The Effects of Nitrogen Deposition and Rainfall Enhancement on Intraspecific and Interspecific Competitive Patterns in Deciduous Broad-Leaved Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experiment Design
2.3. Research Methods
2.3.1. Determination of Dominant Tree Species
2.3.2. Competition Index
2.3.3. Selection of Competitor Trees
2.3.4. One-Way Analysis of Variance
3. Results and Analysis
3.1. Dynamic Changes in Dominant Tree Species
3.2. Determination of Competitive Trees
3.3. Effects of Nitrogen Deposition and Rain Enhancement on Stand Competition
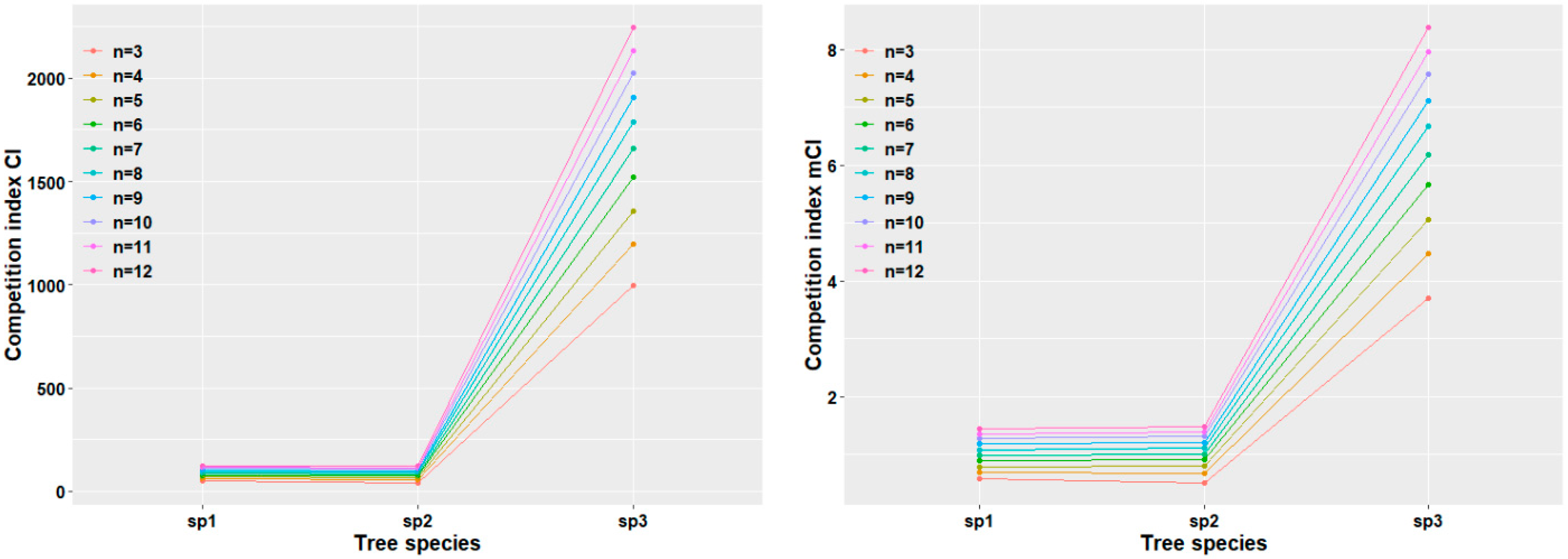
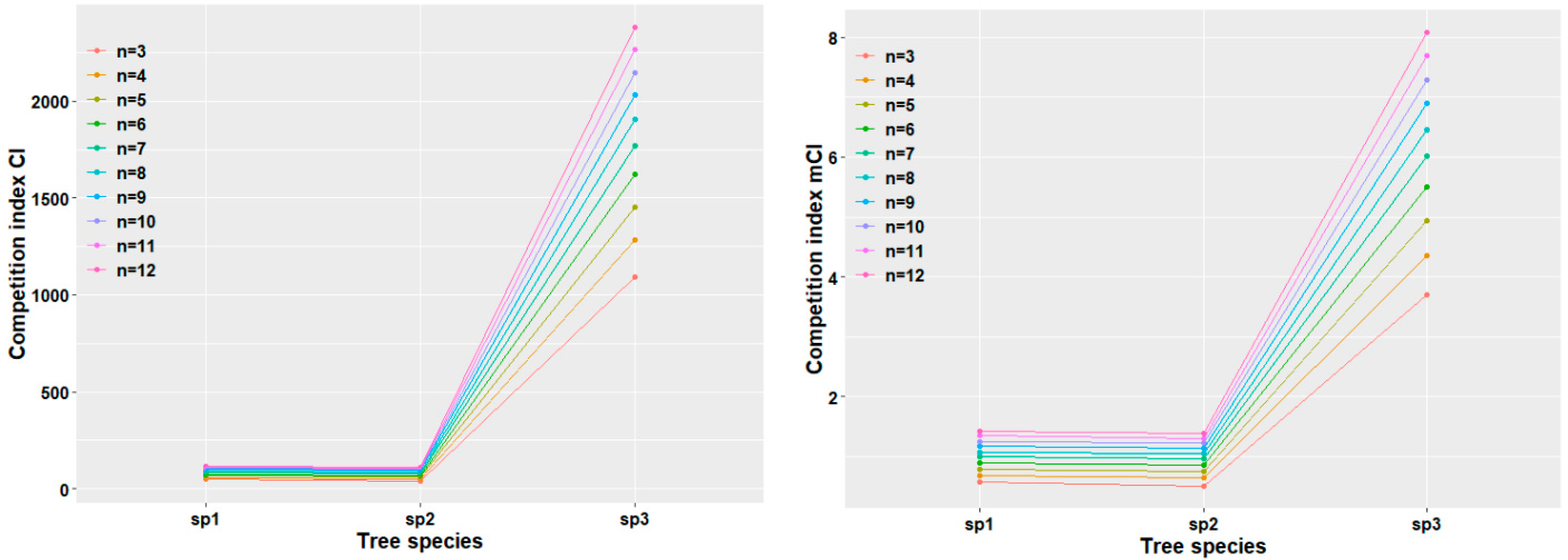
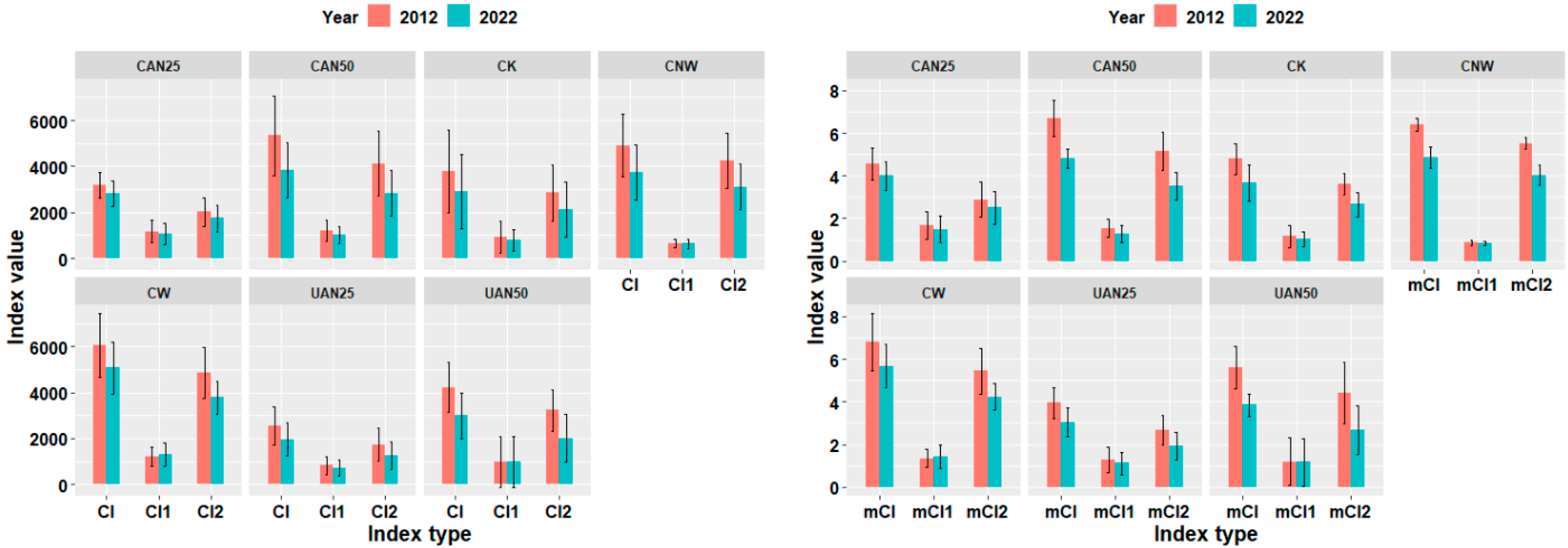
3.3.1. Response of Stand Competition Intensity
3.3.2. Response of Surviving Trees in Plot Level to Competition Intensity
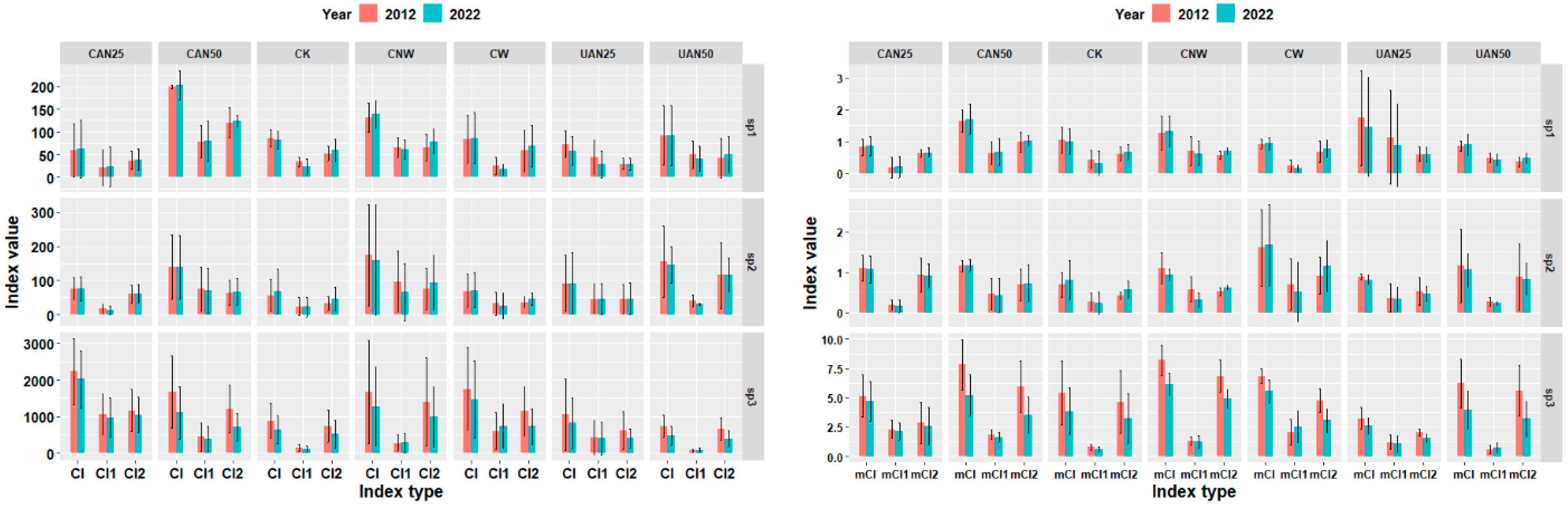
3.3.3. Response of Competitive Intensity of Dominant Tree Species Among Surviving Trees in Plot Level
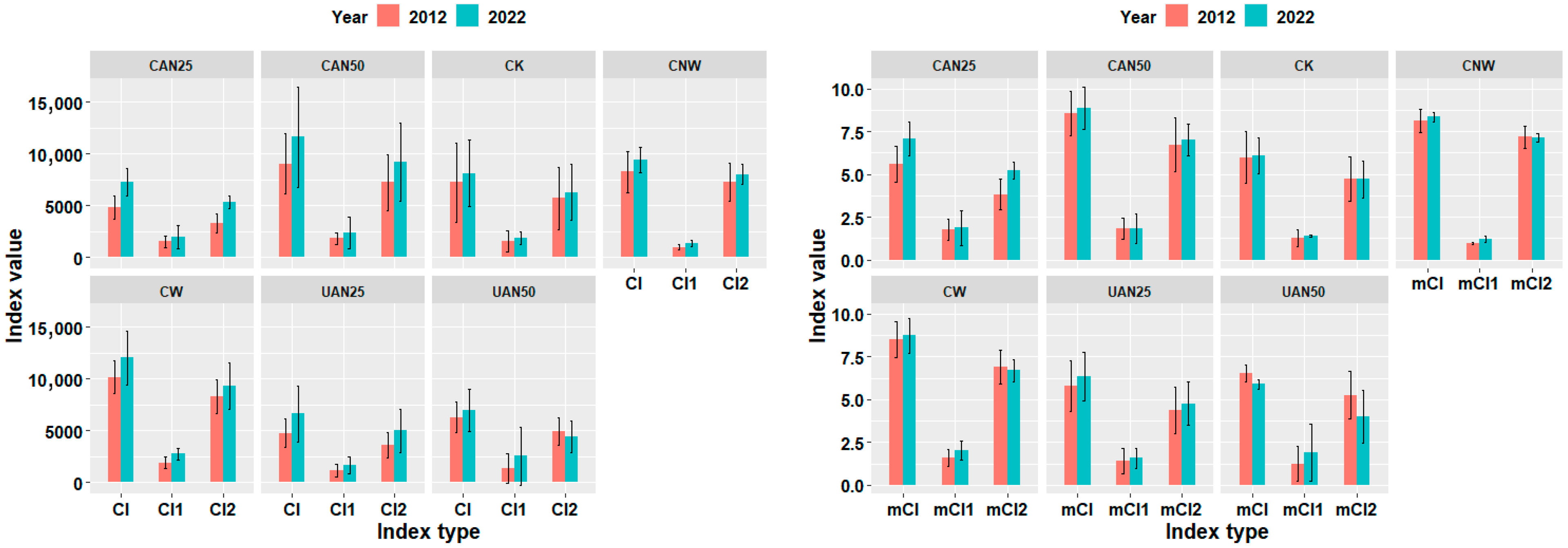
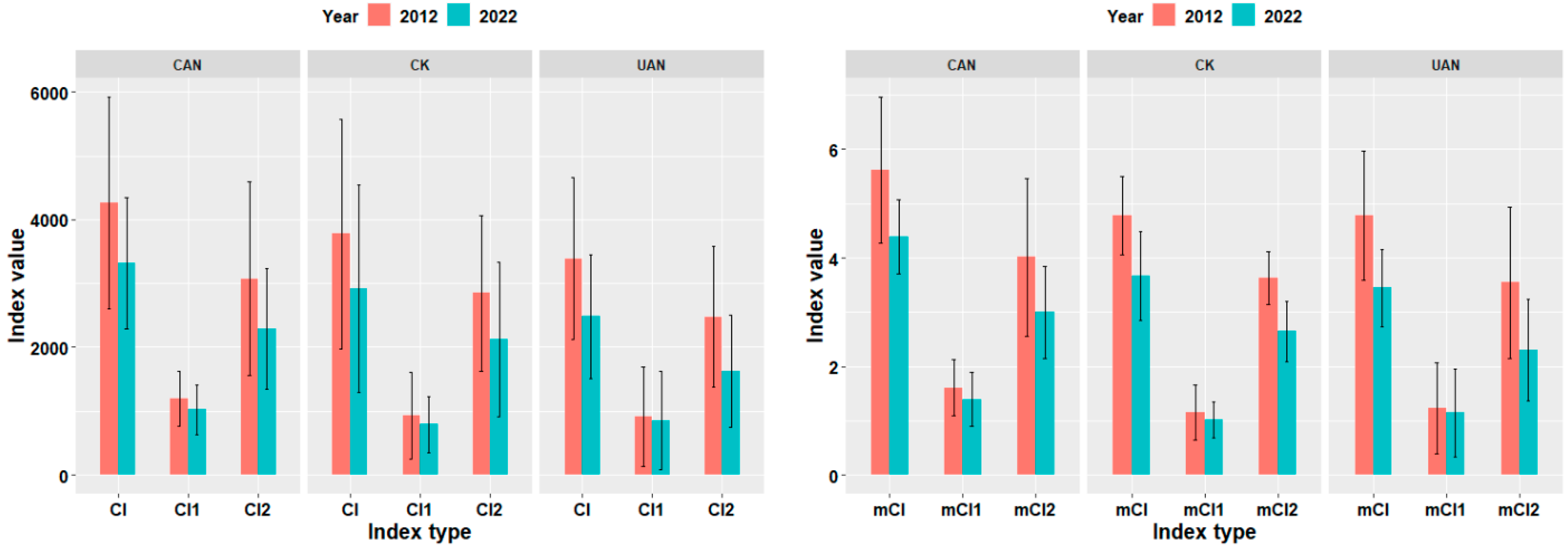
3.4. Effects of Nitrogen Addition Methods on Competition Among Surviving Trees in Plot Level
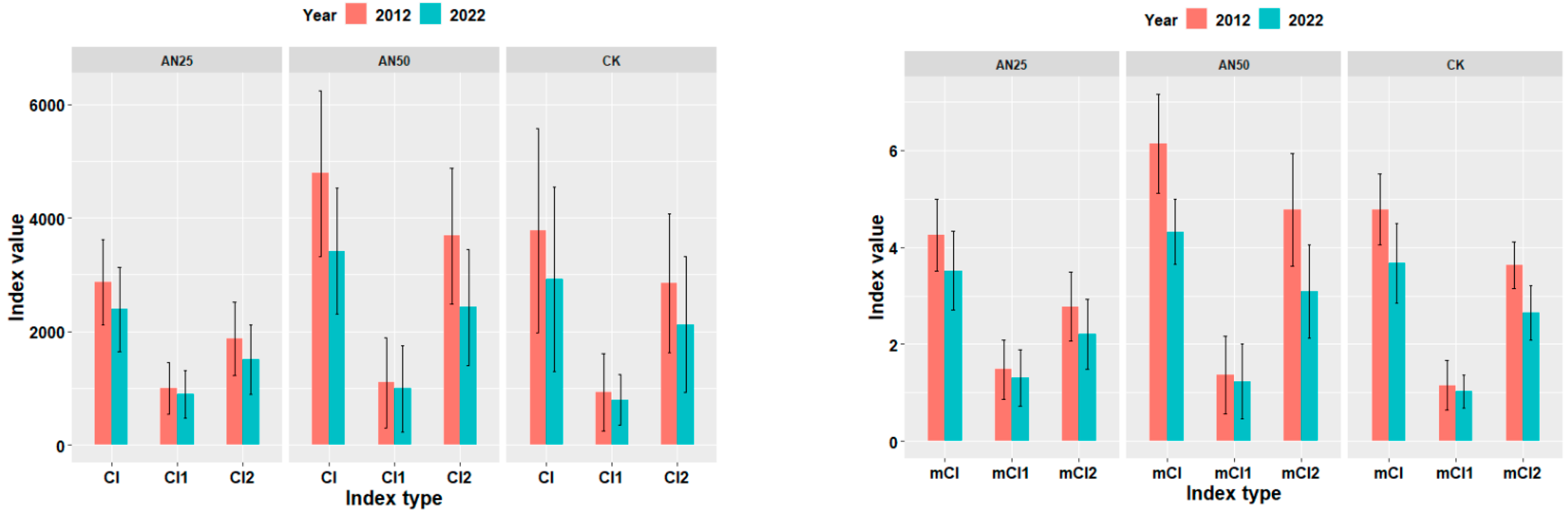
3.5. Effects of Nitrogen Addition Intensity on Competition Among Surviving Trees in Plot Level
4. Discussion
4.1. Competitive Changes in Dominant Tree Species
4.2. Analysis of Changes in Competitive Intensity Under Nitrogen Addition Treatments
4.3. Asymmetric Regulation of the Competitive Landscape of L. formosana by Nitrogen Addition Treatments
4.4. Analysis of Differences in Changes in Competition Intensity Among Surviving Trees in Plot Level Based on Nitrogen Addition Methods and Intensity
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Shen, W.J.; Zhu, S.D.; Wan, S.Q.; Luo, Y.Q.; Yan, J.H.; Wang, K.Y.; Liu, L.; Dai, H.T.; Li, P.X.; et al. Can canopy addition of nitrogen better illustrate the effect of atmospheric nitrogen deposition on forest ecosystem? Sci. Rep. 2015, 5, 11245. [Google Scholar] [CrossRef]
