Genome-Wide Investigation and Expression Analysis of AP2 Gene Subfamily Reveals Its Evolution and Regulatory Role Under Salt Stress in Populus
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of PtAP2 Transcription Factors
2.2. Evolutionary Relationships, Gene Structure, and Conserved Motif Analysis
2.3. Chromosomal Locations and Collinearity in P. trichocarpa and Related Species
2.4. Cis-Elements and Functional Annotation
2.5. Assessment of the Expression Patterns of PtAP2 Genes Across Various Tissues
2.6. Plant Materials and Treatments
2.7. RNA Extraction and qRT-PCR
2.8. Yeast Heterologous Expression and Functional Validation of PtAP2s
3. Results and Analysis
3.1. Characterization of the PtAP2 Gene Family and Evaluation of Protein Physicochemical Properties
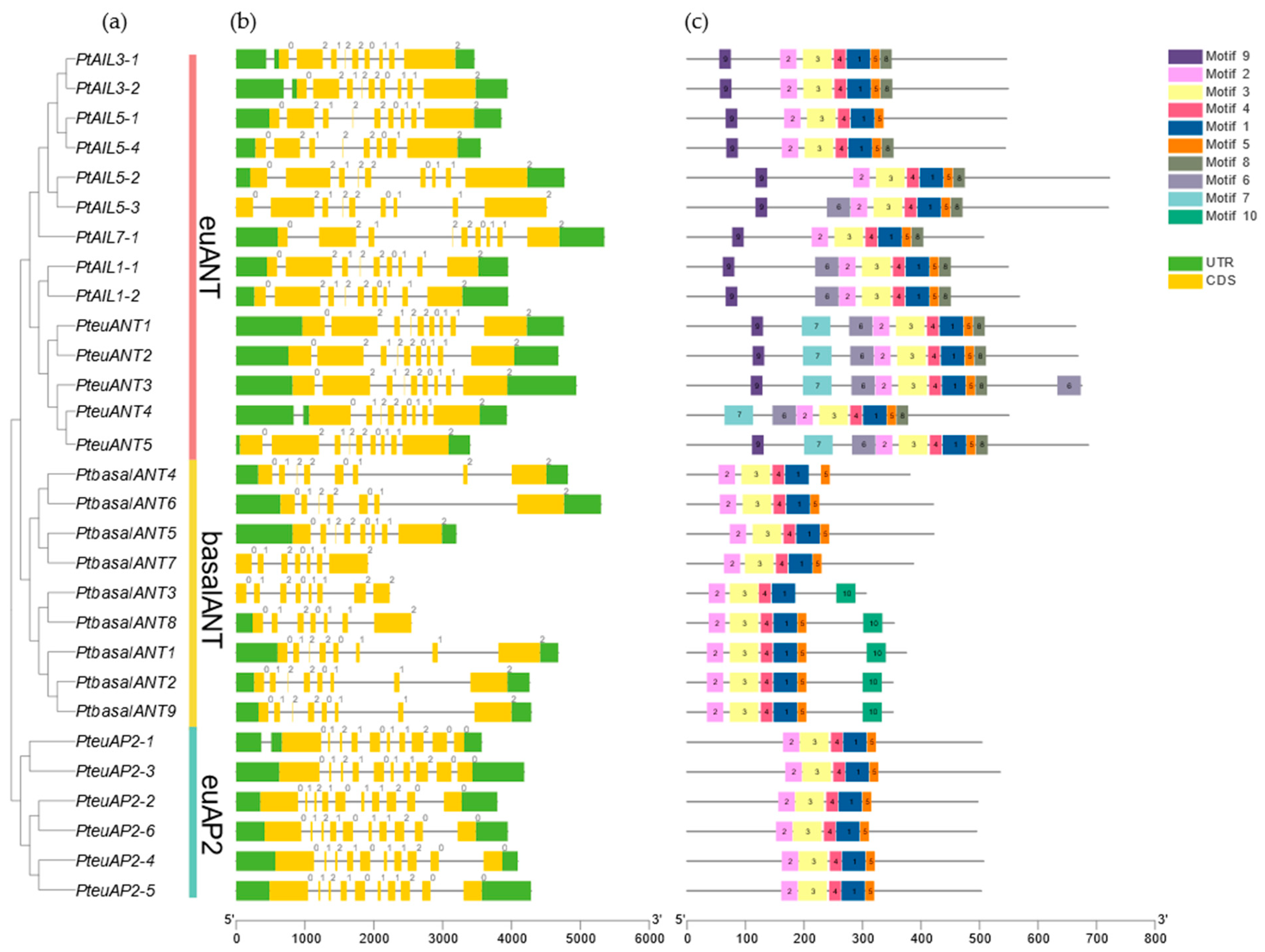
3.2. Phylogenetic Analysis of PtAP2s
3.3. The Analysis of PtAP2 Gene Structure and Motifs Was Conducted to Identify Key Features and Patterns
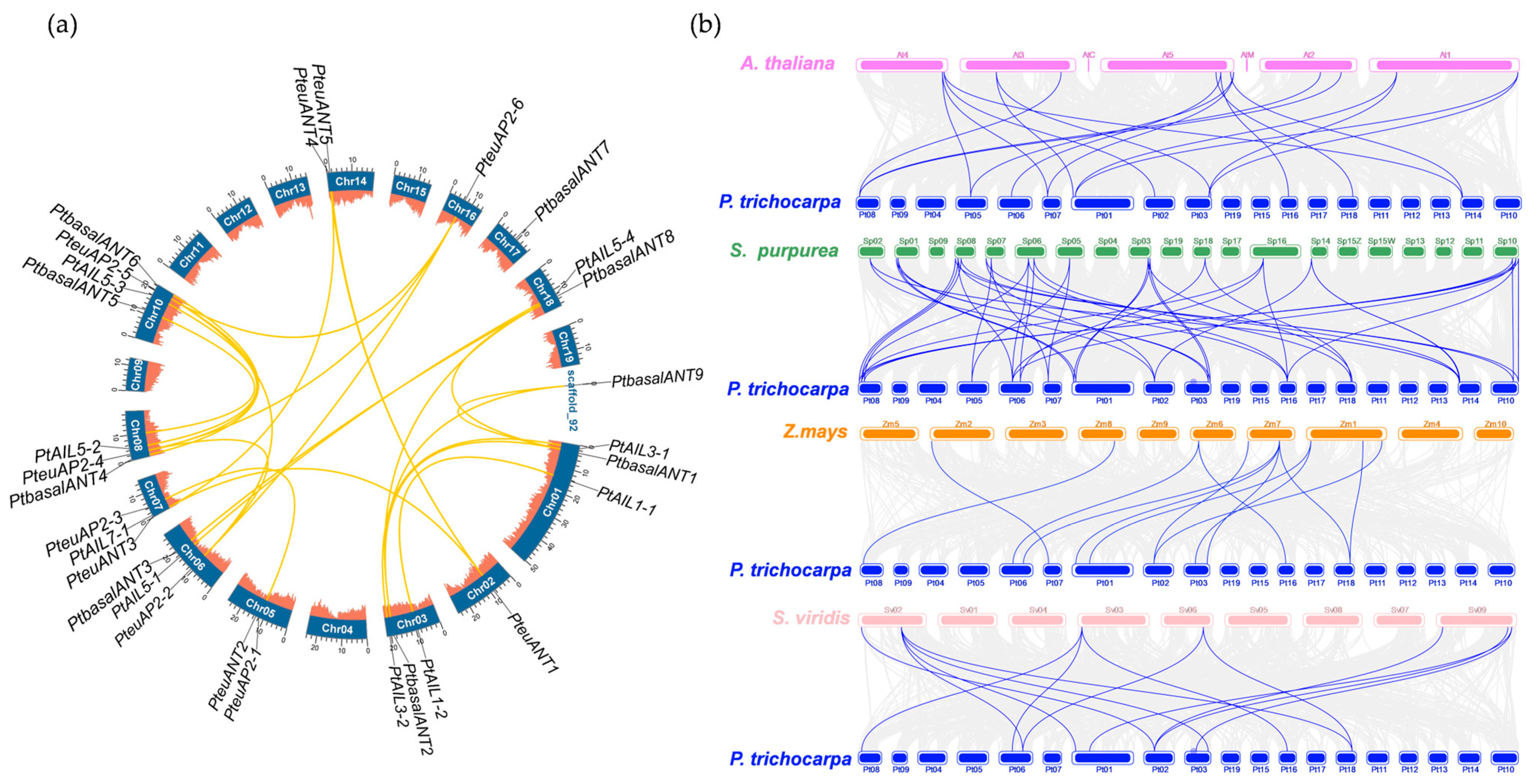
3.4. Chromosomal Localization and Collinearity of PtAP2 Genes
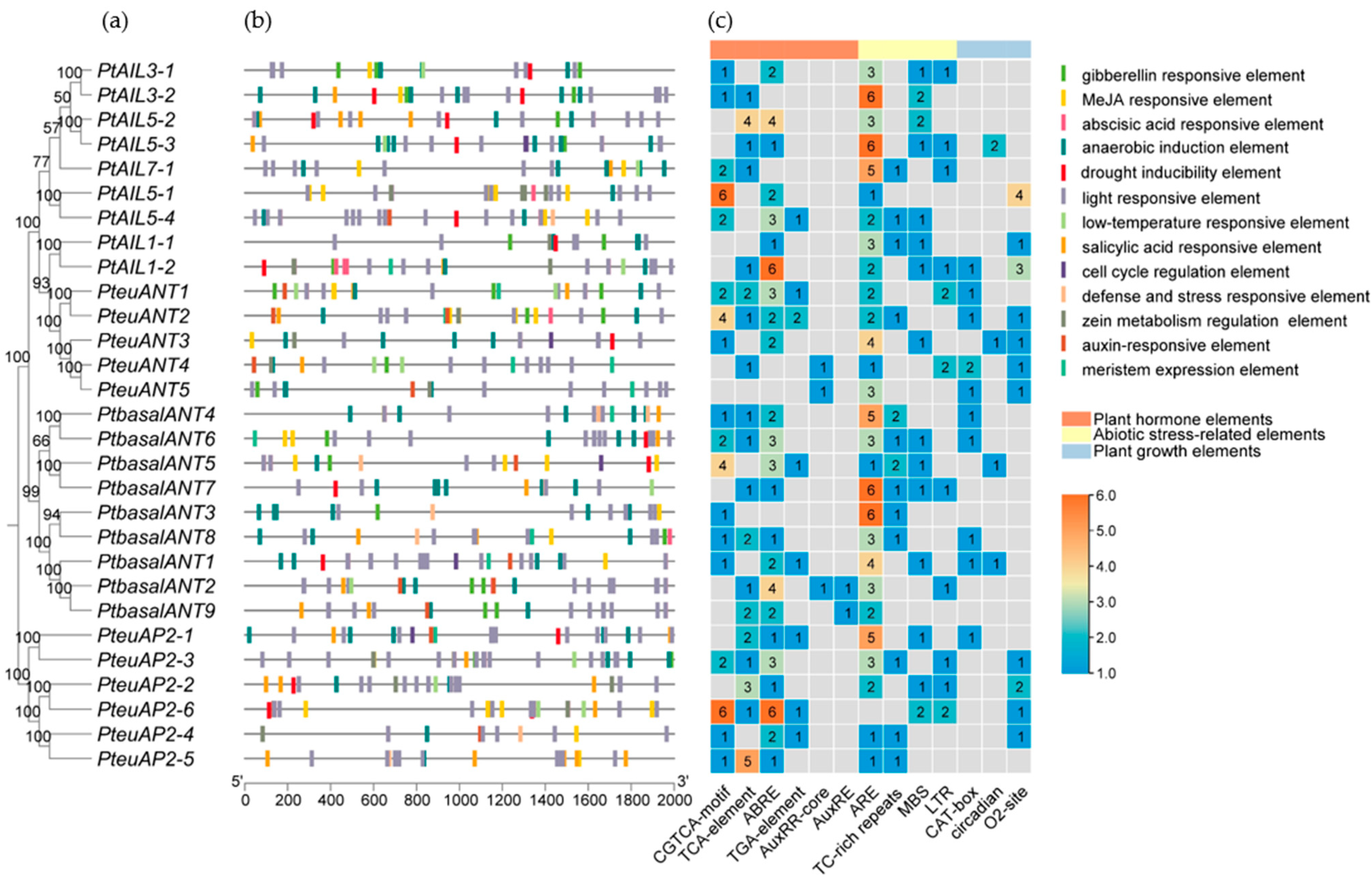
3.5. Analysis of Cis-Elements of PtAP2 Genes
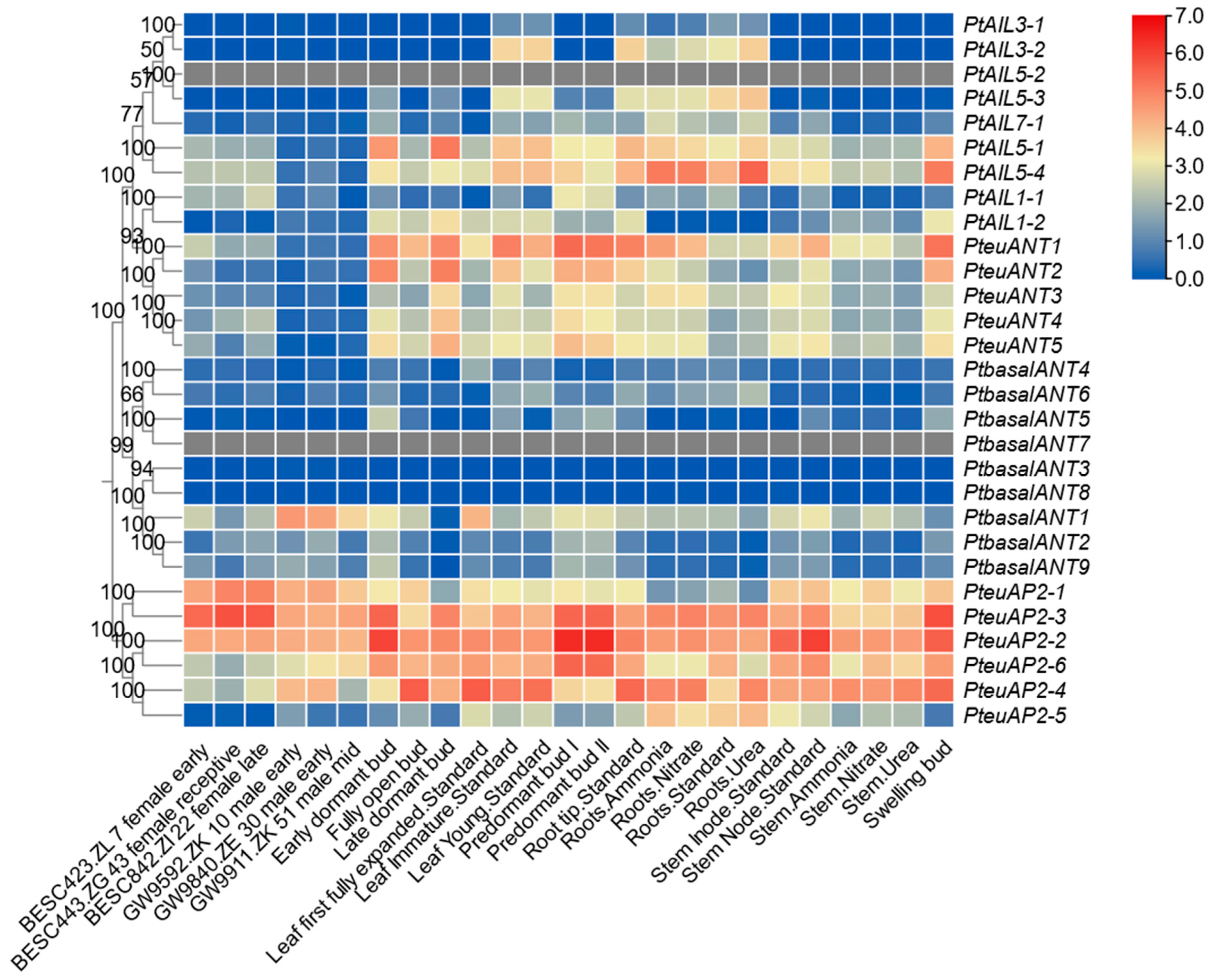
3.6. Analysis of the Expression Patterns of PtAP2 Genes in Various Tissues
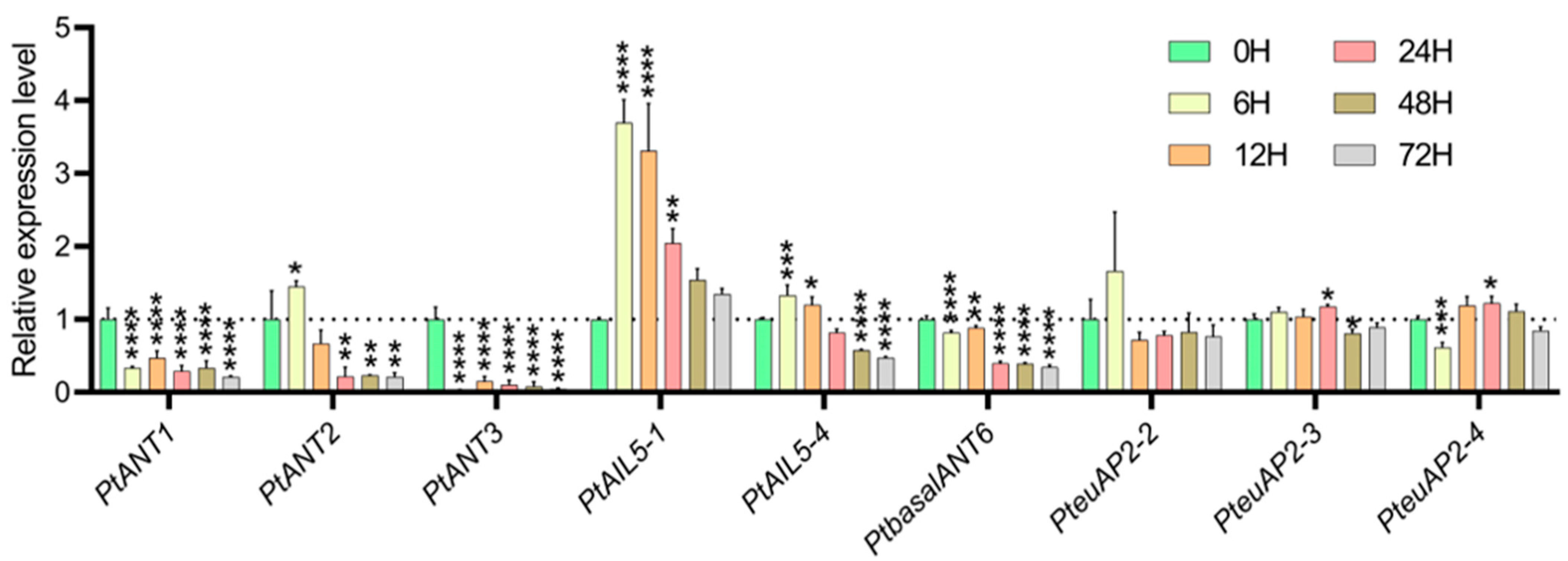
3.7. Analysis of the Expression Patterns of PtAP2 Genes Under NaCl Stress
3.8. PtAP2 Genes Improve Salt Sensitivity of Recombinant Saccharomyces cerevisiae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dietz, K.-J.; Vogel, M.O.; Viehhauser, A. AP2/EREBP Transcription Factors Are Part of Gene Regulatory Networks and Integrate Metabolic, Hormonal and Environmental Signals in Stress Acclimation and Retrograde Signalling. Protoplasma 2010, 245, 3–14. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.-L.; Xing, G.-M.; Liu, J.-X.; Duan, A.-Q.; Xu, Z.-S.; Li, M.-Y.; Zhuang, J.; Xiong, A.-S. Advances in AP2/ERF Super-Family Transcription Factors in Plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Soltis, P.S.; Wall, K.; Soltis, D.E. Phylogeny and Domain Evolution in the APETALA2-like Gene Family. Mol. Biol. Evol. 2006, 23, 107–120. [Google Scholar] [CrossRef]
