Abstract
With global warming, understanding the effect of elevated temperature on the decomposition of Chinese fir needle litter has significant implications for nutrient cycling, yield, and management of economically important Chinese fir plantations. We conducted simulated warming decomposition experiments in incubators at 25 °C, 30 °C, and 35 °C on Chinese fir needle litter from middle-aged, mature, and overmature stands. Changes in litter mass and concentrations of some metallic elements and recalcitrant components were measured in litter sampled at different decomposition time-steps up to 264 days (d). Warming to 35 °C significantly increased the mass loss rate of needle litter from overmature stands throughout the experiment (except at 72 d). The effect of warming on litter mass loss rate for middle-aged and mature stands was lower and is attributed to higher litter quality in these stands. Compared to 25 °C, warming to 30 °C and 35 °C increased the needle litter decomposition rate across all developmental stages by 17.3% and 48.3%, respectively. Potassium (K), calcium (Ca), and magnesium (Mg) were mostly released during needle litter decomposition in all Chinese fir developmental stages. Lignin, condensed tannins, total phenols, and cellulose were enriched in needle litter, while the release of hemicellulose from near the start of the decomposition experiment was attributed to its lower molecular weight compared with other carbohydrates in litter. Compared with 25 °C, warming to 35 °C increased the release rates from litter of K, Ca, and Mg by 14.7%, 24.6%, and 21.5%, and the release rates of lignin, total phenols, cellulose, and hemicellulose by 7.5%, 8.8%, 10.4%, and 13.7%. Needle litter iron (Fe), aluminum (Al), and sodium (Na) in different development stages and manganese (Mn) in the overmature stands were mostly enriched during the experiment. Warming significantly promoted the enrichment of Fe, Al (except for mature stands), and Na, and reduced the enrichment of Mn. In summary, the sensitivity of needle litter to temperature in overmature stands is higher than that in middle-aged and mature stands, suggesting that forest managers can extend the rotation length of Chinese fir plantations to increase the yield of large-diameter timber, litter decomposition, and ecosystem nutrient return.
1. Introduction
Forest litter is an important part of forest productivity and one of the critical links between nutrient cycling and energy flow in the plant–decomposer–soil continuum [1]. Litter decomposition is a key process in the global carbon (C) budget and nutrient dynamics [1], and plays an important feedback role between climate change and global biochemical cycles [2]. Litter decomposition is primarily governed by the interplay between biological and abiotic factors, in particular temperature that directly affects microbial activity and indirectly affects litter chemistry by inducing morphological and physiological changes in plants [3,4].
In the past decade, the response of litter decomposition to warming has been widely studied in terrestrial ecosystems using in situ altitudinal and latitudinal temperature gradients, as well as field heating methods [5,6,7,8]. These studies showed positive, negative, or neutral effects of warming on leaf decomposition. For example, in warm humid ecosystems, where moisture is not limiting, warming accelerated the decomposition of litter from Schima superba and Machilus breviflora, when the litter was translocated from a high-elevation site to a low-elevation site in subtropical forests of China [5]. Conversely, the decrease of litter mass loss rate by 23% in a semiarid shrubland was closely related to warming, which reduced the litter moisture content and microbial biomass [6]. Additionally, litter mass loss in boreal ecosystems exposed to experimental warming was either unchanged [7] or decreased [8], which was partly ascribed to a decline in litter moisture with warming. Therefore, the response of litter decomposition rate to warming varies with plant species, ecosystems, and experimental conditions. The effect of warming on litter decomposition rate is ambiguous and needs further exploration.
More than 90% of nitrogen (N) and phosphorus (P) and more than 60% of metallic elements absorbed by forest growth come from the recycling of elements returned to the soil by forest litter decomposition [9]. Although the demand for metallic elements in plants is not as high as that of N and P, metallic elements are key for regulating plant growth and metabolism [10]. For instance, potassium (K) is vital for maintaining cellular homeostasis, calcium (Ca) is a crucial intracellular signaling molecule, magnesium (Mg) is fundamental for chlorophyll synthesis, iron (Fe) and manganese (Mn) are indispensable for photosynthesis, aluminum (Al) is closely associated with root physiology, and sodium (Na) participates in regulating cell osmosis [11,12]. Whilst K, Ca, and Mg are essential plant nutrients that are crucial to plant physiology, Fe, Al, and Mn can have toxic effects when they exceed certain levels [9]. Litter comprises readily decomposable inorganic components (such as K, Ca, Mg) and relatively recalcitrant organic fractions (such as lignin, cellulose, hemicellulose, polyphenols) [13]. During initial decomposition, approximately 30% of litter mass loss is attributed to the degradation of water-soluble and slightly unstable organic components, whereas lignin and cellulose, being more recalcitrant, significantly influence the later stages of decomposition [14]. Studies indicate that lignin in conjunction with hemicellulose, proteins, and cellulose, forms a cellular wall “barrier” or cross-linked structures, hindering litter decomposition [15]. Additionally, the condensed tannins can form protein/cellulose precipitates or oxidize into difficult-to-decompose complexes [16], which have a toxicological effect on soil organisms involved in litter degradation and inhibit litter decomposition [17]. While much research has focused on the effects of warming on the release of C, N, and P from litter [18,19], studies exploring the dynamics of metallic elements and recalcitrant components during litter decomposition under warming conditions remain scarce, limiting our full understanding of the effect of global climate change on litter decomposition and in turn of forest ecosystems.
Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) is one of the most important timber tree species in China, accounting for 60%–80% of the total area of timber plantations in southeast China and 20%–25% of the national commercial timber output [20]. The timber of Chinese fir is straight and decay-resistant and has a long history as an important construction and furniture material in China. Chinese fir litter comprises needles, branches, flowers, and fruits, of which needles account for the largest proportion (ca. 50%) of total litter production [21]. To date, research on Chinese fir litter has mainly addressed the effects of thinning [22], nitrogen deposition [23], and tree species mixing [24] on litter decomposition. Studies of the effect of warming on litter decomposition in Chinese fir plantations have focused on the release of C, N, and P [21]. However, changes in the metallic elements and recalcitrant components during litter decomposition under warming conditions have not been reported to the best of our knowledge. Nevertheless, understanding the mechanisms by which warming affects litter decomposition in Chinese fir plantations is significant for maintaining the productivity of Chinese fir plantations.
To address this research gap, we investigated the effects of simulated temperatures (25 °C, 30 °C, and 35 °C) on the decomposition rate and release of metallic elements and recalcitrant substances from Chinese fir needle litter from middle-aged, mature, and overmature stands, under equivalent soil and moisture conditions. We hypothesized that, for Chinese fir needle litter at different developmental stages, warming significantly: (1) enhances the litter decomposition rate; (2) promotes the release of metallic elements; (3) accelerates the release of recalcitrant components.
2. Materials and Methods
2.1. Site Description
The materials for this study were obtained from Xinkou National Forest Farm (26°07′–27°13′ N, 117°27′–118°14′ E), Sanming City, Fujian province, China. The region is characterized by a humid subtropical climate (Cfa in the Köppen climate classification). In 2020, the mean annual air temperature was 24 °C, with mean monthly minimum and maximum temperatures of 14 °C and 36 °C, and an annual precipitation of 1700 mm (75% of annual rainfall occurs from March to August). The mean annual evaporation is 1585 mm, the relative humidity is 81.0%, and the frost-free period is 240–300 days. The soil is classified as Silty Oxisol (according to USDA Taxonomy), developed on sandstone and shale parent material [25].
The study area was originally covered by broad-leaved forests that have now been replaced by fast-growing Chinese fir plantations with a short rotation of typically 20–30 years [25]. Three Chinese fir plantations were established in 2003, 1991 and 1979, with ages of 18, 30, and 42 years old, respectively, at the time of our study, and representing middle, mature, and overmature stands, respectively. The location and layout of the different-aged Chinese fir plantations are shown in the Supplementary Material Figure S1. The 18- and 30-year-old stands were located on south-facing slopes, while the 42-year-old stand was on a north-facing slope, all at elevations of ca. 210 m a.s.l. All stands were first rotations, established from seedlings planted in hand-dug holes after clear-cutting and burning of the original forest. In 2012, the 18-year-old stand was still at the initial planting density, but the 30- and 42-year-old stands had been thinned following a regime similar to that prescribed by the Fujian province local standard for thinning conifers. The 18-, 30-, and 42-year-old stands had densities of 2400, 1650, and 1300 stems ha−1, respectively. The understory vegetation of the stands was composed of similar shrubs and herbs, including Callicarpa kochiana, Maesa japonica, and Ilex pubescens, which dominated the shrub layer, and Woodwardia japonica, Selaginella moellendor, and Alpinia japonica, which dominated the herb layer.
