Abstract
The fig (the syconium of the Ficus tree) and its pollinating fig wasp represent exceptional examples for researching plant–insect interactions due to their remarkable specificity in species interaction and mutually beneficial symbiotic relationship. However, the mechanisms underlying the developmental process of monoecious figs in response to the entry of pollinating fig wasps (pollinators) and the metabolic changes occurring during this process remain elusive. Our study employed a combination of controlled experiments in the field and LC-MS methods to investigate the impact of pollinating fig wasp entry on the developmental phase of figs, as well as the metabolic alterations occurring during this process. A total of 381 metabolites and 155 differential metabolites were identified, with the predominant classes of metabolites being organic acids, lipids, and benzene aromatic compounds. The results suggest that in the absence of wasp entry, the receptive phase of fig would exhibit an extended duration. However, upon the entry of fig wasps, the receptive phase of figs would terminate within a span of 1 to 2 days, concomitant with substantial fluctuations in the composition and proportions of metabolites within the fig. Our research focuses on the analysis of linoleic acid metabolism, phenylpropanoid biosynthesis, and flavonoid biosynthesis pathways. Our findings suggest that the entry of wasps triggers alterations in the metabolic regulatory mechanisms of figs. Prior to wasp entry, metabolites primarily regulate fig growth and development. However, after wasp entry, metabolites predominantly govern lipid accumulation and the establishment of defense mechanisms, indicating a transition in fig development. This metabolic perspective explains why figs promptly enter an interflower phase that is not attractive to pollinating fig wasps after their entry, and how figs achieve reproductive balance through the regulation of different metabolic pathways. This study provides scientific evidence for elucidating the stability mechanism of the fig wasp mutualistic system.
1. Introduction
The Ficus genus, a member of the Moraceae family, encompasses approximately 800 species worldwide and predominantly thrives in tropical and subtropical regions, with a minority extending into temperate zones [1]. Renowned for its economic significance, the fig tree holds a prominent position among ornamental plants in gardens [2]. Moreover, it serves as a resource for food production, medicinal applications, industrial utilization, and so on [3,4]. In tropical and subtropical forest ecosystems, fig trees bear figs throughout the year, providing a crucial food source for many frugivorous animals. Additionally, fig trees serve as habitats for various species of parasitic and epiphytic plants, thus occupying a significant ecological niche in tropical and subtropical ecosystems [5]. Moreover, the mutualistic symbiosis between the fig tree and its pollinating fig wasp is an important research subject for studying plant–insect interactions. This specialized mutualism involves a single species of fig wasp that lays eggs in the female flowers of the fig, while each species of fig can only be pollinated by one particular wasp species, with only a few exceptions [6]. Consequently, both the fig tree and its pollinating wasp are mutually dependent on each other for reproduction [7]. The fruits of the Ficus genus, known as figs or syconia, require the specialized species of pollinating fig wasp to pollinate the female flowers within the syconium and deposit eggs in their ovaries, thereby facilitating reproduction for both parties involved in this mutualistic relationship [8]. Figs can be classified as either dioecious or monoecious. Dioecious figs consist of separate female and male figs, with the female producing seeds and the male hosting gall separately. In contrast, monoecious figs produce both seeds and host gall within the same fig, leading to competition for reproductive resources between the seed-producing flowers and the gall-forming flowers [9].
The developmental stages of a monoecious fig can be categorized as the pre-female phase, female phase (receptive phase), interfloral phase, male phase, and post-floral phase. During the pre-flower phase, the bract of the fig is tightly closed, preventing the entry of wasps. Upon reaching the receptive phase, female flowers mature and bract ostiole loosens, which enables fig wasps to enter for egg-laying and pollination [10]. Subsequently, during the interfloral phase, which is characterized by reduced attractiveness to pollinating fig wasps, studies have suggested that the duration of receptivity may vary depending on whether or not fig wasps enter [11]. Following the interfloral phase, both fig wasps and/or seeds reach maturity as the fig transitions into its male and post-floral phases before eventually rotting or being consumed by frugivores (Figure 1). Prior to the entry of pollinators, the female flowers of figs are in the phase of vegetative growth. However, upon pollinator entry for both pollination and egg-laying, the female flowers transition into reproductive growth, initiating fig seed and wasp gall production [12]. Consequently, it can be inferred that the entry of pollinating fig wasps represents a crucial developmental turning point in the life cycle of figs.
