Response of Understory Plant Diversity to Edge Effects in Plantation Forests on the Loess Plateau
Abstract
1. Introduction
2. Materials and Methods
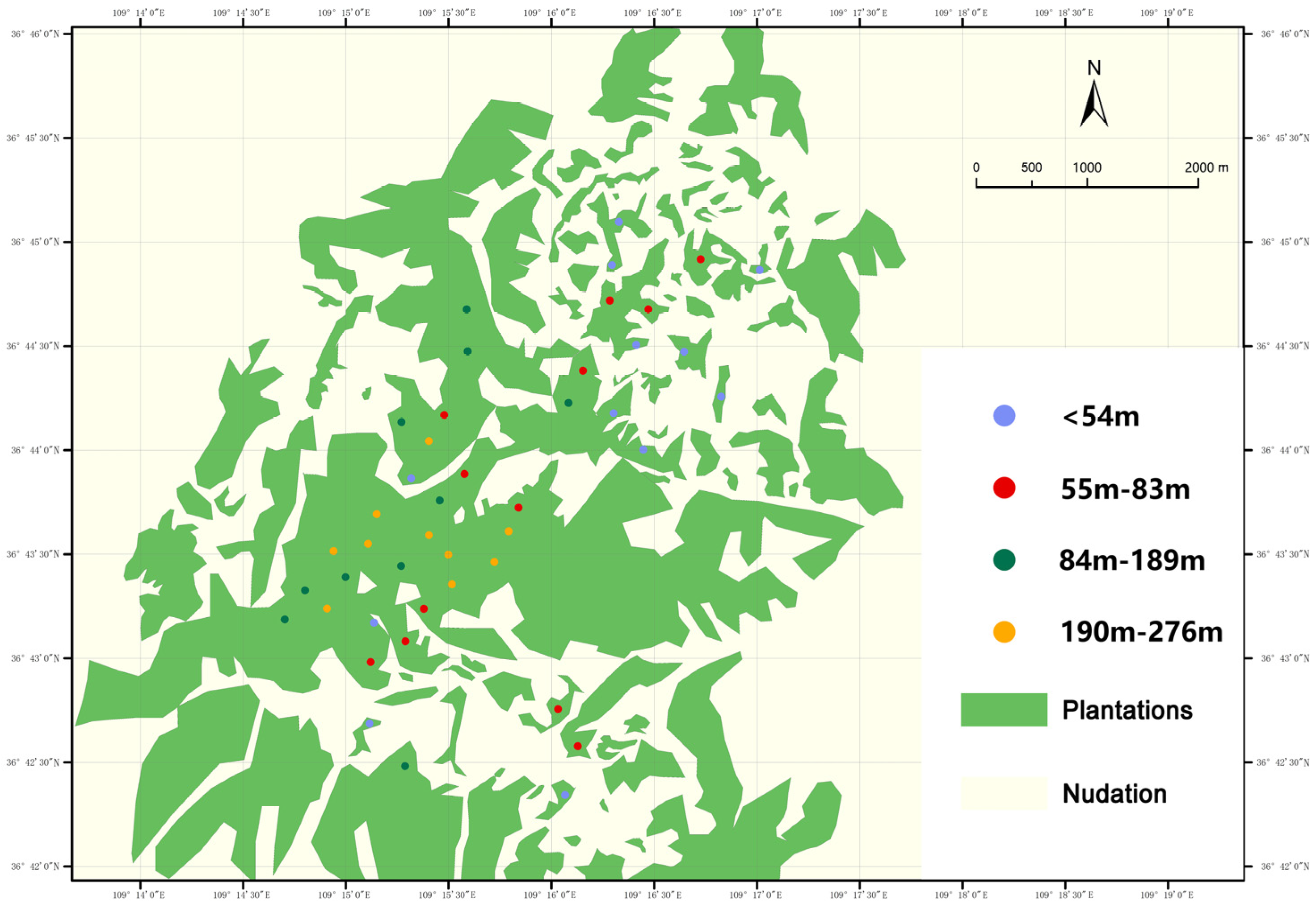
2.1. Study Site
2.2. Experimental Design
2.3. Data Processing and Analysis
3. Results
3.1. Edge Effect Intensity and Depth
3.2. Effect of Edge Distance, Canopy Cover, and Stand Density on Biodiversity
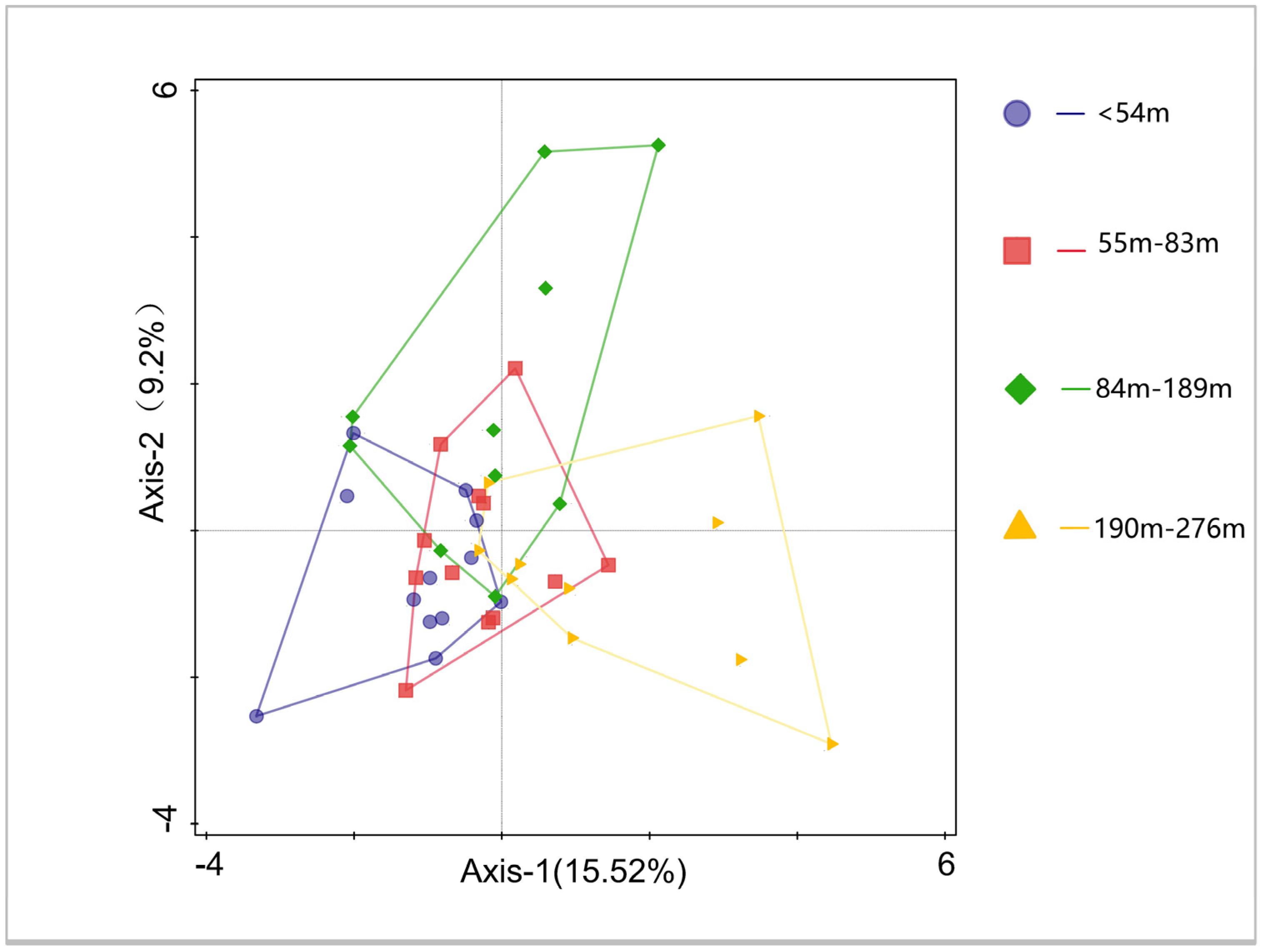
3.3. Species Composition
4. Discussion
4.1. The Strength and Distance of Edge Effects on Each Indicator
4.2. Effect of Edge Distance, Canopy Cover, and Stand Density on Shannon Index
4.3. For the Follow-Up Management Measures Recommended
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Liu, J.G.; Li, S.X.; Ouyang, Z.Y.; Christine, T.; Chen, X.D. Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc. Natl. Acad. Sci. USA 2008, 105, 9477–9482. [Google Scholar] [CrossRef]
- Lü, Y.; Fu, B.; Feng, X.; Zeng, Y.; Liu, Y.; Chang, R.; Sun, G.; Wu, B. A policy-driven large scale ecological restoration: Quantifying ecosystem services changes in the Loess Plateau of China. PLoS ONE 2017, 7, e31782. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Coomes, D.A.; Gibson, L.; Hu, G.; Liu, J.; Luo, Y.; Wu, C.; Yu, M. Forest fragmentation in China and its effect on biodiversity. Biol. Rev. 2019, 94, 1636–1657. [Google Scholar] [CrossRef]
- Carolina, M. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar]
- Chaplin-Kramer, R.; Ramler, I.; Sharp, R.; Haddad, N.M.; Gerber, J.S.; West, P.C.; Mandle, L.; Engstrom, P.; Baccini, A.; Sim, S.; et al. Degradation in carbon stocks near tropical forest edges. Nat. Commun. 2015, 6, 10158. [Google Scholar] [CrossRef]
- Smith, I.A.; Hutyra, L.R.; Reinmann, A.B.; Marrs, J.K.; Thompson, J.R. Piecing together the fragments: Elucidating edge effects on forest carbon dynamics. Front. Ecol. Environ. 2018, 16, 213–221. [Google Scholar] [CrossRef]
- Catherine, L.P.; Stephen, M.T.; Miriam, G. Edge Effects of Linear Canopy Openings on Tropical Rain Forest Understory Microclimate. Biotropica 2007, 39, 62–71. [Google Scholar]
- Hofmeister, J.; Hošek, J.; Brabec, M.; Střalková, R.; Mýlová, P.; Bouda, M.; Pettit, J.L.; Rydval, M.; Svoboda, M. Microclimate edge effect in small fragments of temperate forests in the context of climate change. For. Ecol. Manag. 2019, 48, 48–56. [Google Scholar] [CrossRef]