Soil properties at 0–20 cm depth were determined in the stands at different developmental stages. Soil pH, measured with a precision pH meter (Starter21003C, OHAUS, Parsippany, NJ, USA) in a 2.5:1 water/soil ratio suspension, ranged from 4.43 to 4.62 at different developmental stages. Soil organic matter content was measured using the Walkley–Black K2Cr2O7-H2SO4 wet oxidation method, yielding values from 35.3 to 42.4 g kg−1. Total C and N concentrations (g kg−1) were determined using an Elementar VARIO Micro Cube elemental analyzer (VARIO MAX CN, Elementar, Frankfurt, HE, Germany), and soil C/N ratios ranged from 10.9 to 13.1. Soil bulk density values, determined from oven-drying soil cores at 105–110 °C, were 1.28–1.37 g cm−3 [26].
2.2. Litter Collection and Experimental Design
Within each Chinese fir stand at different development stages, three 20 m × 20 m sampling plots were established, each of which contained 10 randomly located litter traps that had been established for previous studies, such as [26]. The litter traps comprised 1 mm nylon mesh netting stretched across wooden frames and were located at 50 cm above the ground surface so that litter sampled was predominantly from Chinese fir. For this study, the litter traps were prepared and litter was collected from 1 July to 1 October 2021. The study area is often affected by typhoons at this time of year, resulting in high litterfall production. Litter samples from the 10 litter traps within each of the three 20 m × 20 m plots in each stand were mixed to create a composite sample so that there were three litter replicates for each Chinese fir development stage. The composite litter samples were taken to the laboratory, where bark, excess debris, fresh leaves, and leaves that had begun to decompose were removed, so that the litter retained for the incubation experiments contained only Chinese fir needles and twigs. Some of this litter material was analyzed to determine the initial litter dry mass and composition at the start of the decomposition experiment (day 0).
The litter decomposition experiment was conducted in custom-made cuboid decomposition boxes (17 cm long, 11 cm wide, 5 cm deep, surface area 0.0187 m2), each containing 200 g of surface soil (0–20 cm depth) that was randomly sampled for each of the three 20 m × 20 m plots in each stand of the different development stages on the same day in October 2021 when the litter traps were emptied. The surface soil samples were matched to the composite litter sample for that sampling plot. Our previous investigation found the mean annual yield of dry mass of Chinese fir needle at different developmental stages was 118.58–206.97 g m−2 [27], so 5 g fresh needle litter, a slightly higher needle mass weighted for surface area, was placed in each decomposition box. The soil and needle litter in the boxes were separated by a 1 mm nylon mesh to facilitate the collection of needle litter for analysis at different decomposition times. The design of the decomposition experiment comprised needle litter from the three different Chinese fir developmental stages (middle-aged, mature, overmature) in plant incubators (BPC500-2H, Fujian Jubo Biotechnology Co., Fuzhou, China) incubated at 25 °C, 30 °C, and 35 °C and for different lengths of time. These temperatures were selected to encompass the mean annual average and mean monthly maximum air temperatures in the study area in 2020. The incubator has an air-exchange and circulation system that automatically regulates the incubator environment to maintain a stable oxygen content and ca. 80% humidity throughout the experiment. In order to maintain a 60% water-holding capacity throughout the incubation, soil moisture content was checked by weighing the decomposition boxes every 3–5 days and adjusted by adding distilled water when necessary. At 15, 30, 72, 118, 172, and 264 days (d) from the start of the experiment, litter decomposition boxes from the different Chinese fir developmental stages under different temperature treatments were randomly selected and removed for chemical analysis. There were three replicate decomposition boxes for each temperature–decomposition time combination for every stand developmental stage, giving a total of 162 decomposition boxes.
2.3. Litter Mass and Chemical Analysis
The litter materials in the decomposition boxes removed at each timestep in the experiment were placed in an oven, dried at 75 °C to constant weight, and then mass measured. Dried samples were then ground uniformly using a grinder (A11, Guangzhou YiKe Experimental Technology Co., Guangzhou, China), passed through a 0.149 mm mesh sieve, and stored in a sealed container for chemical analysis of selected metallic elements and recalcitrant components within 1 month. To determine the concentrations of K, Ca, Mg, Fe, Mn, Al, and Na in the litter samples at the start of the experiment and at different decomposition times under different temperature treatments, 5 mL HNO3 and 1 mL H2O2 were added to 0.2 g of the prepared litter material and placed in a microwave digestor (ETHOS UP, Milestone, Modena, Italy) at 160 °C for 2 h. The digested samples were then analyzed using inductively coupled plasma spectroscopy (PE OPTIMA 8000, PerkinElmer, Waltham, MA, USA). The concentrations of lignin, cellulose, hemicellulose, total phenols, and condensed tannins in needle litter were determined using a rapid detection kit (Shanghai ZCIBIO Technology Co., Shanghai, China), as described in detail in the Supplementary Material Section S1. In brief, dry needle litter samples of different masses (three replicates each of 3 mg, 300 mg, 20 mg, 100 mg, and 50 mg) were extracted according to the manufacturer’s instructions. After chromogenic reaction, the optical density of the extractions for lignin, cellulose, hemicellulose, total phenols, and condensed tannins were measured at 280, 620, 540, 275, and 760 nm, respectively, using a multi-mode microplate reader (SpectraMax iD5, Molecular Devices, Sunnyvale, CA, USA). The concentrations of lignin, cellulose, hemicellulose, total phenols, and condensed tannins were calculated from calibration curves after subtraction of values for reagent blanks.
2.4. Data Processing and Analyses
The mass loss rate (ML), and release rate (R) for metallic elements and recalcitrant components in all litter samples from the experiment were calculated as follows [28]:
where M0 and Mt represent, respectively, the initial oven-dried litter mass and the remaining litter mass after each sampling collection during the decomposition experiment (g). C0 represents the initial litter concentration of metallic elements and recalcitrant components (mg g−1), and Ct represents the concentrations of metallic elements and recalcitrant components in the litter after each sampling collection during the decomposition experiment. Positive and negative values of R indicate net release and net enrichment, respectively.
The litter decomposition rate was calculated from the fraction of litter mass remaining using an exponential decay model [29]:
where Mt/M0 is the fraction of litter mass remaining at time t, M0 and Mt are as defined above, e represents the base of the natural logarithm, k represents the decomposition coefficient, and t represents the time of each sampling collection.
The times at which 50% and 95% litter mass loss occurred were estimated using the following equation [29]:
where T (years, y) represents the time of litter mass loss, n represents the litter decomposition level (50% and 95%), and k is as defined above.
The data were analyzed after testing for homogeneity of variance using Levene’s test. When constant variance was not satisfied, a log10 or square transformation was used. The differences in initial metallic elements and recalcitrant components concentration, mass loss rate, and release rate of metallic elements and recalcitrant components for litter between different temperature treatments and Chinese fir developmental stages were analyzed by one-way analysis of variance (ANOVA) and Duncan multiple range tests. The association between litter mass loss rate and litter chemical composition was assessed using the Pearson correlation coefficient in Supplementary Material Table S1, and if there was a significant correlation, a scatter plot was used to assess whether to fit a linear or nonlinear model. Model suitability was further evaluated using p-values and R2. Generally, a p-value < 0.05 and an R2 value approaching 1 indicate that the model’s fit is statistically significant. A three-way analysis of variance (ANOVA) was used to test the effects of stand developmental stage, decomposition time, and temperature, and their interactions on the release rate of metallic elements and recalcitrant components from needle litter in Supplementary Material Table S2. All statistical analyses were performed using SPSS version 21.0.
3. Results
3.1. Initial Concentrations of Some Metallic Elements and Recalcitrant Components in Needle Litter from Different Chinese Fir Plantation Developmental Stages
The initial concentrations of metallic elements and recalcitrant components in needle litter at the start of the decomposition experiment were significantly different between the different Chinese fir developmental stages (Table 1). With increasing stand age, the concentrations of K, Mg, Mn, and Na in needle litter decreased significantly, while the concentrations of Fe, lignin, condensed tannins, total phenols, and cellulose increased significantly. The concentration of Ca in the overmature stand was significantly higher than that in middle-aged stands, with the opposite pattern displayed for Al concentrations, whilst hemicellulose concentration in the middle-aged stand was significantly lower than that in mature and overmature stands.

Table 1.
Initial concentrations of metallic elements and recalcitrant components in needle litter from Chinese fir plantations at different developmental stages. Values are mean ± standard deviation (n = 3).