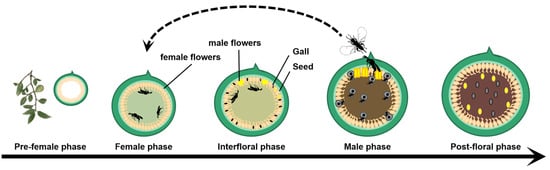
Figure 1.
Life history of monoecious fig and its pollinating fig wasps.
The fig literature is rich in experiments showing that the egg-laying and pollination activities of fig wasps deeply modify the development of figs [13], while the study of metabolic pathways following pollination and insect galling has not been reported. Upon entry into monoecious figs, fig wasps parasitize the ovary of female flowers through egg deposition, thereby imposing pressure on their normal development. However, regardless of the number of wasps that enter, not all flowers within the fig will fully develop into galls, instead, certain female flowers will grow to seed [10]. Currently, the allocation of female flowers and the underlying biological mechanisms governing this regulation have garnered attention from researchers [11,14,15]. Metabolomics has emerged as a widely employed approach to investigate plant response mechanisms toward parasitic galls. For instance, it was used to clarify changes in the Metabolome of secondary metabolites in rubber trees due to Pseudphodiplosis rubi (Diptera: Cecidomyiidae). Additionally, it has shown that increased aromatic amino acids are key to tomato plants’ resistance to nematodes. Metabolomics studies also indicate that Haloxylon persicum exhibits a stronger defense response, with higher levels of fatty acids, dialkyl ethers, and carbohydrates, when infected by Asiodiplosis ulkunkalkani and Caillardia notata, compared to Haloxylon aphyllum, improving our understanding of plant defense mechanisms [16,17,18]. Currently, research on the impact of pollinating fig wasps’ entry on fig developmental stages primarily focuses on morphological structures [19,20], ecological phenomena [21,22], volatile compounds [23], and a limited number of studies investigating molecular mechanisms [24]. However, there is still a dearth of studies exploring the metabolic regulatory mechanisms underlying changes in fig developmental stages.
This study aims to investigate the impact of pollinating fig wasps on fig development through controlled experiments in the field. Additionally, we will utilize metabolomics technology to compare the metabolic changes in figs before and after the introduction of pollinating fig wasps. The objective is to elucidate the mediating role of pollinating fig wasps in fig development and unravel the defense mechanisms employed by figs against wasp parasitism from a metabolic perspective. This research provides the theoretical underpinning for comprehending the stability mechanisms of the fig-wasp system and also serves as a reference for studying plant–insect cooperation and antagonism in nature.
2. Materials and Methods
2.1. Study Sites and Materials
The study site is located in Kunming City, the capital of Yunnan Province, China (N: 25°6′, E: 102°44′, average elevation: 1907 m). It is situated in the southwestern region of China and falls within the low-latitude subtropical highland monsoon climate zone. The area experiences abundant sunshine hours, a short frost-free period, an average annual temperature of 15 °C, approximately 2200 h of annual sunshine duration, and an annual precipitation of 1450 mm. The climate exhibits mild and sunny characteristics [25]. For this research project, Ficus hookeriana was selected as the experimental species due to its wide distribution in tropical and subtropical regions of Asia. This tall evergreen tree bears figs throughout the year, and its figs are monecious syconium. Additionally, it holds significant importance as an ornamental and landscape tree species [26]. The pollinating fig wasps of Ficus hookeriana are Platyscapa sp. (Hymenoptera: Chalcidoidea, Agaonidae), exhibiting sexual dimorphism [27]. The pollinating fig wasps for the controlled experiment are females. They were collected as follows: F. hookeriana figs in the male phase were placed in netting bags before the field experiment. The wasps naturally emerged around 6–8 a.m. the next morning and were then taken to the field for the experiment.
