differences in sulphur assimilation and allocation in poplar (Poplus tremula × P. alba) leaves. J. Exp. Bot. 2000, 51, 1077–1088. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Trüper, H.G.; Rogers, L.A. Purification and properties of adenylyl sulfate reductase from the phototrophic sulfur bacterium, Thiocapsa roseopersicina. J. Bacteriol. 1971, 108, 1112–1121. [Google Scholar] [CrossRef]
- Ju, X.H.; Tang, S.; Jia, Y.; Guo, J.; Ding, Y.; Song, Z.; Zhao, Y. Determination and characterization of cysteine, glutathione and phytochelatins (PC2-6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. J. Chromatogr. B 2011, 879, 1717–1724. [Google Scholar] [CrossRef]
- Brychkova, G.; Grishkevich, V.; Fluhr, R.; Sagi, M. An essential role for tomato sulfite oxidase and enzymes of the sulfite network in maintaining leaf sulfite homeostasis. Plant Physiol. 2013, 161, 148–164. [Google Scholar] [CrossRef]
- De Kok, L.J. Sulfur metabolism in plants exposed to atmospheric sulfur. In Sulfur Nutrition and Sulfur Assimilation in Higher Plants; Rennenberg, H., Brunold, C., De Kok, L.J., Stulen, I., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 111–130. [Google Scholar]
- Calderwood, A.; Morris, R.J.; Kopriva, S. Predictive sulfur metabolism—A field in flux. Front. Plant Sci. 2014, 5, 646. [Google Scholar] [CrossRef]
- Vauclare, P.; Kopriva, S.; Fell, D.; Suter, M.; Sticher, L.; von Ballmoos, P.; Krahenbuhl, U.; den Camp, R.O.; Brunold, C. Flux control of sulphate assimilation in Arabidopsis thaliana: Adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. Plant J. 2002, 31, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, S.; Koprivova, A. Plant adenosine 5′-phosphosulphate reductase: The past, the present, and the future. J. Exp. Bot. 2004, 55, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot. 2006, 97, 479–495. [Google Scholar] [CrossRef]
- Harms, K.; Von Ballmoos, P.; Brunold, C.; Höfgen, R.; Hesse, H. Expression of a bacterial serine bacetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J. 2000, 22, 335–343. [Google Scholar] [CrossRef]
- Ruiz, J.; Blumwald, E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002, 214, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.; Hell, R. Functional analysis of the cysteine synthase protein complex from plants: Structural, biochemical and regulatory properties. J. Plant Physiol. 2006, 163, 273–286. [Google Scholar] [CrossRef]
- Kaiser, G.; Martinoia, E.; Schroppelmeier, G.; Heber, U. Active-transport of sulfate into the vacuole of plant cells provideshalotolerance and can detoxify SO2. J. Plant Physiol. 1989, 133, 756–763. [Google Scholar] [CrossRef]
- Kaiser, W.; Dittrich, A.; Heber, U. Sulfate concentrations in Norway spruce needles in relation to atmospheric SO2: A comparison of trees from various forests in Germany with trees fumigated with SO2 in growth chambers. Tree Physiol. 1993, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Kiso, K. Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur. J. Biochem. 1973, 33, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Alseher, R. Effects of SO on light-modulated enzyme reactions. In Gaseous Air Pollutants and Plant Metabolism; Koziol, M.J., Whatley, F.R., Eds.; Butterworths: London, UK, 1984; pp. 181–200. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wang, L.; Liu, W.; Gao, Y.; Wan, X. Age-Dependent Differences in Leaf Sulfur Assimilation and Relationship with Resistance to Air Pollutant SO2. Forests 2025, 16, 1582. https://doi.org/10.3390/f16101582
Feng J, Wang L, Liu W, Gao Y, Wan X. Age-Dependent Differences in Leaf Sulfur Assimilation and Relationship with Resistance to Air Pollutant SO2. Forests. 2025; 16(10):1582. https://doi.org/10.3390/f16101582
Chicago/Turabian StyleFeng, Jinxia, Luyi Wang, Wenxin Liu, Ying Gao, and Xianchong Wan. 2025. "Age-Dependent Differences in Leaf Sulfur Assimilation and Relationship with Resistance to Air Pollutant SO2" Forests 16, no. 10: 1582. https://doi.org/10.3390/f16101582
APA StyleFeng, J., Wang, L., Liu, W., Gao, Y., & Wan, X. (2025). Age-Dependent Differences in Leaf Sulfur Assimilation and Relationship with Resistance to Air Pollutant SO2. Forests, 16(10), 1582. https://doi.org/10.3390/f16101582