3.2. Effects of Warming on Mass Loss Rate of Needle Litter at Different Chinese Fir Plantation Developmental Stages
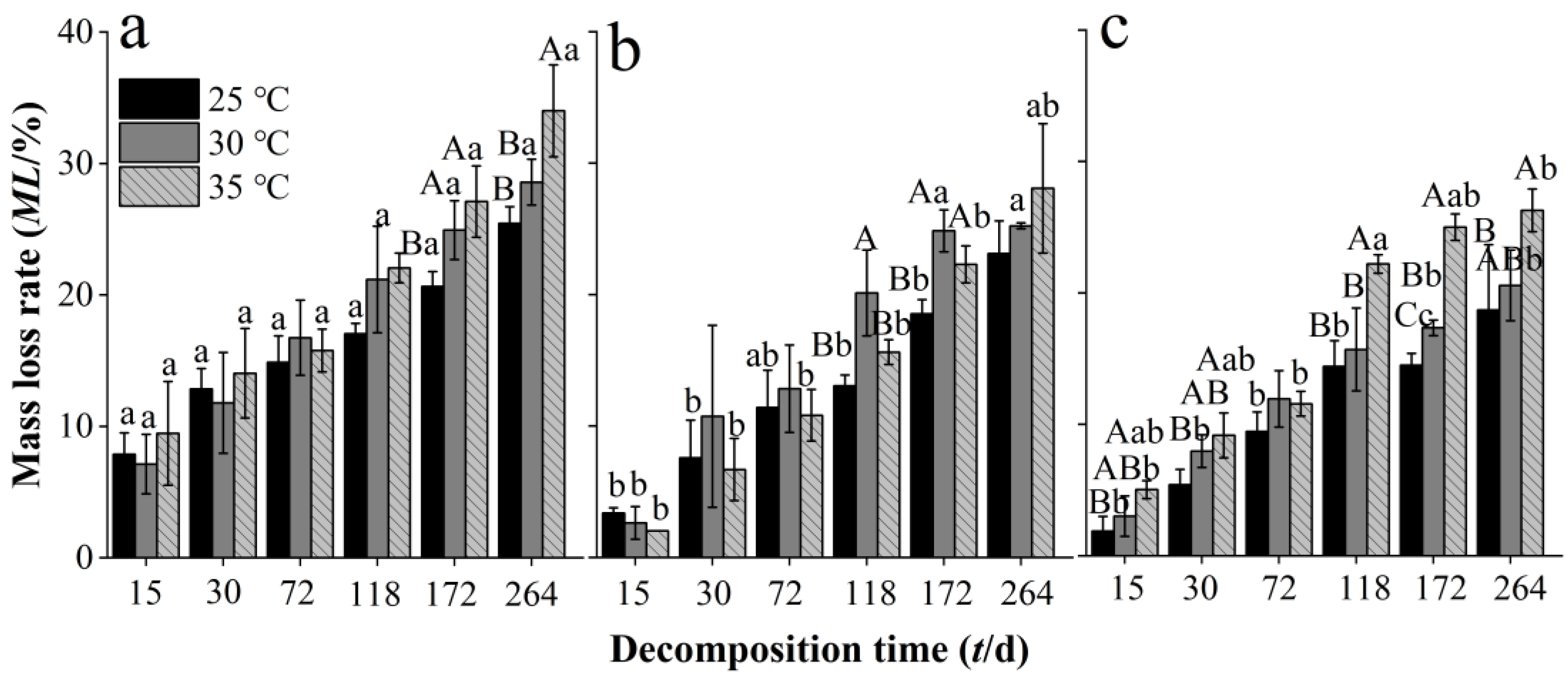
In our decomposition experiment, litter mass loss occurred in all treatments at all decomposition time durations, incubation temperatures, and Chinese fir plantation developmental stages. The mass loss rate of needle litter at different developmental stages increased with decomposition time at all incubation temperatures (Figure 1). Warming to 35 °C significantly increased the mass loss rate of needle litter from the overmature stand throughout the experiment (except at 72 d) (Figure 1a), while significant increases in mass loss rate of litter occurred later in the experiment in the middle-aged (at 172 and 264 d) (Figure 1b) and mature stands (at 118 and 172 d) (Figure 1c). There were some significant differences in the mass loss rate of needle litter between the different developmental stages, with significantly higher values in the middle-aged stand compared with the mature and overmature stands, particularly at the shorter decomposition times. At the end of the decomposition experiment, the mean mass loss rates of needle litter in the middle-aged, mature, and overmature stands across the different incubation temperatures were 25.5%–32.0%, 23.1%–28.1%, and 18.7%–26.3%, respectively. Decomposition coefficients increased with temperature within each development stage (Table 2). The estimated times for 50% and 95% decomposition decreased with temperature and were longer for the overmature stand compared with the middle-aged and mature stands.
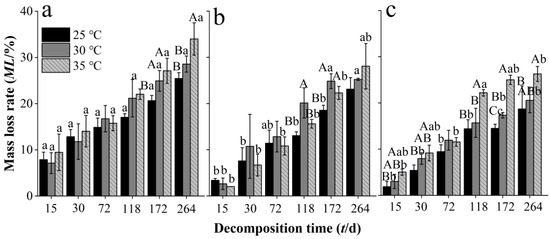
Figure 1.
Mass loss rate of Chinese fir needle litter at different developmental stages at different temperatures during the decomposition experiment for the different developmental stages: (a) middle-aged, (b) mature, and (c) overmature stands. Bars show mean values and error bars represent standard deviation (n = 3). Different uppercase letters indicate significant differences among temperatures at the same developmental stage at each decomposition time (p < 0.05). Different lowercase letters indicate significant differences among developmental stages at the same temperature treatment at each decomposition time (p < 0.05). Letters are shown only where there are significant differences.

Table 2.
Chinese fir needle litter decomposition rate coefficients (k) and predicted time (years, y) to 50% and 95% mass loss for different developmental stages and temperatures across all the decomposition period, calculated using Equations (3) and (4).
3.3. Correlation Between the Mass Loss Rate of Chinese Needle Litter and the Concentrations of Some Metallic Elements and Recalcitrant Components
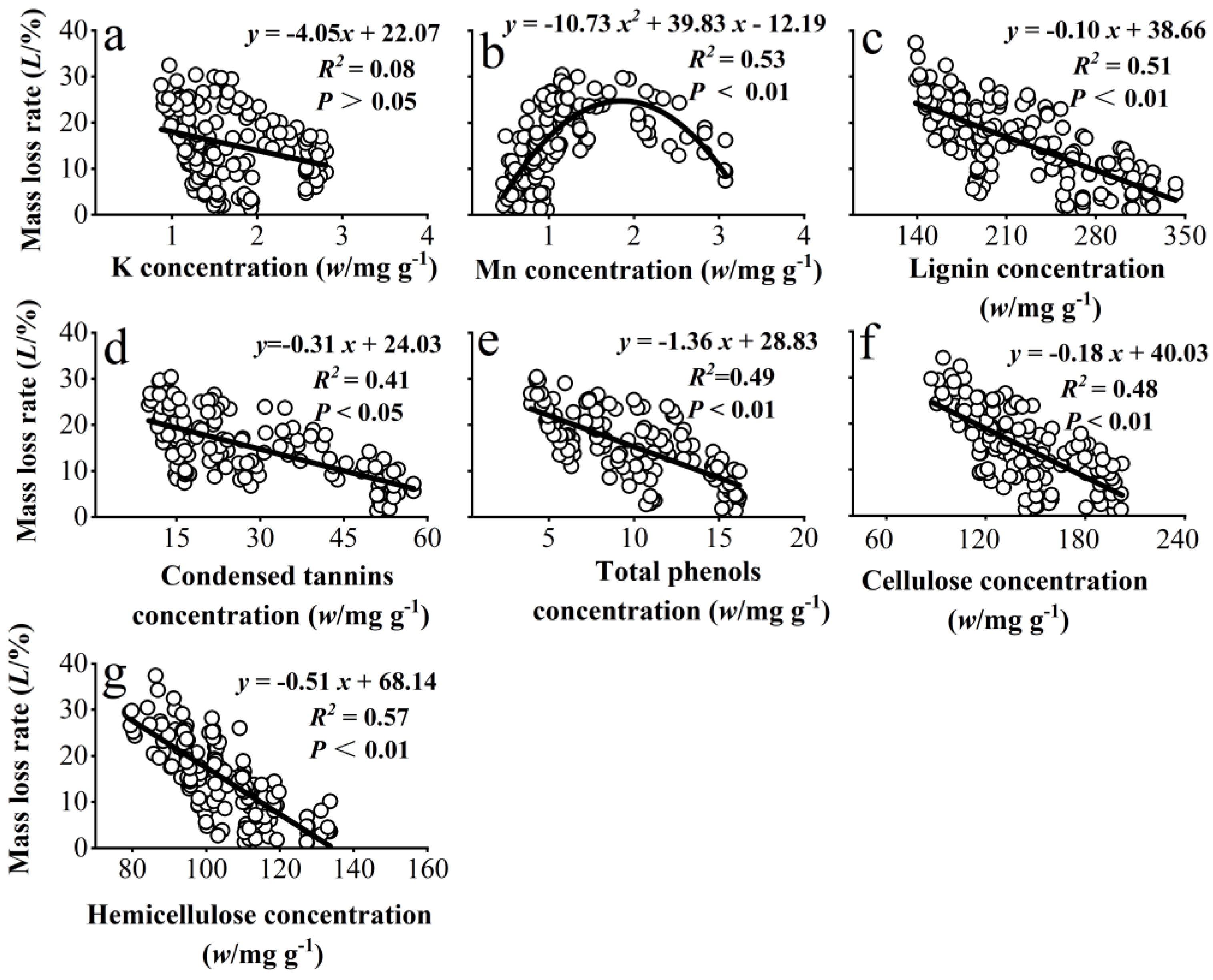
Modeling of the significant correlations in Supplementary Material Table S1 showed that needle litter mass loss rate had a significant parabolic relationship with litter Mn concentration (Figure 2b) and a significant linear negative relationship with concentrations of lignin, condensed tannins, total phenols, hemicellulose, and cellulose (Figure 2c–g).