- Jofuku, K.D.; BoerI, B.G.W.; den MontaguI, M.V.; Okamuro, J.K. Control of Arabidopsis Flower and Seed Development by the Homeotic Gene APETALA2. Plant Cell 1994, 6, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Wang, X.; Liu, J.; Guo, X.; Mu, J.; Tian, J.; Wang, X. Defective APETALA2 Genes Lead to Sepal Modification in Brassica Crops. Front. Plant Sci. 2018, 9, 367. [Google Scholar] [CrossRef]
- TIAN, M.; XU, Z.; LIU, X.; SUI, S.; LI, M.; LI, Z. Identification of the AP2 Subfamily Transcription Factors in Chimonanthus Praecox and the Functional Study of CpAP2-L11. Acta Hortic. Sin. 2023, 50, 382–396. [Google Scholar] [CrossRef]
- Jofuku, K.D.; Omidyar, P.K.; Gee, Z.; Okamuro, J.K. Control of Seed Mass and Seed Yield by the Floral Homeotic Gene APETALA2. Proc. Natl. Acad. Sci. USA 2005, 102, 3117–3122. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, S.; Ning, K.; Chen, Z.; Wang, Y.; Yang, J.; Wang, Q. LsAP2 Regulates Leaf Morphology by Inhibiting CIN-like TCP Transcription Factors and Repressing LsKAN2 in Lettuce. Hortic. Res. 2021, 8, 184. [Google Scholar] [CrossRef]
- Rigal, A.; Yordanov, Y.S.; Perrone, I.; Karlberg, A.; Tisserant, E.; Bellini, C.; Busov, V.B.; Martin, F.; Kohler, A.; Bhalerao, R.; et al. The AINTEGUMENTA LIKE1 Homeotic Transcription Factor PtAIL1 Controls the Formation of Adventitious Root Primordia in Poplar. Plant Physiol. 2012, 160, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Raha, S.; Tripathi, V.; Chattopadhyay, D. Expression of CAP2, an APETALA2-Family Transcription Factor from Chickpea, Enhances Growth and Tolerance to Dehydration and Salt Stress in Transgenic Tobacco. Plant Physiol. 2006, 142, 113–123. [Google Scholar] [CrossRef]
- Hawku, M.D.; Goher, F.; Islam, M.A.; Guo, J.; He, F.; Bai, X.; Yuan, P.; Kang, Z.; Guo, J. TaAP2-15, An AP2/ERF Transcription Factor, Is Positively Involved in Wheat Resistance to Puccinia striiformis f. Sp. Tritici. Int. J. Mol. Sci. 2021, 22, 2080. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-S.; Wang, Y.-B.; Yao, S.-Q.; Liu, A. Arabidopsis AINTEGUMENTA Mediates Salt Tolerance by Trans-Repressing SCABP8. J. Cell Sci. 2015, 128, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dai, Y.; Li, Y.; Yang, J.; Jiang, Y.; Liu, G.; Yu, C.; Zhong, F.; Lian, B.; Zhang, J. Overexpression of the Salix Matsudana SmAP2-17 Gene Improves Arabidopsis Salinity Tolerance by Enhancing the Expression of SOS3 and ABI5. BMC Plant Biol. 2022, 22, 102. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Li, D.; He, Y.; Li, S.; Shi, S.; Li, L.; Liu, Y.; Chen, H. Genome-Wide Characterization and Expression Analysis of AP2/ERF Genes in Eggplant (Solanum melongena L.). Plant Physiol. Biochem. 2021, 167, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, W.; Ma, Z.; Zheng, T.; Huang, L.; Wu, Q.; Zhao, G.; Tang, Z.; Bu, T.; Li, C.; et al. Genome-Wide Investigation of the AP2/ERF Gene Family in Tartary Buckwheat (Fagopyum tataricum). BMC Plant Biol. 2019, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Giorgi, F.M.; Zenoni, S.; Osti, F.; Pezzotti, M.; Perata, P. Genomic and Transcriptomic Analysis of the AP2/ERF Superfamily in Vitis Vinifera. BMC Genom. 2010, 11, 719. [Google Scholar] [CrossRef]
- Li, P.; Chai, Z.; Lin, P.; Huang, C.; Huang, G.; Xu, L.; Deng, Z.; Zhang, M.; Zhang, Y.; Zhao, X. Genome-Wide Identification and Expression Analysis of AP2/ERF Transcription Factors in Sugarcane (Saccharum spontaneum L.). BMC Genom. 2020, 21, 685. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang Shi, S.; Jiang, Y.; Zhong, F.; Liu, G.; Yu, C.; Lian, B.; Chen, Y. Genome-Wide Investigation of the AP2/ERF Superfamily and Their Expression under Salt Stress in Chinese Willow (Salix matsudana). PeerJ 2021, 9, e11076. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xiao, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in Understanding the Physiological and Molecular Responses of Populus to Salt Stress. Int. J. Mol. Sci. 2019, 20, 1312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Alexandre, G.; Christine, H.; Ivan, I.; Ron, D.A.; Amos, B. ExPASy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, J.; Ling, Q.; Xi, Y.; Qian, Y. Genome-Wide Identification and Expression Profiling Analysis of Maize AP2/ERF Superfamily Genes Reveal Essential Roles in Abiotic Stress Tolerance. BMC Genom. 2022, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Koressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating Masking of Template Sequence with Primer Design Software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthou, S.; Tang, Q.; Lee, J.; Taylor, S.J.; Zhao, Y.; Steidl, U.; Zheng, D.; Dawlaty, M.M. The DNA Dioxygenase Tet1 Regulates H3K27 Modification and Embryonic Stem Cell Biology Independent of Its Catalytic Activity. Nucleic Acids Res. 2022, 50, 3169–3189. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Zou, X.; Mu, D.; Li, H.; Zang, D.; Wang, Y. Expression of Ethylene Response Factors (ERFs) from Betula platyphylla and the Confer Salt and Drought Tolerance Analysis in a Yeast. J. Plant Biochem. Biotechnol. 2017, 26, 35–42. [Google Scholar] [CrossRef]