- Tian, D.; Du, E.Z.; Jiang, L.; Ma, S.H.; Zeng, W.J.; Zou, A.L.; Feng, C.Y.; Xu, L.C.; Xing, A.J.; Wang, W.F.; et al. Responses of forest ecosystems to increasing N deposition in China: A critical review. Environ. Pollut. 2018, 243 Pt A, 75–86. [Google Scholar] [CrossRef]
- Chen, X.N.; Zhao, N.Q.; Duan, N.; Gegen, B.; Zhang, J.B.; Shi, S.Y.; Duan, R.B.; Gao, S.C. Research progress on plant responses to water and nitrogen addition. Temper. For. Res. 2022, 5, 7–11. [Google Scholar]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; Zhou, X.B.; He, C.Y.; Zhang, F.S. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Long, J.N.; Dean, T.J.; Roberts, S.D. Linkages between silviculture and ecology: Examination of several important conceptual models. For. Ecol. Manag. 2004, 200, 249–261. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef]
- Simpson, D.; Arneth, A.; Mills, G.; Solberg, S.; Uddling, J. Ozone—The persistent menace: Interactions with the N cycle and climate change. Curr. Opin. Environ. Sustain. 2014, 9–10, 9–19. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, D.; Ma, S.H.; Zhou, X.L.; Xu, L.C.; Zhu, J.X.; Jing, X.; Zheng, C.Y.; Shen, H.H.; Zhu, B.; et al. The response of tree growth to nitrogen and phosphorus additions in a tropical montane rainforest. Sci. Total Environ. 2018, 618, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, D.S.; Yang, H.; Niu, S.L. Size-dependent nutrient limitation of tree growth from subtropical to cold temperate forests. Funct. Ecol. 2018, 32, 95–105. [Google Scholar] [CrossRef]
- Tian, D.; Li, P.; Fang, W.J.; Xu, J.; Luo, Y.K.; Yan, Z.B.; Zhu, B.; Wang, J.J.; Xu, X.N.; Fang, J.Y. Growth responses of trees and understory plants to nitrogen fertilization in a subtropical forest in China. Biogeosciences 2017, 14, 3461–3469. [Google Scholar] [CrossRef]
- Hong, L.; Tang, S.Z.; Li, T.; Fu, L.Y.; Song, X.Y.; Duan, G.S.; Fu, J.M.; Ma, L. Determinants of Deadwood Biomass under the Background of Nitrogen and Water Addition in Warm Temperate Forests. Forests 2024, 15, 1464. [Google Scholar] [CrossRef]
- Hou, L.; Dong, Z.J.; Yang, Y.Y.; Zhang, D.H.; Zhang, S.L.; Zhang, S.X. Applying foliar stoichiometric traits of plants to determine fertilization for a mixed pine-oak stand in the Qinling Mountains, China. PeerJ 2018, 6, e4628. [Google Scholar] [CrossRef]