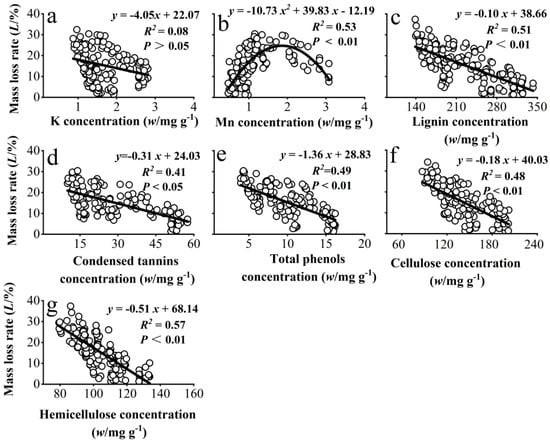
Figure 2.
Fitted relationships between the mass loss rate of needle litter and the concentrations of K and Mn (a,b) and the recalcitrant components (c–g) in needle litter across all developmental stages, temperatures, and decomposition periods in the experiment.
3.4. Effects of Warming on the Release Rate of Some Metallic Elements from Chinese Fir Needle Litter at Different Developmental Stages
To help understand the drivers of the enrichment and release of some metallic elements and recalcitrant compounds during the litter decomposition experiment, data for litter mass loss and litter concentrations for all treatments are shown in Supplementary Material Figures S2–S4. Since litter mass loss occurred in all treatments (Figure 1), the enrichment of some metallic elements/recalcitrant compounds was driven by increases in concentration as the element/compound accumulated in litter, probably as a result of microbial activity. In contrast, the release of some metallic elements/recalcitrant compounds was driven by decreases in concentration and/or litter mass as the element/compound was released from litter during decomposition.
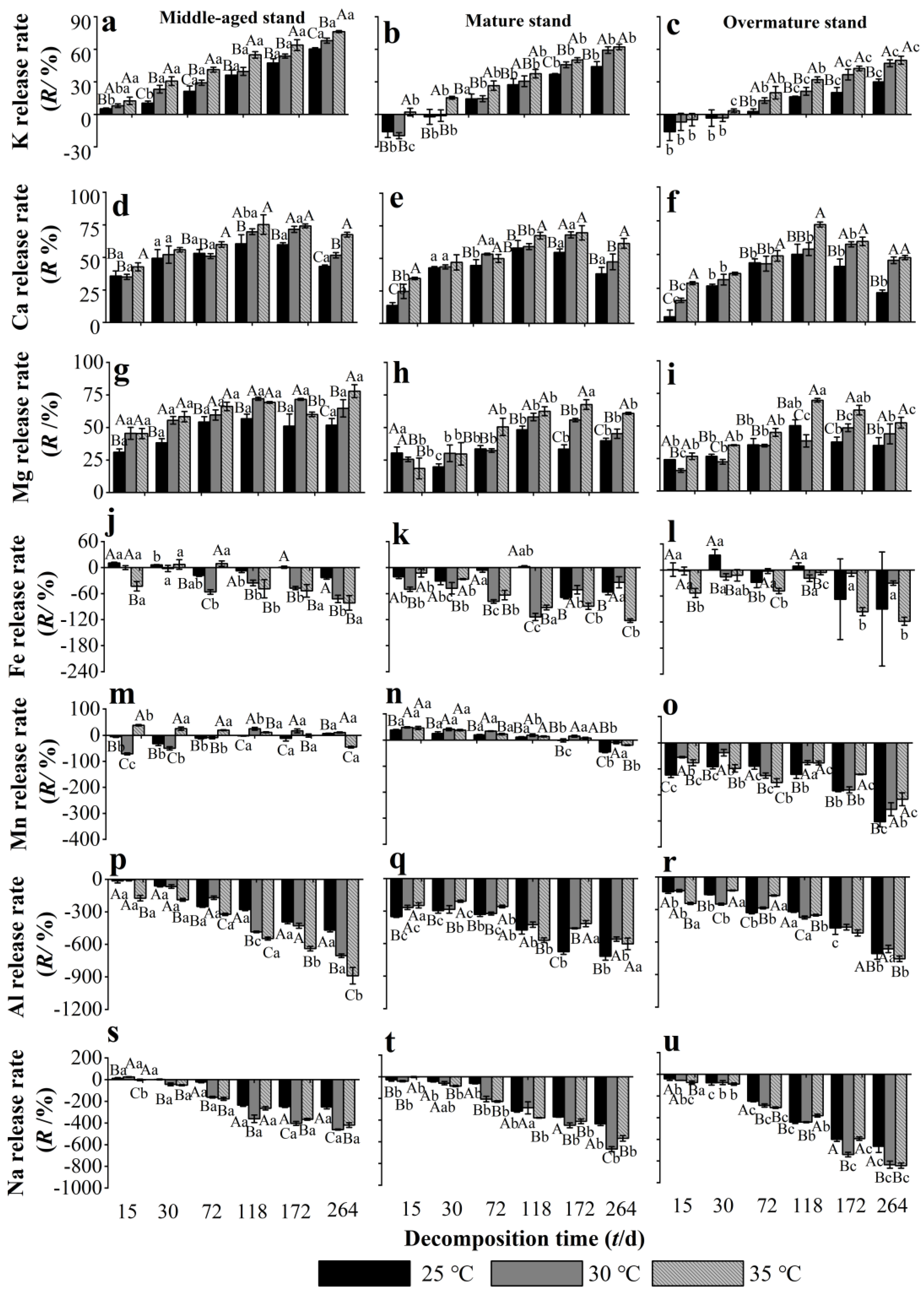
During the decomposition experiment, K was released from the middle-aged stand needle litter, whilst in the mature and overmature stand litters, K was initially enriched and then released (Figure 3a–c). The release rate of K from needle litter increased with increasing decomposition time, and overall was negatively associated with stand age, with significantly highest values in the middle-aged stand. Warming to 30–35 °C significantly increased the K release rate of litter from the middle-aged stand throughout the experiment, and in the mature and overmature stands after 72 d. Needle litter Ca was released throughout the experiment from all stand developmental stages (Figure 3d–f). The Ca release rate of needle litter in the middle-aged stand was significantly higher at 25 °C and 30 °C than that in the overmature stand during 15–72 d. Warming to 30–35 °C significantly increased the Ca release rate of litter from stands of all developmental stages throughout the experiment, except for at 30 d and for the mature stand at 72 d. Needle litter Mg was also released throughout the experiment from all stand developmental stages, and the release rate of Mg in the middle-aged stand was higher than that in mature and overmature stands (Figure 3g–i). At 72–118 d, warming to 30–35 °C significantly promoted the release of Mg from needle litter of all developmental stages. At the end of the decomposition experiment, warming to 35 °C had increased the release rates of K, Ca, and Mg from needle litter of all developmental stages by 14.7%, 24.6%, and 21.5%, respectively, compared with at 25 °C.