- McLeay, R.C.; Bailey, T.L. Motif Enrichment Analysis: A Unified Framework and an Evaluation on ChIP Data. BMC Bioinform. 2010, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Fan, P.; Liu, H.; Tan, P.; Ma, W.; Mo, Z.; Zhao, J.; Chu, G.; Peng, F. Insight into the CBL and CIPK Gene Families in Pecan (Carya illinoinensis): Identification, Evolution and Expression Patterns in Drought Response. BMC Plant Biol. 2022, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. Cis-Regulatory Elements in Plant Development, Adaptation, and Evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef]
- Kim, J.-S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE Promoter Sequence Is Involved in Osmotic Stress-Responsive Expression of the DREB2A Gene, Which Encodes a Transcription Factor Regulating Drought-Inducible Genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Y.; Chen, W.; Xu, M.; Zhou, R.; Shou, H.; Chen, J. High-Resolution Anatomical and Spatial Transcriptome Analyses Reveal Two Types of Meristematic Cell Pools within the Secondary Vascular Tissue of Poplar Stem. Mol. Plant 2023, 16, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Cai, B.; Peng, R.-H.; Zhu, B.; Jin, X.-F.; Xue, Y.; Gao, F.; Fu, X.-Y.; Tian, Y.-S.; Zhao, W.; et al. Genome-Wide Analysis of the AP2/ERF Gene Family in Populus trichocarpa. Biochem. Biophys. Res. Commun. 2008, 371, 468–474. [Google Scholar] [CrossRef]
- Okamuro, J.K.; Caster, B.; Villarroel, R.; Van Montagu, M.; Jofuku, K.D. The AP2 Domain of APETALA2 Defines a Large New Family of DNA Binding Proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 7076–7081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jin, H.; Chen, J.; Li, C.; Wang, J.; Luo, J.; Wang, Z. Identification and Analysis of the AP2 Subfamily Transcription Factors in the Pecan (Carya illinoinensis). Int. J. Mol. Sci 2021, 22, 13568. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, R.; Xu, D.; Bi, H.; Xia, Z.; Peng, H. Genome-Wide Identification and Analysis of the AP2 Transcription Factor Gene Family in Wheat (Triticum aestivum L.). Front. Plant Sci. 2019, 10, 1286. [Google Scholar] [CrossRef]