- Yang, H. Effects of nitrogen and phosphorus addition on leaf nutrient characteristics in a subtropical forest. Trees 2018, 32, 383–391. [Google Scholar] [CrossRef]
- You, C.M.; Wu, F.Z.; Yang, W.Q.; Xu, Z.F.; Tan, B.; Yue, K.; Ni, X.Y. Nutrient-limited conditions determine the responses of foliar nitrogen and phosphorus stoichiometry to nitrogen addition: A global meta-analysis. Environ. Pollut. 2018, 241, 740–749. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Razaq, M.; Zhang, P.; Shen, H.L.; Salahuddin. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Zhang, H.C.; Li, Y.Q.; Xu, K.Q.; Yu, L.H.; He, P.; Zeng, S.P.; Song, Y.X.; Liu, R.; Sun, Y. Effects of Anthropogenic Disturbance on the Structure, Competition, and Succession of Abies ziyuanensis Communities. Forests 2024, 15, 1001. [Google Scholar] [CrossRef]
- Daniels, R.F. Simple Competition Indices and Their Correlation with Annual Loblolly Pine Tree Growth. For. Sci. 1976, 22, 454–456. [Google Scholar]
- Adler, P.B.; Smull, D.; Beard, K.H.; Choi, R.T.; Furniss, T.; Kulmatiski, A.; Meiners, J.M.; Tredennick, A.T.; Veblen, K.E. Competition and coexistence in plant communities: Intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018, 21, 1319–1329. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Filipescu, C.N.; Comeau, P.G. Competitive interactions between aspen and white spruce vary with stand age in boreal mixedwoods. For. Ecol. Manag. 2007, 247, 175–184. [Google Scholar] [CrossRef]
- Weiner, J.; Thomas, S.C. Size Variability and Competition in Plant Monocultures. Oikos 1986, 47, 211–222. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. Size-symmetric versus size-asymmetric competition and growth partitioning among trees in forest stands along an ecological gradient in central Europe. Can. J. For. Res. 2010, 40, 370–384. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Rihm, B. Growth trends of beech and Norway spruce in Switzerland: The role of nitrogen deposition, ozone, mineral nutrition and climate. Sci. Total Environ. 2017, 599–600, 637–646. [Google Scholar] [CrossRef]