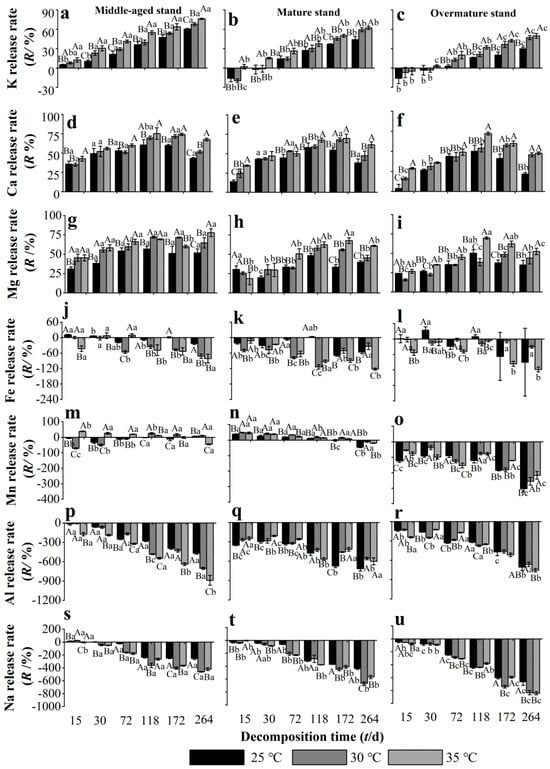
Figure 3.
Release rate of metallic elements from Chinese fir needle litter at different temperatures during the decomposition experiment at different developmental stages. (a–c) represent the K release rate of middle-aged, mature, and overmature stands, respectively; (d–f) represent the Ca release rate of middle-aged, mature, and overmature stands, respectively; (g–i) represent the Mg release rate of middle-aged, mature, and overmature stands, respectively; (j–l) represent the Fe release rate of middle-aged, mature, and overmature stands, respectively; (m–o) represent the Mn release rate of middle-aged, mature, and overmature stands, respectively; (p–r) represent the Al release rates of middle-aged, mature, and overmature stand, respectively; (s–u) represent the Na release rates of middle-aged, mature, and overmature stand, respectively. Bars show mean values and error bars represent standard deviation (n = 3). Different uppercase letters indicate significant differences among temperatures at the same developmental stage at each decomposition time (p < 0.05). Different lowercase letters indicate significant differences among developmental stages at the same temperature treatment at each decomposition time (p < 0.05). Letters are shown only where there are significant differences.
Iron in needle litter was mostly enriched throughout the decomposition experiment, apart from a small amount of release at 25 °C from litter of middle-aged and overmature stands at 15–30 d (Figure 3j–l). At 72–264 d, warming to 30–35 °C significantly promoted the enrichment of Fe in the middle-aged and mature stands, but not in the overmature stand. The release pattern of Mn in needle litter varied between the different development stage stands. In the middle-aged stand, Mn fluctuated between release and enrichment, whilst Mn was mostly released from mature stand litter, and enrichment occurred in overmature stand litter (Figure 3m–o). Warming also had variable effects on litter Mn release/enrichment in the different age stands. Higher temperatures appeared to promote the release of litter Mn in middle-aged and mature stands during most of the experiment, and to mostly reduce the litter Mn enrichment in the overmature stand. Needle litter Al and Na were enriched in all stand developmental stages throughout the experiment, and their enrichment rates increased with decomposition time (Figure 3p–u). Warming to 30–35 °C generally promoted the enrichment of Na and Al in middle-aged and overmature stands, but mostly reduced the enrichment of Al in the mature stand. At 15–118 d, the enrichment rate of Na and Al in needle litter of the middle-aged stand was higher than that of the mature and overmature stands.
3.5. Effects of Warming on the Release Rate of Recalcitrant Components from Chinese Fir Needle Litter at Different Developmental Stages
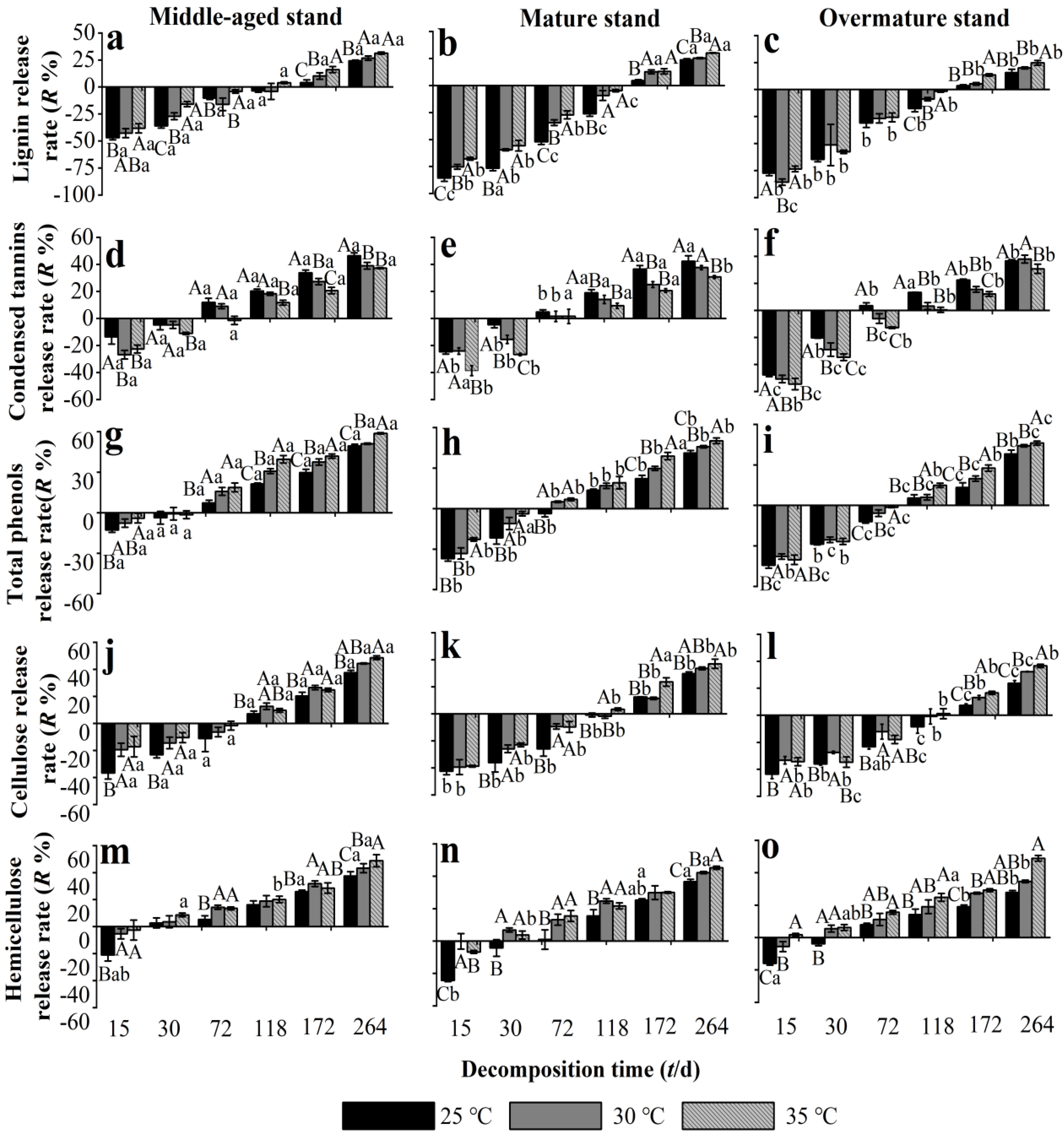
In all stand development stages, needle litter lignin was enriched until 118 d and then released at 172–264 d, although the enrichment rate in middle-aged stands was lower than that in mature and overmature stands (Figure 4a–c). Warming to 30–35 °C significantly decreased the net enrichment rate of lignin in middle-aged and mature stands, but not in the overmature stands. At 172–264 d, the release of lignin in all stand developmental stages was promoted by warming to 30–35 °C. The patterns of release rates of total phenols and condensed tannins in needle litter during the experiment were near identical (Figure 4d–i). At 15–30 d, total phenols and condensed tannins were enriched in needle litter, and the enrichment rate was mostly significantly lower in middle-aged forest than in mature forest and overmature forest. Warming to 30–35 °C promoted the enrichment of condensed tannins and reduced the enrichment of total phenols in all developmental stages, apart from total phenols in the overmature stand at 30 d. The condensed tannins and total phenols in litter switched from enrichment to release in the middle-aged and mature stands at 72 d and in the overmature stand at 118 d. During 118–264 d, total phenols and condensed tannins were released from needle litter in all developmental stages, with mostly higher release rates from middle-aged stands compared with mature and overmature stands. Warming to 30–35 °C significantly reduced the release of condensed tannins (Figure 4d–f) and promoted the release of total phenols in all stand developmental stages (Figure 4g–i).