2.2. Controlled Experiment in the Field
To prevent interference from fig wasps and other insects, the pre-female floral phase figs were isolated using netting bags. The ostiolar bract of each fig was observed daily, and upon noticing loosening, the bag was removed, and an active fig wasp was introduced onto the fig wall. If the fig wasp crawled toward the bract and exhibited behavior indicating an attempt to enter the fig, it was promptly removed using tweezers, marking that day as the first receptive day. A total of 30 figs with 2 wasps entry and 30 figs without wasps entry were set up for experimentation. This process was repeated daily to record the behavior of the fig wasps. The experiment could only be concluded once the receptive phase of all figs had ceased. If the pollinator does not show any entry behavior to the ostiole within 5 min, the receptive phase is considered to have stopped [11]. A total of 3 trees were utilized in the experiment.
2.3. Data Analysis
The normality of the data was assessed using SPSS 20.0. If the data met the criteria for a normal distribution, an independent sample t-test was conducted. Otherwise, the Mann–Whitney non-parametric test was employed. A p-value less than 0.05 indicates a statistically significant difference.
2.4. Metabolomic Analysis
2.4.1. LC-MS Sample Preparation
The metabolomic techniques were conducted in collaboration with BioNovoGene Technology Co., Ltd. (Suzhou, China). In this study, a total of 10 figs were selected that had not been entered by wasps (BF), while another set of 10 figs was also selected after being intentionally pollinated by pollinating fig wasps for two days (AF). The female flowers were meticulously dissected from these figs and subsequently placed into 0.5 mL test tubes, with each treatment being replicated three times. The samples were rapidly frozen using liquid nitrogen for a duration of 10 min, followed by storage at −80 °C. Subsequently, they underwent further processing through grinding into a fine powder. To this powder, 40 mg of the sample, along with 600 µL of methanol containing 2-chlorophenylalanine (4 ppm), and 100 mg of small steel ball were added in 2 mL centrifugal tubes. Then, the mixture was vortexed for 30 s, ground at a frequency of 60 Hz for 90 s, and subsequently subjected to ultrasonic extraction for 15 min. Afterward, it was centrifuged at 12,000 rpm at 4 °C for 10 min, the supernatant was filtered through a 0.22 μm membrane filter, and the filtrate was preserved for LC-MS detection.
2.4.2. LC Conditions and MS Method
The LC-MS analysis was performed using the Waters ACQUITY ultra-performance liquid chromatography system (Waters, Milford, MA, USA) coupled with a Thermo Q Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The samples were introduced onto an ACQUITY UPLC® HSS T3 column (2.1 × 150 mm, 1.8 µm) (Waters, Milford, MA, USA) at a flow rate of 0.25 mL/min and maintained at a temperature of 40 °C with an injection volume of 2 µL. In positive ion (POS) mode, the mobile phase consisted of acetonitrile with 0.1% formic acid (B1) and water with 0.1% formic acid (A1). In negative ion mode, the mobile phase comprised acetonitrile (B2) and water with 5 mM ammonium formate (A2). The elution gradient was conducted as follows: 0–1 min at 2% phase B; 1–9 min from 2% to 50% phase B; 9–12 min from 50% to 98% phase B; 12–13.5 min at 98% phase B; 13.5–14 min from 98% to 2% phase B; and 14–20 min at 2% phase B. The mass spectrometry conditions were applied as follows: the electrospray ionization source (ESI) was operated in both positive and negative ion modes, with a spray voltage set at +3.50 kV and −2.50 kV, respectively. The sheath gas was maintained at 30 arb, the auxiliary gas at 10 arb, and the temperature was kept constant at 325 °C.
2.4.3. Data Processing and Multivariate Analysis
The R XCMS software (version 4.4.1) suite was utilized for feature identification, retention time calibration, and alignment. Metabolite identification was accomplished by precise mass measurements (within <30 ppm) and MS/MS data that were matched with HMDB (Human Metabolome Database), MassBank, LipidMaps, mzCloud, and KEGG (Kyoto Encyclopedia of Genes and Genomes). To normalize the data to mitigate any systematic biases, the robust LOESS signal correction (QC-RLSC) was implemented. Post-normalization, only ion peaks exhibiting relative standard deviations (RSDs) of less than 30% in quality control were retained to ensure accurate metabolite identification.