- Lian, H.; Wang, L.; Ma, N.; Zhou, C.-M.; Han, L.; Zhang, T.-Q.; Wang, J.-W. Redundant and Specific Roles of Individual MIR172 Genes in Plant Development. PLoS Biol. 2021, 19, e3001044. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of Duplicate Genes in Exon–Intron Structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Z.; Hu, G.; Yao, X. Genome-Wide Identification and Characterization of AP2/ERF Gene Superfamily during Flower Development in Actinidia eriantha. BMC Genom. 2022, 23, 650. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Genome-Wide Survey and Expression Analysis of the Plant-Specific NAC Transcription Factor Family in Soybean during Development and Dehydration Stress. DNA Res. 2011, 18, 263–276. [Google Scholar] [CrossRef]
- Liu, H.; Lyu, H.-M.; Zhu, K.; Van de Peer, Y.; (Max) Cheng, Z.-M. The Emergence and Evolution of Intron-Poor and Intronless Genes in Intron-Rich Plant Gene Families. Plant J. 2021, 105, 1072–1082. [Google Scholar] [CrossRef]
- Panchy, N.L.; Azodi, C.B.; Winship, E.F.; O’Malley, R.C.; Shiu, S.-H. Expression and Regulatory Asymmetry of Retained Arabidopsis thaliana Transcription Factor Genes Derived from Whole Genome Duplication. BMC Evol. Biol. 2019, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Zou, C.; Lehti-Shiu, M.D.; Shinozaki, K.; Shiu, S.-H. Importance of Lineage-Specific Expansion of Plant Tandem Duplicates in the Adaptive Response to Environmental Stimuli. Plant Physiol. 2008, 148, 993–1003. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Wang, C.; Li, X.; Man, Y.; Ruan, H.; Zhao, Y. Genome-Wide Analysis of the Populus trichocarpa Laccase Gene Family and Functional Identification of PtrLAC23. Front. Plant Sci. 2023, 13, 1063813. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Yu, J.; Huang, S.; Zhang, Y.; Wei, H.; Wei, Z. Genome-Wide Identification and Characterization of Auxin Response Factor (ARF) Gene Family Involved in Wood Formation and Response to Exogenous Hormone Treatment in Populus trichocarpa. Int. J. Mol. Sci. 2023, 24, 740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Chen, Y.; Huang, M.; Zhu, S. Comprehensive Genome-Wide Analyses of Poplar R2R3-MYB Transcription Factors and Tissue-Specific Expression Patterns under Drought Stress. Int. J. Mol. Sci. 2023, 24, 5389. [Google Scholar] [CrossRef] [PubMed]