- DeForest, J.L.; Snell, R.S. Tree growth response to shifting soil nutrient economy depends on mycorrhizal associations. N. Phytol. 2020, 225, 2557–2566. [Google Scholar] [CrossRef]
- Bu, W.S.; Wang, F.C.; Zhang, C.C.; Bruelheide, H.; Fang, X.M.; Wang, H.M.; Chen, F.S. The contrasting effects of nitrogen and phosphorus fertilizations on the growth of Cunninghamia lanceolata depend on the season in subtropical China. For. Ecol. Manag. 2021, 482, 118874. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, C.C.; Deng, J.; Zhang, B.W.; Chen, F.S.; Chen, W.; Fang, X.M.; Li, J.J.; Zu, K.L.; Bu, W.S. Response of tree growth to nutrient addition is size dependent in a subtropical forest. Sci. Total Environ. 2024, 923, 171501. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, P.; Zhou, L.; Zhang, L.; Lin, Y.B.; Wang, Y.J.; Wang, J.; Hui, D.F.; Ren, H.; Lu, H.F. Multi-ecosystem services differently affected by over-canopy and understory nitrogen additions in a typical subtropical forest. Glob. Change Biol. 2024, 30, e17192. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, C.L.; Zhang, B.B.; Wu, D.; Zhu, D.D.; Zhang, W.; Ye, Q.; Yan, J.H.; Fu, J.M.; Fang, C.L.; et al. Nitrogen deposition and increased precipitation interact to affect fine root production and biomass in a temperate forest: Implications for carbon cycling. Sci. Total Environ. 2021, 765, 144497. [Google Scholar] [CrossRef]
- Hong, L.; Duan, G.S.; Fu, S.L.; Fu, L.Y.; Ma, L.; Li, X.W.; Fu, J.M. Response of Stand Spatial Structure to Nitrogen Addition in Deciduous Broad-Leaved Forest in Jigong Mountain. Sustainability 2024, 16, 5137. [Google Scholar] [CrossRef]
- Ohsawa, M. Differentiation of vegetation zones and species strategies in the subalpine region of Mt. Fuji. Vegetatio 2004, 57, 15–52. [Google Scholar] [CrossRef]
- Hu, X.F.; Duan, G.S.; Zhang, H.R.; Lu, J.; Zhang, X.H. Response of tree competition in natural secondary Quercus mongolica forest to thinning treatment. Forest Res. 2021, 34, 1–9. [Google Scholar] [CrossRef]