- Schmidt, M.; Jochheim, H.; Kersebaum, K.C.; Lischeid, G.; Nendel, C. Gradients of microclimate, carbon and nitrogen in transition zones of fragmented landscapes—A review. Agric. For. Meteorol. 2017, 232, 659–671. [Google Scholar] [CrossRef]
- Briant, G.; Gond, V.; Laurance, S.G.W. Habitat fragmentation and the desiccation of forest canopies: A case study from eastern Amazonia. Biol. Conserv. 2010, 143, 2763–2769. [Google Scholar] [CrossRef]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.; Ewers, R.M.; Harms, K.E.; Luizão, R.C.C.; Ribeiro, J.E. Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2007, 2, e1017. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Zhang, D. Forest parcelization in the United States—A study of contributingfactors. J. For. 2001, 99, 30–34. [Google Scholar]
- Willmer, J.N.G.; Püttker, T.; Prevedello, J.A. Global impacts of edge effects on species richness. Biol. Conserv. 2022, 272, 109654. [Google Scholar] [CrossRef]
- Franklin, C.M.A.; Harper, K.A.; Clarke, M.J. Trends in studies of edge influence on vegetation at human-created and natural forest edges across time and space. Can. J. For. Res. 2021, 51, 274–282. [Google Scholar] [CrossRef]
- Solar, R.R.d.C.; Barlow, J.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Thomson, J.R.; Louzada, J.; Maués, M.; Moura, N.G.; Oliveira, V.H.F.; et al. How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecol. Lett. 2015, 18, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Lefebvre, V.; Peres, C.A.; Banks, L.C.; Wearn, O.R.; Marsh, C.J.; Butchart, S.H.M.; Arroyo-Rodríguez, V.; Barlow, J.; Cerezo, A.; et al. Creation of forest edges has a global impact on forest vertebrates. Nature 2017, 551, 187. [Google Scholar] [CrossRef] [PubMed]
- Sfair, J.C.; Arroyo-Rodríguez, V.; Santos, B.A.; Tabarelli, M. Taxonomic and functional divergence of tree assemblages in a fragmented tropical forest. Ecol. Appl. 2016, 26, 1816–1826. [Google Scholar] [CrossRef]
- Collins, C.D.; Banks-Leite, C.; Brudvig, L.A.; Foster, B.L.; Cook, W.M.; Damschen, E.I.; Andrade, A.; Austin, M.; Camargo, J.L.; Driscoll, D.A.; et al. Fragmentation affects plant community composition over time. Ecography 2017, 40, 119–130. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Esseen, P.-A. Edge Influence on Forest Structure and Composition in Fragmented Landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Paulson, A.K.; Peña, H.; Alexander, H.D.; Davydov, S.P.; Loranty, M.M.; Mack, M.C.; Natali, S.M. Understory plant diversity and composition across a postfire tree density gradient in a Siberian Arctic boreal forest. Can. J. For. Res. 2021, 51, 720–731. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Guo, J.; Tan, Z.; Dong, W.; Wang, H. Changes in the understory diversity of secondary Pinus tabulaeformis forests are the result of stand density and soil properties. Glob. Ecol. Conserv. 2021, 28, e01628. [Google Scholar] [CrossRef]
- Hedwall, P.O.; Holmström, E.; Lindbladh, M.; Felton, A. Concealed by darkness: How stand density can override the biodiversity benefits of mixed forests. Ecosphere 2019, 10, e02835. [Google Scholar] [CrossRef]
- Mestre, L.; Toro-Manríquez, M.; Soler, R.; Huertas-Herrera, A.; Martínez-Pastur, G.; Lencinas, M.V. The influence of canopy-layer composition on understory plant diversity in southern temperate forests. For. Ecosyst. 2017, 4, 6. [Google Scholar] [CrossRef]
- Jagodzinski, A.M.; Oleksyn, J. Ecological consequences of silviculture at variable stand densities. III. Stand stability, phytoclimate and biodiversity. SYLWAN 2009, 153, 219–230. [Google Scholar]
- Benchimol, M.; Peres, C.A.; Bellingham, P. Edge-mediated compositional and functional decay of tree assemblages in Amazonian forest islands after 26 years of isolation. J. Ecol. 2015, 103, 408–420. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological Responses to Habitat Edges: Mechanisms, Models, and Variability Explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Tabarelli, M.; Peres, C.A.; Melo, F.P.L. The ‘few winners and many losers’ paradigm revisited: Emerging prospects for tropical forest biodiversity. Biol. Conserv. 2012, 155, 136–140. [Google Scholar] [CrossRef]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Fearnside, A.C.; Fearnside, P.M.; Fearnside, P.M.; Ribeiro, J.E.L.; Capretz, R.L. Rain forest fragmentation and the proliferation of successional trees. Ecology 2006, 87, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Víctor, A.R.; Melo, F.P.L.; Miguel, M.R.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. Camb. Philos. Soc. 2017, 92, 326–340. [Google Scholar]
- Normile, D. Getting at the Roots of Killer Dust Storms. Science 2007, 317, 314–316. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Liu, Q.J.; Qin, L.H.; Liu, F.Z.; Siqing, B.L. Ecological Beneft Assessment of the Grain for Green Project in Yan an. J. Northwest For. Univ. 2022, 37, 259–266. [Google Scholar]
- Zhang, C.X.; Lu, X.; Gao, S. Residual Chi-square Test and DAVAR Based on Fault Diagnosis and Positioning. Navig. Control. 2018, 17, 25–31. [Google Scholar]