- Nole-Wilson, S.; Tranby, T.L.; Krizek, B.A. AINTEGUMENTA-like (AIL) Genes Are Expressed in Young Tissues and May Specify Meristematic or Division-Competent States. Plant Mol. Biol. 2005, 57, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Lin, H.-H.; Yu, C.-P.; Chang, C.-K.; Chen, H.-J.; Lin, J.-J.; Lu, M.-Y.J.; Tu, S.-L.; Shiu, S.-H.; Wu, S.-H.; et al. Maize ANT1 Modulates Vascular Development, Chloroplast Development, Photosynthesis, and Plant Growth. Proc. Natl. Acad. Sci. USA 2020, 117, 21747–21756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Cho, J.-I.; Han, M.; Ahn, C.-H.; Jeon, J.-S.; An, G.; Park, P.B. The ABRE-Binding bZIP Transcription Factor OsABF2 Is a Positive Regulator of Abiotic Stress and ABA Signaling in Rice. J. Plant Physiol. 2010, 167, 1512–1520. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Jun, M.; Kong, A.-N.T. Nrf2: A Potential Molecular Target for Cancer Chemoprevention by Natural Compounds. Antioxid. Redox Signal. 2006, 8, 99–106. [Google Scholar] [CrossRef]
- Scholes, A.N.; Lewis, J.A. Comparison of RNA Isolation Methods on RNA-Seq: Implications for Differential Expression and Meta-Analyses. BMC Genom. 2020, 21, 249. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, K.; Tian, Y.; Han, K.; El-Kassaby, Y.A.; Yang, H.; Si, H.; Sun, Y.; Li, Y. Time-Course Transcriptomics Analysis Reveals Key Responses of Populus to Salt Stress. Ind. Crops Prod. 2023, 194, 116278. [Google Scholar] [CrossRef]
- Gao, L.-W.; Yang, S.-L.; Wei, S.-W.; Huang, D.-F.; Zhang, Y.-D. Supportive Role of the Na+ Transporter CmHKT1;1 from Cucumis Melo in Transgenic Arabidopsis Salt Tolerance through Improved K+/Na+ Balance. Plant Mol. Biol. 2020, 103, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zhou, M.; Tang, T.; Hassan, M.J.; Zhou, J.; Tan, M.; Li, Z.; Peng, Y. A Trifolium Repens Flavodoxin-like Quinone Reductase 1 (TrFQR1) Improves Plant Adaptability to High Temperature Associated with Oxidative Homeostasis and Lipids Remodeling. Plant J. 2023, 115, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Li, H.; Wang, Y.; Zhang, Y.; Wood, A.J. EsDREB2B, a Novel Truncated DREB2-Type Transcription Factor in the Desert Legume Eremosparton songoricum, Enhances Tolerance to Multiple Abiotic Stresses in Yeast and Transgenic Tobacco. BMC Plant Biol. 2014, 14, 44. [Google Scholar] [CrossRef]