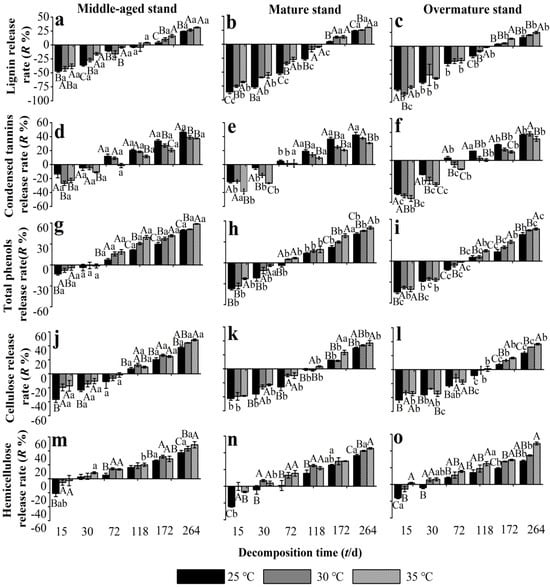
Figure 4.
Release rate of recalcitrant components from Chinese fir needle litter at different temperatures during the decomposition experiment at different developmental stages. (a–c) represent the lignin release rate of middle-aged, mature, and overmature stands, respectively; (d–f) represent the condensed tannins release rate of middle-aged, mature, and overmature stands, respectively; (g–i) represent the total phenols release rate of middle-aged, mature, and overmature stands, respectively; (j–l) represent the cellulose release rate of middle-aged, mature, and overmature stands, respectively; (m–o) represent the hemicellulose release rate of middle-aged, mature, and overmature stands, respectively. Bars show mean values and error bars represent standard deviation (n = 3). Different uppercase letters indicate significant differences among temperatures at the same developmental stage at each decomposition time (p < 0.05). Different lowercase letters indicate significant differences among developmental stages at the same temperature treatment at each decomposition time (p < 0.05). Letters are shown only where there are significant differences.
The enrichment-release pattern of cellulose in needle litter was very similar to that of condensed tannins and total phenols, apart from the switch from enrichment to release that occurred later during the experiment at 118 d (Figure 4j–l). At 15–72 d, net enrichment in cellulose occurred in needle litter, and warming mostly reduced the enrichment of cellulose. During 172–264 d, cellulose was released from needle litter, with higher release rates from middle-aged stands than from mature and overmature stands. Warming to 30–35 °C significantly promoted the release of cellulose from litter in all stand developmental stages. Hemicellulose from needle litter was released at 15 d, but switched to enrichment at 30 d, with enrichment rates increasing for the remainder of the experiment (Figure 4m–o). Warming to 30–35 °C significantly promoted the release of hemicellulose from litter in all stand developmental stages, except in the middle-aged stand at 118 d and the mature stand at 172 d. At the end of the decomposition experiment, warming to 35 °C had increased the release rates of lignin, total phenols, cellulose, and hemicellulose from needle litter of all developmental stages by 7.5%, 8.8%, 10.4%, and 13.7%, respectively, compared with 25 °C.
4. Discussion
4.1. Warming Increases Chinese Fir Needle Litter Decomposition Rate at Different Developmental Stages
Our results mostly verified our first hypothesis that warming enhances the decomposition rate of Chinese fir needle litter. Warming to 35 °C significantly increased the mass loss rate of needle litter from the overmature stand throughout the experiment (except at 72 d), while significant increases in mass loss rate of litter occurred later in the experiment in the middle-aged (at 172 and 264 d) and mature stands (at 118 and 172 d) (Figure 1). This is consistent with the finding that warming significantly promoted the decomposition rate of litter from seven different tundra plant types in controlled environment soil–litter microcosm incubations [30]. However, it is contrary to the results of [31], a 5-year study in which the negative effect of warming on mass loss in litter bags of four alpine plants deployed in open-top chambers (OTCs) in the field was attributed to the creation of low soil moisture content in the OTCs. Under humid conditions, it has been reported that the decomposition rate of litter increases with temperature [32], and the decomposition rate of high-quality litter is less sensitive to increasing temperature than that of low-quality litter [33]. Since our study focused on the effect of temperature as a variable and controlled the humidity at 80%, warming under suitable environmental conditions may significantly increase the diversity and activity of microorganisms and promote litter decomposition. The initial litter quality of the middle-aged and mature stands was higher than that of the overmature stand, with higher concentrations of beneficial metallic elements and lower concentrations of recalcitrant components (Table 1), accounting for the limited effects of warming on the mass loss rates of needle litter from the middle-aged and mature stands in our experiment.
Nevertheless, at the same temperature, the mass loss rate of needle litter from the middle-aged stand was significantly higher than that of mature and overmature stands (Figure 1). Furthermore, the times for decomposition of 50% and 95% litter mass in overmature stands were longer than those in middle-aged and mature stands (Table 2). Throughout the decomposition experiment, the litter mass loss rate was significantly negatively related to concentrations of lignin, condensed tannins, total phenols, hemicellulose, and cellulose (Figure 2c–g), which indicates that the decomposition rate of litter is closely related to litter quality. Compared with low-quality litter, high-quality litter has lower concentrations of recalcitrant components (e.g., lignin, cellulose), resulting in a faster decomposition rate [34]. The needle litter of the overmature stand had higher concentrations of recalcitrant compounds, and these recalcitrant compounds had complex structures that were not readily used by soil animals and microorganisms, providing insufficient nutrient sources for the growth and reproduction of soil animals and microorganisms, and thus inhibiting litter decomposition in overmature stands [35].
4.2. Warming Promotes the Release of Some Metallic Elements in Chinese Fir Needle Litter and the Enrichment of Other Elements
Our results partially validated our second hypothesis that warming promotes the release of metallic elements during the decomposition of Chinese fir needle litter. Warming (30–35 °C) significantly promoted the release of K, Ca, and Mg from the litter of all stand developmental stages at 72–264 d in the experiment (Figure 3a–i). K is neither a component of organic matter nor an intermediate metabolite in plants, existing in the form of ions that are released by leaching during decomposition [36]. Mg is an important component of chlorophyll, mainly occurring in the ionic state in the cell fluid, and is easily released by microorganisms [37]. Although Ca mainly occurs in plants in the form of chelates, it has a low degree of coupling with organic carbon and is easily utilized by microorganisms [38]. Warming can directly promote the leaching and migration of K in needle litter, increase the relative abundance and species diversity of microbial community, improve the utilization of Ca and Mg by microorganisms, and promote the release of Ca and Mg in needle litter [30].
In contrast, enrichment of Fe, Mn, Al, and Na in Chinese fir needle litter from all stand developmental stages was observed during the experiment, contradicting our second hypothesis. Warming to 35 °C significantly promoted the enrichment of Al, Na, and Fe (except in the overmature stand) (Figure 3j–l, p–u). Although a small part of Fe and Al exists in the ionic state, they predominantly form stable recalcitrant components with humic acid substances produced in the process of litter decomposition, so are often enriched in litter [39]. The combined effect of the release of K, Ca, and Mg nutrients from needle litter and enrichment of Fe and Al may acidify the decomposition environment [40], thus enhancing the ability of acidophilic iron- and aluminum-reducing bacteria to reduce Fe and Al to recalcitrant forms [41]. During litter decomposition, litter consumers must accumulate Na up to approximately 100- to 1000-fold over the litter they consume to maintain Na balance [11]. Warming may increase the acquisition of soil Na by microorganisms, thereby promoting the accumulation of Na in needle litter. Needle litter Mn was released or enriched in our experiment, depending on stand development stage. In mature forest litter, Mn was predominantly released, whilst in litter of the overmature stands enrichment occurred throughout the experiment, and in middle-aged stand litter Mn fluctuated between release and enrichment (Figure 3m–o). Warming increased the release rate of Mn and reduced Mn enrichment in needle litter. In the process of litter decomposition, lignin is decomposed by the enzyme manganese peroxidase produced by the majority of all wood-degrading basidiomycetes fungi [12]. Because litter Mn concentration in the middle-aged and mature stands was significantly higher than that of the overmature stand (Table 1), it could meet the demand of the basidiomycetes to produce the manganese peroxidase enzyme, resulting in Mn release. In contrast, the low concentration of Mn in overmature stand litter may have stimulated Mn enrichment from soil by microorganisms [42]. Warming may increase the activity of basidiomycetes and promote the release of Mn from litter [5]. These processes may also explain the highly significant parabolic relationship between litter mass loss rate and needle litter Mn concentration during the decomposition experiment (Figure 2b).