- Hegyi, F. A simulation model for managing jack-pine stands simulation. Royal Coll. For. Res. Notes 1974, 30, 74–90. [Google Scholar]
- Hui, G.Y.; Hu, Y.B.; Zhao, Z.H.; Yuan, S.Y. A tree competition index based on intersection angle. Scient. Silvae Sin. 2013, 49, 68–73. [Google Scholar] [CrossRef]
- Staupendahl, K.; Zucchini, W. Estimating the spatial distribution in forest stands by counting small angles between nearest neighbours. Allg. Forst Jagdztg. 2006, 177, 160–168. [Google Scholar]
- Assuncao, R. Testing spatial randomness by means of angles. Biometrics. 1994, 50, 531–537. [Google Scholar] [CrossRef]
- Davies, O.; Pommerening, A. The contribution of structural indices to the modelling of Sitka spruce (Picea sitchensis) and birch (Betula spp.) crowns. For. Ecol. Manag. 2008, 256, 68–77. [Google Scholar] [CrossRef]
- Tang, S.Z. Multivariate Statistical Analysis Methods; China Forestry Publish House: Beijing, China, 1986. [Google Scholar]
- Liu, F.; Tan, C.; Yang, Z.G.; Li, J.J.; Xiao, H.S.; Tong, Y. Regeneration and growth of tree seedlings and saplings in created gaps of different sizes in a subtropical secondary forest in southern China. For. Ecol. Manag. 2022, 511, 120143. [Google Scholar] [CrossRef]
- Guan, Y.Y.; Fei, F.; Guan, Q.W.; Chen, B. Research progress on forest gap ecology. Sci. Silvae Sin. 2016, 52, 91–99. [Google Scholar]
- Liu, Y.X.; Zeng, Y.; Wang, P.; He, J.; Li, P.P.; Liang, Y.J. The spatial pattern of Populus euphratica competition based on competitive exclusion theory. Front. Plant Sci. 2024, 15, 1276489. [Google Scholar] [CrossRef] [PubMed]
- Castagneri, D.; Vacchiano, G.; Lingua, E.; Motta, R. Analysis of intraspecific competition in two subalpine Norway spruce (Picea abies (L.) Karst.) stands in Paneveggio (Trento, Italy). For. Ecol. Manag. 2007, 255, 651–659. [Google Scholar] [CrossRef]
- Carr, S.; Larocque, G.R.; Luckai, N.J.; Bell, F.W. Effect of competition on individual white spruce production in young boreal mixedwood forests. Can. J. For. Res. 2020, 50, 726–735. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.H.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Osei, R.; Mao, Z.K.; Ye, J.; Lin, F.; Fang, S.; Wang, X.G.; Hao, Z.Q.; Ali, A. Determinants of tree population temporal stability in a temperate mixed forest over a gradient of nitrogen addition. J. Environ. Manag. 2024, 368, 122198. [Google Scholar] [CrossRef]