- Ma, J.; Wang, R.; Zhao, H.; Li, L.; Zeng, F.; Wang, Y.; Chen, M.; Chang, J.; He, G.; Yang, G.; et al. Genome-Wide Characterization of the VQ Genes in Triticeae and Their Functionalization Driven by Polyploidization and Gene Duplication Events in Wheat. Int. J. Biol. Macromol. 2023, 243, 125264. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Song, X.; Yue, W.; Liu, L.; Ge, F.; Wang, J. Identification and Functional Characterization of Abiotic Stress Tolerance-Related PLATZ Transcription Factor Family in Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2024, 25, 10191. [Google Scholar] [CrossRef] [PubMed]


| Gene Accession Number | Gene Name | Gene | Protein | Clade | ||||
|---|---|---|---|---|---|---|---|---|
| Chromosome Location | CDS (bp) | Size (aa) | MW (kDa) | pI | Subcellular Localization | |||
| Potri.001G169500 | PtAIL1-1 | Chr01:4692443..14305089 | 1647 | 549 | 60.66 | 6.50 | nucleus | euANT |
| Potri.003G064700 | PtAIL1-2 | Chr03:8039191..9350664 | 1704 | 568 | 63.03 | 8.44 | nucleus | |
| Potri.001G018400 | PtAIL3-1 | Chr01:1345695..1349157 | 1638 | 546 | 60.39 | 6.18 | nucleus | |
| Potri.003G205700 | PtAIL3-2 | Chr03:11385939..20649330 | 1647 | 549 | 60.57 | 6.20 | nucleus | |
| Potri.006G167700 | PtAIL5-1 | Chr06:18079435..17286293 | 1638 | 546 | 59.52 | 8.18 | nucleus | |
| Potri.008G076400 | PtAIL5-2 | Chr08:29793053..4764074 | 2166 | 722 | 79.23 | 6.00 | nucleus | |
| Potri.010G181000 | PtAIL5-3 | Chr10:33139801..17939215 | 2160 | 720 | 78.66 | 6.05 | nucleus | |
| Potri.018G091600 | PtAIL5-4 | Chr18:44853419..12089067 | 1632 | 544 | 59.22 | 6.97 | nucleus | |
| Potri.007G011600 | PtAIL7-1 | Chr07:23099557..924669 | 1521 | 507 | 55.60 | 5.90 | nucleus | |
| Potri.002G114800 | PteuANT1 | Chr02:6365817..8647364 | 1992 | 664 | 73.05 | 6.47 | nucleus | |
| Potri.005G148400 | PteuANT2 | Chr05:14732687..13163410 | 2004 | 668 | 73.26 | 6.93 | nucleus | |
| Potri.007G007400 | PteuANT3 | Chr07:21426183..520362 | 2028 | 676 | 74.80 | 6.52 | nucleus | |
| Potri.014G008100 | PteuANT4 | Chr14:38159923..839194 | 1650 | 550 | 61.15 | 7.08 | nucleus | |
| Potri.014G012200 | PteuANT5 | Chr14:39833297..1269003 | 2058 | 686 | 75.94 | 6.35 | nucleus | |
| Potri.001G041500 | PtbasalANT1 | Chr01:3019069..3023748 | 1125 | 375 | 42.34 | 6.52 | chloroplast | basalANT |
| Potri.003G185300 | PtbasalANT2 | Chr03:9712565..19101511 | 1056 | 352 | 39.71 | 7.14 | chloroplast | |
| Potri.006G179900 | PtbasalANT3 | Chr06:19752809..19412247 | 918 | 306 | 34.85 | 8.72 | chloroplast | |