- Liu, M.; Wang, Y.Y. Exploring in Durbin-Watson Autocorrelation Test. J. Quant. Tech. Econ. 2014, 31, 153–160. [Google Scholar]
- Li, H.; Li, C.; Zha, T.; Liu, J.; Jia, X.; Wang, X.; Chen, W.; He, G. Patterns of biomass allocation in an age-sequence of secondary Pinus bungeana forests in China. For. Chron. 2014, 90, 169–176. [Google Scholar] [CrossRef]
- Krishnadas, M.; Bagchi, R.; Sridhara, S.; Comita, L.S. Weaker plant-enemy interactions decrease tree seedling diversity with edge-effects in a fragmented tropical forest. Nat. Commun. 2018, 9, 4523. [Google Scholar] [CrossRef] [PubMed]
- Zaninovich, S.C.; Gatti, M.G. Carbon stock densities of semi-deciduous Atlantic forest and pine plantations in Argentina. Sci. Total Environ. 2020, 747, 41085. [Google Scholar] [CrossRef]
- Meeussen, C.; Govaert, S.; Vanneste, T.; Haesen, S.; Van Meerbeek, K.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.A.O.; Diekmann, M.; et al. Drivers of carbon stocks in forest edges across Europe. Sci. Total Environ. 2020, 759, 143497. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, L.D.M.; Arturi, M.F.; Goya, J.F.; Pérez, C.A.; Frangi, J.L. Eucalyptus grandis plantations: Effects of management on soil carbon, nutrient contents and yields. J. For. Res. 2018, 31, 601–611. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y. Medium and Long-Term Influences of Typical Plantations on Surface Soil Nutrients in the Temperate Zone of Mount Meng, China. Appl. Ecol. Environ. Res. 2018, 16, 6385–6393. [Google Scholar] [CrossRef]
- Stine, A.R.; Huybers, P. Implications of Liebig’s law of the minimum for tree-ring reconstructions of climate. Environ. Res. Lett. 2017, 12, 114018. [Google Scholar] [CrossRef]
- Laurance, W.F.; Laurance, S.G.; Ferreira, L.V.; Merona, J.M.R.D.; Gascon, C.; Lovejoy, T.E. Biomass Collapse in Amazonian Forest Fragments. Science 1997, 278, 1117–1118. [Google Scholar] [CrossRef]
- Grandpré, L.D.; Gagnon, D.; Bergeron, Y. Changes in the Understory of Canadian Southern Boreal Forest after Fire. J. Veg. Sci. 1993, 4, 803–810. [Google Scholar] [CrossRef]
- Hart, S.A.; Chen, H.Y.H. Understory Vegetation Dynamics of North American Boreal Forests. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Aussenac, G. Interactions between forest stands and microclimate: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Rambo, T.R.; North, M.P. Canopy microclimate response to pattern and density of thinning in a Sierra Nevada forest. For. Ecol. Manag. 2009, 257, 435–442. [Google Scholar] [CrossRef]