- Zou, A.L.; Li, X.P.; Ni, X.F.; Ji, C.J. Responses of tree growth to nitrogen addition in Quercus wutaishanica forests in Mt. Dongling, Beijing, China. Chin. J. Plant Ecol. 2019, 43, 783–792. [Google Scholar]
- Li, W.; Su, Y.Z.; Qin, Y.W.; Li, F.W.; Zhou, T.L.; He, Y.Q.; Mao, J.N.; Li, M.K.; Liu, C.; Shi, Y.F.; et al. Canopy nitrogen and water addition affect fine-root survival strategies and carbon allocation in a warm-temperate forest in China. For. Ecol. Manag. 2025, 590, 122791. [Google Scholar] [CrossRef]
- Liu, T.; Mao, P.; Shi, L.L.; Wang, Z.Y.; Wang, X.L.; He, X.X.; Tao, L.B.; Liu, Z.F.; Zhou, L.X.; Shao, Y.H.; et al. Contrasting effects of nitrogen deposition and increased precipitation on soil nematode communities in a temperate forest. Soil Biol. Biochem. 2020, 148, 107869. [Google Scholar] [CrossRef]
- Qiu, T.; James, S.C.; Kyle, R.K.; Philip, A.T.; Jennifer, J.S. Remotely sensed crown nutrient concentrations modulate forest reproduction across the contiguous United States. Ecology 2024, 105, e4366. [Google Scholar] [CrossRef]
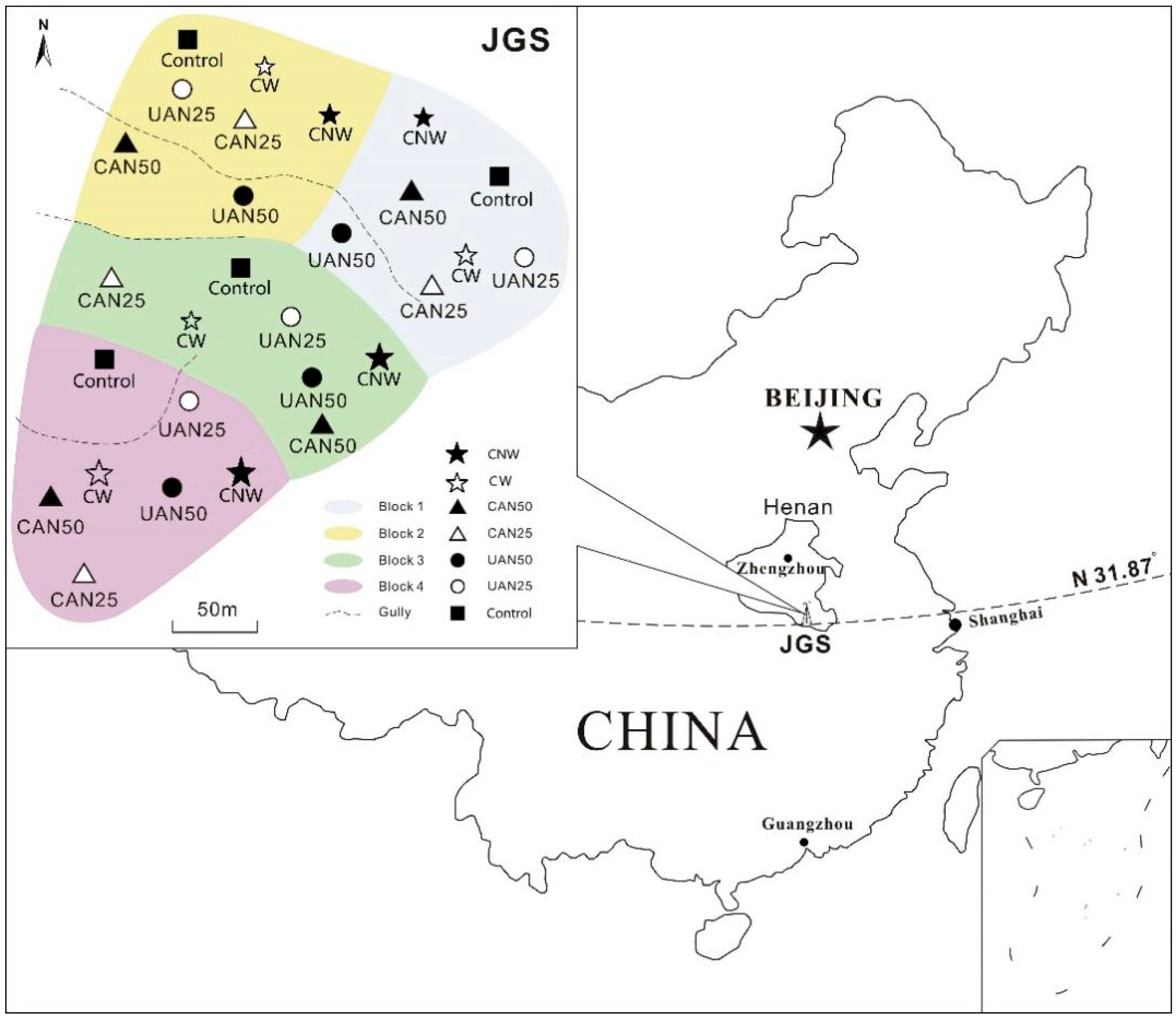
| Nitrogen Addition Treatments | Abbreviation | Nitrogen Addition Intensities/kg N ha−1 yr−1 | Nitrogen Addition Methods | Rainfall/mm ha−1 |
|---|---|---|---|---|
| Canopy low nitrogen addition | CAN 25 | 25 | canopy | 21 |
| Canopy high nitrogen addition | CAN 50 | 50 | canopy | 21 |
| Understory low nitrogen addition | UAN 25 | 25 | understory | 21 |
| Understory high nitrogen addition | UAN 50 | 50 | understory | 21 |
| Canopy water addition | CW | 0 | canopy | 330 |
| Canopy low nitrogen and water addition | CNW | 25 | canopy | 21 + 330 |
| Control | CK | 0 | / | 0 |
| Tree Species | Basal Area/m2ha−1 | Percent/% | Tree Number/Stems ha−1 | Percent/% | Surviving Trees Number/Stems ha−1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2022 | 2012 | 2022 | 2012 | 2022 | 2012 | 2022 | ||