| Potri.008G011900 | PtbasalANT4 | Chr08:26446305..664128 | 1143 | 381 | 40.61 | 5.91 | chloroplast | |
| Potri.010G092800 | PtbasalANT5 | Chr10:31466427..11580707 | 1266 | 422 | 47.69 | 8.69 | nucleus | |
| Potri.010G247200 | PtbasalANT6 | Chr10:36486549..22108246 | 1263 | 421 | 46.68 | 5.59 | chloroplast | |
| Potri.017G078600 | PtbasalANT7 | Chr17:43180045..9177502 | 1161 | 387 | 43.74 | 8.22 | nucleus | |
| Potri.018G102200 | PtbasalANT8 | Chr18:46526793..12967019 | 1062 | 354 | 39.87 | 6.67 | chloroplast | |
| Potri.T081332 | PtbasalANT9 | scaffold92:48200167..82174 | 1056 | 352 | 39.71 | 7.14 | chloroplast | |
| Potri.005G140700 | PteuAP2-1 | Chr05:13059313..11809464 | 1512 | 504 | 55.92 | 6.48 | nucleus | euAP2 |
| Potri.006G132400 | PteuAP2-2 | Chr06:16406061..10884850 | 1491 | 497 | 54.48 | 6.34 | nucleus | |
| Potri.007G046200 | PteuAP2-3 | Chr07:24772931..4214823 | 1605 | 535 | 59.51 | 6.75 | nucleus | |
| Potri.008G045300 | PteuAP2-4 | Chr08:28119679..2606980 | 1521 | 507 | 56.14 | 6.84 | nucleus | |
| Potri.010G216200 | PteuAP2-5 | Chr10:34813175..20337268 | 1512 | 504 | 55.32 | 6.40 | nucleus | |
| Potri.016G084500 | PteuAP2-6 | Chr16:41506671..6602171 | 1485 | 495 | 53.96 | 8.33 | nucleus | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liang, X.; Hao, Z.; Feng, Q.; Li, H.; Liu, Y. Genome-Wide Investigation and Expression Analysis of AP2 Gene Subfamily Reveals Its Evolution and Regulatory Role Under Salt Stress in Populus. Forests 2025, 16, 94. https://doi.org/10.3390/f16010094
Wang Z, Liang X, Hao Z, Feng Q, Li H, Liu Y. Genome-Wide Investigation and Expression Analysis of AP2 Gene Subfamily Reveals Its Evolution and Regulatory Role Under Salt Stress in Populus. Forests. 2025; 16(1):94. https://doi.org/10.3390/f16010094
Chicago/Turabian StyleWang, Zhengbo, Xiaolin Liang, Ziyuan Hao, Qi Feng, Hongying Li, and Yingli Liu. 2025. "Genome-Wide Investigation and Expression Analysis of AP2 Gene Subfamily Reveals Its Evolution and Regulatory Role Under Salt Stress in Populus" Forests 16, no. 1: 94. https://doi.org/10.3390/f16010094
APA StyleWang, Z., Liang, X., Hao, Z., Feng, Q., Li, H., & Liu, Y. (2025). Genome-Wide Investigation and Expression Analysis of AP2 Gene Subfamily Reveals Its Evolution and Regulatory Role Under Salt Stress in Populus. Forests, 16(1), 94. https://doi.org/10.3390/f16010094