4.3. Warming Accelerates the Release Rate of Recalcitrant Components from Chinese Fir Needle Litter After 6 Months, Apart from Condensed Tannins
Our third hypothesis that warming accelerates the release of recalcitrant components from Chinese fir needle litter was mostly verified by our results. Warming (30–35 °C) significantly promoted the release of lignin, cellulose, hemicellulose, and total phenols from litter of all stand developmental stages, particularly towards the end of the experiment, while inhibiting the release of condensed tannins (Figure 4). The suggested reason for the different response of condensed tannins is that warming activated more enzymes that promoted complexation of condensed tannins with proteins, which are not easily decomposed by microorganisms [43]. Lignin, cellulose, and total phenols in needle litter at different developmental stages all showed an enrichment-release pattern, with initial enrichment declining until 30–118 d before a switch to increasing release (Figure 4a–c,g–l). In contrast, the earlier release of hemicellulose from needle litter starting at 30 d (Figure 4m–o) is attributed to its lower molecular weight, making it easier to decompose and release compared with other carbohydrates in litter [44]. The delay in release of lignin, cellulose, and total phenols from litter decomposition could be due to the time taken for changes in the soil microbial and macrofaunal community that promote their decomposition, particularly for lignin. The decomposition of lignin is mainly realized by three decomposers—white rot fungi, soft rot fungi, and brown rot fungi. When litter begins to decompose, it takes a period of microbial transformation to form the main fungal community suitable for lignin decomposition and produce the corresponding enzymes [45], which accounts for the enrichment of lignin at the start of the decomposition experiment (15–118 d). In the subsequent decomposition process (172–264 d), microorganisms destroy the molecular structure of lignin, and the litter loses the protection of the peripheral “barrier” and decomposes rapidly [46]. Warming causes the rapid growth and reproduction of soil microorganisms, and the extracellular enzymes capable of degrading lignin are secreted in large quantities to promote the degradation of lignin into small molecular aromatic compounds [47]. In the early stage of cellulose decomposition (15–72 d), most of the cellulose was protected from degradation by physical and chemical methods due to the enrichment of lignin [15]. In the later stage of decomposition (118–264 d), hydrogen peroxide, continuously produced due to the decomposition of hemicellulose, diffuses into the cell wall to release cellulose by oxidative decomposition [48]. Warming increases the activity of extracellular enzymes secreted by fungi and bacteria that promote the degradation of cellulose and hemicellulose into smaller oligosaccharides and glucose [44]. Total phenols were first slowly degraded by leaching from needle litter, and then rapidly lost through biodegradation [14,49]. Warming can increase the activity of polyphenol oxidase and promote the release of total phenols [50]. The total number of soil nematodes and bacterial-feeding nematodes has been reported to increase with decreasing litter total phenols concentration [51], indicating that the release of total phenols can increase the activity of decomposers and promote the decomposition of needle litter.
5. Conclusions
Our results indicate that warming to 35 °C shortened the decomposition time of 95% of Chinese fir litter at different developmental stages, and increased the release rates of K, Ca, and Mg from needle litter of all developmental stages by 14.7%, 24.6%, and 21.5%, respectively. The release rates of lignin, total phenols, cellulose, and hemicellulose from needle litter of all developmental stages increased by 7.5%, 8.8%, 10.4%, and 13.7%, respectively. The effects of warming on litter decomposition rates and the release rates of metallic elements and recalcitrant components in litter among different Chinese fir developmental stages were significantly different, which were closely related to the litter quality. The decomposition rate of needle litter in the overmature stand was considerably lower than that in middle-aged and mature stands due to its initial lower K and Mn concentrations and higher recalcitrant components concentrations. The sensitivity of needle litter decomposition to temperature in an overmature stand was higher than that in middle-aged and mature stands, which could allow extending the rotations of Chinese fir plantations. The effect of longer rotation length may improve the yield of large-diameter timber, and increase litter decomposition rate and nutrient return under higher temperatures, reducing the site disturbance and increasing carbon sequestration for Chinese fir plantations. Our research results can serve as a reference for the effect of warming on the decomposition rate of needle litter and implications for biogeochemical cycling in evergreen coniferous forest ecosystems in the south-eastern USA, southern Brazil, and coastal areas of Australia with a similar climate to our study site. Limitations of the work include the small scale of the experiment conducted in incubators and the maintenance of constant and high soil moisture, so moisture was not considered. However, litter decomposition is affected by combined physical (temperature and moisture) and biological factors (soil microbiology and macrofauna) and their interactions, which will all be affected by climate change. Besides temperature, the effect of other factors on biogeochemical cycling in Chinese fir plantation litter should be examined, such as precipitation, extreme drought, and nitrogen deposition. Among them, studying the impacts of precipitation and atmospheric nitrogen deposition on the decomposition rate and the chemical composition of needle litter will be the focus of our future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15122151/s1, Section S1: Method for determination of concentrations of recalcitrant components in Chinese fir needle litter; Figure S1: Location and layout of the different-aged Chinese fir plantations from which litter was sampled for the decomposition experiments; Figure S2: The actual measured Mass loss (g) of Chinese fir needle litter at different decomposition times under different temperature treatments at different development stages; Figure S3: The actual measured concentrations of metal elements in Chinese fir needle litter at different decomposition times under different temperature treatments at different development stages; Figure S4: The actual measured concentrations of recalcitrant compounds in Chinese fir needle litter at different decomposition times under different temperature treatments at different development stages; Table S1: Pearson correlation coefficients between the mass loss rate of Chinese fir needle litter and the concentration of metallic elements and recalcitrant components across all developmental stages and temperatures for each decomposition period. Table S2: Three-way analysis of variance (ANOVA) of the effect of temperature, developmental stage, decomposition time, and their interactions on release rates of some metallic elements and recalcitrant components from Chinese fir needle litter (F value).