- Holbo, H.R.; Childs, S.W. Summertime Radiation Balances of Clearcut and Shelterwood Slopes in Southwest Oregon. For. Sci. 1987, 33, 504–516. [Google Scholar] [CrossRef]
- Moore, R.; Spittlehouse, D.L.; Story, A. Riparian microclimate and stream temperature response to forest harvesting: A review. JAWRA J. Am. Water Resour. Assoc. 2005, 41, 813–834. [Google Scholar] [CrossRef]
- Park, S.G.; Kurosawa, K. Evaluation of Regeneration Potential for an Artificial Larch Forest Using Light Condition, Plant Species Diversity, and Plant Coverage and Biomass of the Forest Understory. J. Fac. Agric. Kyushu Univ. 2017, 62, 47–52. [Google Scholar] [CrossRef]
- Xu, J. China’s new forests aren’t as green as they seem. Nature 2011, 477, 371. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Deng, N.; Ma, F.; Song, Q.; Tian, Y. Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China. Open Life Sci. 2023, 18, 20220574. [Google Scholar] [CrossRef]



| R2 = 0.34 | Unstandardized Coefficient | Standardized Coefficient | t | Significance | 95.0% Confidence Interval of B | ||
|---|---|---|---|---|---|---|---|
| B | SE | Beta | Lower limit | Upper limit | |||
| intercept distance | 1.16 | ±0.27 | 4.32 | 0.00 * | 0.62 | 1.70 | |
| Distance (m) | 0.00 | ±0.00 | 0.00 | 0.01 | 0.99 | 0.00 | 0.00 |
| Tree density (/0.01 ha) | 0.06 | ±0.03 | 0.49 | 2.24 | 0.03 * | 0.01 | 0.12 |
| Canopy (%) | 0.46 | ±0.69 | 0.16 | 0.67 | 0.51 | −0.92 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Zheng, B.; Lei, H.; Guo, H.; Li, X. Response of Understory Plant Diversity to Edge Effects in Plantation Forests on the Loess Plateau. Forests 2025, 16, 87. https://doi.org/10.3390/f16010087
Du S, Zheng B, Lei H, Guo H, Li X. Response of Understory Plant Diversity to Edge Effects in Plantation Forests on the Loess Plateau. Forests. 2025; 16(1):87. https://doi.org/10.3390/f16010087
Chicago/Turabian StyleDu, Sixuan, Bo Zheng, Hangyu Lei, Huifeng Guo, and Xiang Li. 2025. "Response of Understory Plant Diversity to Edge Effects in Plantation Forests on the Loess Plateau" Forests 16, no. 1: 87. https://doi.org/10.3390/f16010087
APA StyleDu, S., Zheng, B., Lei, H., Guo, H., & Li, X. (2025). Response of Understory Plant Diversity to Edge Effects in Plantation Forests on the Loess Plateau. Forests, 16(1), 87. https://doi.org/10.3390/f16010087