| Q. acutissima | 10.37 | 11.65 | 36.72 | 35.15 | 88 | 85 | 5.34 | 3.91 | 82 |
| Q. variabilis | 8.44 | 9.71 | 29.89 | 29.30 | 83 | 81 | 5.04 | 3.73 | 79 |
| L. formosana | 4.90 | 6.58 | 17.36 | 19.87 | 354 | 410 | 21.49 | 18.86 | 256 |
| A. buergerianum | 0.85 | 1.32 | 3.02 | 3.98 | 296 | 471 | 17.97 | 21.67 | 135 |
| Q. aliena | 0.79 | 0.85 | 2.80 | 2.57 | 21 | 18 | 1.28 | 0.83 | 14 |
| Sophora japonica | 0.69 | 0.28 | 2.43 | 0.84 | 16 | 8 | 0.97 | 0.37 | 7 |
| C. sinensis | 0.53 | 0.85 | 1.88 | 2.57 | 246 | 371 | 14.94 | 17.07 | 111 |
| Pinus massoniana | 0.47 | 0.39 | 1.67 | 1.19 | 10 | 6 | 0.61 | 0.28 | 6 |
| Cerasus szechuanica | 0.17 | 0.29 | 0.59 | 0.88 | 48 | 48 | 2.91 | 2.21 | 35 |
| Lindera glauca | 0.14 | 0.26 | 0.51 | 0.77 | 166 | 306 | 10.08 | 14.08 | 58 |
| Vernicia fordii | 0.14 | 0.15 | 0.49 | 0.46 | 66 | 74 | 4.01 | 3.40 | 27 |
| Competitive Index | 2012 Year | 2022 Year | Annual Variation | |||
|---|---|---|---|---|---|---|
| p-Value | Significance | p-Value | Significance | p-Value | Significance | |
| CI | 0.4865 | 0.0262 | * | 0.1284 | ||
| CI1 | 0.3243 | 0.6166 | 0.2213 | |||
| CI2 | 0.1485 | 0.0136 | * | 0.1537 | ||
| mCI | 0.0151 | * | 0.0007 | *** | 0.3121 | |
| mCI1 | 0.2139 | 0.5918 | 0.3177 | |||
| mCI2 | 0.0003 | *** | 0.0014 | ** | 0.3003 | |
| Competitive Index | 2012 Year | 2022 Year | Annual Variation | |||
|---|---|---|---|---|---|---|
| p-Value | Significance | p-Value | Significance | p-Value | Significance | |
| CI | 0.0146 | * | 0.0225 | * | 0.0176 | * |
| CI1 | 0.7975 | 0.6845 | 0.0680 | . | ||
| CI2 | 0.0026 | ** | 0.0110 | * | 0.0097 | ** |
| mCI | 0.0003 | *** | 0.0006 | *** | 0.0230 | * |
| mCI1 | 0.6285 | 0.6999 | 0.0272 | * | ||
| mCI2 | 0.0002 | *** | 0.0012 | ** | 0.0153 | * |
| Competitive Index | L. formosana | Q. variabilis | Q. acutissima | |||
|---|---|---|---|---|---|---|
| p-Value | Significance | p-Value | Significance | p-Value | Significance | |
| CI | 0.3756 | 0.9659 | 0.4138 | |||
| CI1 | 0.0179 | * | 0.1423 | 0.5153 | ||
| CI2 | 0.2892 | 0.9775 | 0.7332 | |||
| mCI | 0.0008 | *** | 0.8312 | 0.0589 | . | |
| mCI1 | 0.0313 | * | 0.3573 | 0.1707 | ||