Author Contributions
Conceptualization: L.Z. (Lixian Zhang) and L.Z. (Lili Zhou); Methodology: L.Z. (Lixian Zhang), W.G., Y.C., Z.L., Q.L. and K.V.H.; Formal Analysis: L.Z. (Lixian Zhang), W.G., Y.C., Z.L. and Q.L.; Investigation: L.Z. (Lixian Zhang), W.G., Y.C., Z.L. and Q.L.; Validation: L.Z. (Lixian Zhang), W.G., Y.C. and Z.L.; Software: Y.C. and Z.L.; Writing—Original Draft Preparation: L.Z. (Lixian Zhang), W.G., Y.C. and K.V.H.; Writing—Review and Editing: L.Z. (Lixian Zhang), L.Z. (Lili Zhou), W.G., Q.L., K.V.H. and S.L.; Date curation: L.Z. (Lixian Zhang) and L.Z. (Lili Zhou); Project Administration: L.Z. (Lili Zhou) and S.L.; Funding Acquisition: L.Z. (Lili Zhou). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32271864), the Forestry Science and Technology Special Project of Fuzhou City (No. 81, 2022), the Science and Technology Plan Project of Fuzhou City (2022-S-004), the Research Project of Fashu Foundation (MFK24035), and the Science and Technology Innovation Special Project of Fujian Agriculture and Forestry University (KFB23056).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We acknowledge Sanming Xinkou National Forest Farm in Fujian Province for providing the experimental sites, and Yu Lin for the field surveys.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, S.Q.; Yang, R.; Peng, X.D.; Hou, C.L.; Ma, J.B.; Guo, J.R. Contributions of plant litter decomposition to soil nutrients in ecological tea gardens. Agriculture 2022, 12, 957. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, S.D.; Li, X.J.; Yang, Z.J.; Xiong, D.C.; Xu, C.; Wanek, W.G.; Yang, Y.S. Soil warming delays leaf litter decomposition but exerts no effect on litter nutrient release in a subtropical natural forest over 450 days. Geoderma 2022, 427, 116139. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Malik, A.A.; Swenson, T.; Weihe, C.; Morrison, E.W.; Martiny, J.B.H.; Brodie, E.L.; Northen, T.R.; Allison, S.D. Drought and plant litter chemistry alter microbial gene expression and metabolite production. SME J. 2020, 14, 2236–2247. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Liu, S.G.; Li, Y.Y.; Liu, S.Z.; Yin, G.C.; Huang, J.; Xu, Y.; Zhou, G.Y. Warming effects on the decomposition of two litter species in model subtropical forests. Plant Soil 2017, 420, 277–287. [Google Scholar] [CrossRef]
- Prieto, I.; Almagro, M.; Bastida, F.; Querejeta, J.I. Altered leaf litter quality exacerbates the negative impact of climate change on decomposition. J. Ecol. 2019, 107, 2364–2382. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Haugwitz, M.S.; Priemé, A.; Nielsen, C.S.; Elberling, B.; Michelsen, A.; Grogan, P.; Blok, D. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Chang. Biol. 2016, 23, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Romero-Olivares, A.L.; Allison, S.D.; Treseder, K.K. Decomposition of recalcitrant carbon under experimental warming in boreal forest. PLoS ONE 2017, 12, e0179674. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Du Laing, G.; Van Ryckegem, G.; Tack, F.M.; Verloo, M. Metal accumulation in intertidal litter through decomposing leaf blades, sheaths and stems of Phragmites australis. Chemosphere 2006, 63, 1815–1823. [Google Scholar] [CrossRef]
- Brun, C.B.; Åström, M.E.; Peltola, P.; Johansson, M.B. Trends in major and trace elements in decomposing needle litters during a long-term experiment in Swedish forests. Plant Soil 2008, 306, 199–210. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Peng, Y.; Zhang, C.; Huang, C.; Xu, Z.F.; Tan, B.; Wu, F.Z. Dynamics of multiple metallic elements during foliar litter decomposition in an alpine forest river. Ann. For. Sci. 2016, 73, 547–557. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.J.; Jian, Z.; Zhou, H.Y.; Zhao, Y.B.; Wei, D.P. Litter decomposition and degradation of recalcitrant components in Pinus massoniana plantations with various canopy densities. J. For. Res. 2019, 30, 1395–1405. [Google Scholar] [CrossRef]
- Loranger, G.; Ponge, J.F.; Imbert, D.; Lavelle, P. Leaf decomposition in two semi-evergreen tropical forests: Influence of litter quality. Biol. Fert. Soils 2002, 35, 247–252. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballaré, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; Mckey, D. Extraction and quantification of "condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Richardson, S.J.; Press, M.C.; Parsons, A.N.; Hartley, S.E. How do nutrients and warming impact on plant communities and their insect herbivores? A 9-year study from a sub-Arctic heath. J. Ecol. 2002, 90, 544–556. [Google Scholar] [CrossRef]
- Day, T.A.; Ruhland, C.T.; Xiong, F.S. Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra. Glob. Chang. Biol. 2008, 14, 1827–1843. [Google Scholar] [CrossRef]
- BI, J.; Blanco, J.A.; Seely, B.; Kimmins, J.P.; Ding, Y.; Welham, C. Yield decline in Chinese-fir plantations: A simulation investigation with implications for model complexity. Can. J. For. Res. 2007, 37, 1615–1630. [Google Scholar] [CrossRef]
- Li, A.G.; Fan, Y.X.; Chen, S.L.; Song, H.W.; Lin, C.F.; Yang, Y.S. Soil warming did not enhance leaf litter decomposition in two subtropical forests. Soil Biol. Biochem. 2022, 170, 108716. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. CATENA 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, X.F.; Wang, S.L. Fate of Chinese-fir litter during decomposition as a result of inorganic N additions. Appl. Soil Ecol. 2014, 74, 30–36. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Huang, Y. Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. For. Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Wu, Z.L. Chinese Fir; China Forestry Publishing House: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Zhou, L.L.; Shalom, A.D.D.; Wu, P.F.; Li, S.B.; Jia, Y.Y.; Ma, X.Q. Litterfall production and nutrient return in different-aged Chinese fir (Cunninghamia lanceolata) plantations in South China. J. For. Res. 2015, 26, 79–89. [Google Scholar] [CrossRef]
- Ma, X.Q.; Liu, C.J.; Hannu, I.; Westman, C.J.; Liu, A.Q. Biomass, litterfall and the nutrient fluxes in Chinese fir stands of different age in subtropical China. J. For. Res. 2002, 13, 165–170. [Google Scholar]
- Yan, G.Y.; Dong, X.D.; Huang, B.B.; Wang, H.L.; Hong, Z.M.; Zhang, J.H.; Xing, Y.J.; Wang, Q.G. Effects of nitrogen deposition on litter decomposition and nutrient release mediated by litter types and seasonal change in a temperate forest. Can. J. Soil Sci. 2019, 100, 11–25. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Hobbie, S.E. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol. Monogr. 1996, 66, 503–522. [Google Scholar] [CrossRef]
- Hong, J.T.; Lu, X.Y.; Ma, X.X.; Wang, X.D. Five-year study on the effects of warming and plant litter quality on litter decomposition rate in a Tibetan alpine grassland. Sci. Total Environ. 2021, 750, 142306. [Google Scholar] [CrossRef]
- Robinson, C.H. Controls on decomposition and soil nitrogen availability at high latitudes. Plant Soil 2002, 242, 65–81. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.M.; McLauchlan, K.; Schimel, J.P. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 86, 320–326. [Google Scholar] [CrossRef]
- Sanchez, F.G. Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. For. Ecol. Manag. 2001, 152, 85–96. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Decomposition as a Process—Some Main Features. In Plant Litter—Decomposition, Humus Formation, Carbon Sequestration; Springer: Cham, Switzerland, 2020; pp. 13–43. [Google Scholar] [CrossRef]
- Edmonds, R.L.; Tuttle, K.M. Red alder leaf decomposition and nutrient release in alder and conifer riparian patches in western Washington, USA. For. Ecol. Manag. 2010, 259, 2375–2381. [Google Scholar] [CrossRef]
- Laskowskim, R.; Niklinskam, M.; Maryanski, M. The dynamics of chemical elements in forest litter. Ecology 1995, 76, 1393–1406. [Google Scholar] [CrossRef]
- Yue, K.; Ni, X.Y.; Fornara, D.A.; Peng, Y.; Liao, S.; Tan, S.Y.; Wang, D.Y.; Wu, F.Z.; Yang, Y.S. Dynamics of calcium, magnesium, and manganese during litter decomposition in alpine forest aquatic and terrestrial ecosystems. Ecosystems 2021, 24, 516–529. [Google Scholar] [CrossRef]
- Windham, L.M.; Weis, J.S.; Weis, P. Metal dynamics of plant litter of Spartina alterniflora and Phragmites australis in Metal-Contaminated salt marshes. Part 1: Patterns of decomposition and metal uptake. Environ. Toxicol. Chem. 2004, 23, 1520–1528. [Google Scholar] [CrossRef]
- Bockheim, J.G.; Jepsen, E.A.; Heisey, D.M. Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in northwestern Wisconsin. Can. J. For. Res. 1991, 21, 803–812. [Google Scholar] [CrossRef]
- Gray, D.M.; Dighton, J. Mineralization of forest litter nutrients by heat and combustion. Soil Biol. Biochem. 2006, 38, 1469–1477. [Google Scholar] [CrossRef]
- Berg, B.; Steffen, K.T.; McClaugherty, C. Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 2007, 82, 29–39. [Google Scholar] [CrossRef]
- Maie, N.; Behrens, A.; Knicker, H.; Kögel-Knabner, I. Changes in the structure and protein binding ability of condensed tannins during decomposition of fresh needles and leaves. Soil Biol. Biochem. 2003, 35, 577–589. [Google Scholar] [CrossRef]
- Schädel, C.; Blöchl, A.; Richter, A.; Hoch, G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol. Biochem. 2010, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osono, T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Cox, P.; Wilkinson, S.P.; Anderson, J.M. Effects of fungal inocula on the decomposition of lignin and structural polysaccharides in Pinus sylvestris litter. Biol. Fertil. Soils 2001, 33, 246–251. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 15. [Google Scholar] [CrossRef]
- Berg, B.; Hannus, K.; Popoff, T.; Theander, O. Changes in organic chemical components of needle litter during decomposition. Long-term decomposition in a Scots pine forest. I. Can. J. Bot. 1982, 60, 1310–1319. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Newman, R.H. 13C NMR study of pine needle decomposition. Plant Soil 2000, 219, 273–278. [Google Scholar] [CrossRef]
- Polyakova, O.; Billor, N. Impact of deciduous tree species on litterfall quality, decomposition rates and nutrient circulation in pine stands. For. Ecol. Manag. 2007, 253, 11–18. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).