| mCI2 | 0.0008 | *** | 0.5460 | 0.8045 | ||
| Competitive Index | 2012 Year | 2022 Year | Annual Variation | |||
|---|---|---|---|---|---|---|
| p-Value | Significance | p-Value | Significance | p-Value | Significance | |
| CI | 0.5295 | 0.3642 | 0.9686 | |||
| CI1 | 0.6441 | 0.7642 | 0.2330 | |||
| CI2 | 0.6620 | 0.3987 | 0.9386 | |||
| mCI | 0.3248 | 0.0488 | * | 0.9007 | ||
| mCI1 | 0.4293 | 0.5893 | 0.1726 | |||
| mCI2 | 0.7569 | 0.2752 | 0.7941 | |||
| Competitive Index | 2012 Year | 2022 Year | Annual Variation | |||
|---|---|---|---|---|---|---|
| p-Value | Significance | p-Value | Significance | p-Value | Significance | |
| CI | 0.0300 | * | 0.2032 | 0.0037 | ** | |
| CI1 | 0.9038 | 0.8503 | 0.9399 | |||
| CI2 | 0.0084 | ** | 0.1573 | 0.0017 | ** | |
| mCI | 0.0014 | ** | 0.1250 | 0.0048 | ** | |
| mCI1 | 0.7482 | 0.7664 | 0.8489 | |||
| mCI2 | 0.0014 | ** | 0.1266 | 0.0034 | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, L.; Duan, G.; Yang, Y.; Fu, S.; Fu, L.; Ma, L.; Li, X.; Fu, J. The Effects of Nitrogen Deposition and Rainfall Enhancement on Intraspecific and Interspecific Competitive Patterns in Deciduous Broad-Leaved Forests. Forests 2025, 16, 1505. https://doi.org/10.3390/f16101505
Hong L, Duan G, Yang Y, Fu S, Fu L, Ma L, Li X, Fu J. The Effects of Nitrogen Deposition and Rainfall Enhancement on Intraspecific and Interspecific Competitive Patterns in Deciduous Broad-Leaved Forests. Forests. 2025; 16(10):1505. https://doi.org/10.3390/f16101505
Chicago/Turabian StyleHong, Liang, Guangshuang Duan, Yanhua Yang, Shenglei Fu, Liyong Fu, Lei Ma, Xiaowei Li, and Juemin Fu. 2025. "The Effects of Nitrogen Deposition and Rainfall Enhancement on Intraspecific and Interspecific Competitive Patterns in Deciduous Broad-Leaved Forests" Forests 16, no. 10: 1505. https://doi.org/10.3390/f16101505
APA StyleHong, L., Duan, G., Yang, Y., Fu, S., Fu, L., Ma, L., Li, X., & Fu, J. (2025). The Effects of Nitrogen Deposition and Rainfall Enhancement on Intraspecific and Interspecific Competitive Patterns in Deciduous Broad-Leaved Forests. Forests, 16(10), 1505. https://doi.org/10.3390/f16101505