The components eluted from the chromatography were introduced into the mass spectrometer, and the mass spectrometer conducted continuous scanning to collect data for obtaining the base peak chromatogram. To preliminarily determine the overall metabolic differences and variability among each group of samples, the normalized metabolite data were employed to generate a hierarchical clustering heatmap (HCA). The Pearson correlation coefficient was employed for the evaluation of reproducibility and correlation. To determine the extent of intergroup differences for the identified metabolites, (orthogonal) partial least squares-discriminant analysis (OPLS-DA) was performed to calculate the variable importance in projection (VIP) and fold change (FC). A significant threshold for variable importance of metabolites exhibiting notable differences in expression levels was established based on VIP > 1 and p-value < 0.05. The differential metabolites were thoroughly analyzed in terms of quantity and attributes, accompanied by the generation of volcano plots and bar charts to visually represent their differential expression patterns. Based on the results obtained from the screening of differential metabolites, we performed pathway annotation and analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG). The KEGG pathway mapper functionality was employed to annotate and present significant differential metabolites (VIP > 1, p-value < 0.01) along with their corresponding pathways. In order to enhance visual clarity, color-coding based on upregulation and downregulation information of these differential metabolites was incorporated into the display.
3. Results
3.1. The Duration of Fig Receptivity Following Pollinator Entry and in the Absence of Pollinators
After pollinator entry, the attractiveness of figs to pollinators rapidly diminishes, indicating a loss of receptivity upon pollinators. However, in the absence of the pollinator entry, the receptive phase can extend beyond one week. Based on observations of pollinator behavior at the ostiole, figs that had been visited by two foundresses exhibited significantly shorter receptivity compared to those without any pollinator entry (p < 0.05) (Figure 2).
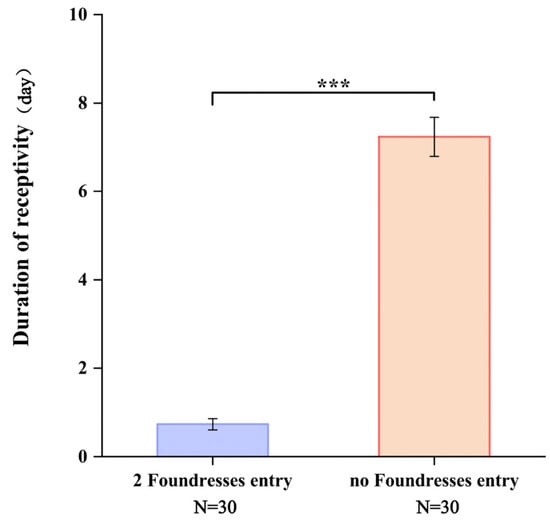
Figure 2.
The duration of fig receptivity following pollinator entry and in the absence of pollinators. Note: ***: p < 0.01.
3.2. Metabolomic Analysis of Pre- and Post-Pollinator Entry Figs
3.2.1. Data Quality Assessment
The metabolites of female flowers before and after the entry of pollinating fig wasps into the figs were analyzed using LC-MS. After quality control inspection, the total ion chromatogram (TIC) plots for data quality control revealed a significant overlap in the ground curves of metabolite detection. Furthermore, the retention times and peak intensities of the metabolites exhibited consistent patterns, indicating optimal stability in the signals obtained from quality control detection and ensuring reliable experimental outcomes (Figure 3a). To investigate the accumulation pattern of metabolites before and after the entry of pollinating fig wasps into the figs, this study employed a cluster heat map analysis, revealing significant differences in metabolite expression among the six clusters. Metabolites in cluster 2 were up-regulated in the BF group but down-regulated in the AF group. Conversely, metabolites in clusters 5 and 6 were down-regulated in the BF group and up-regulated in the AF group.
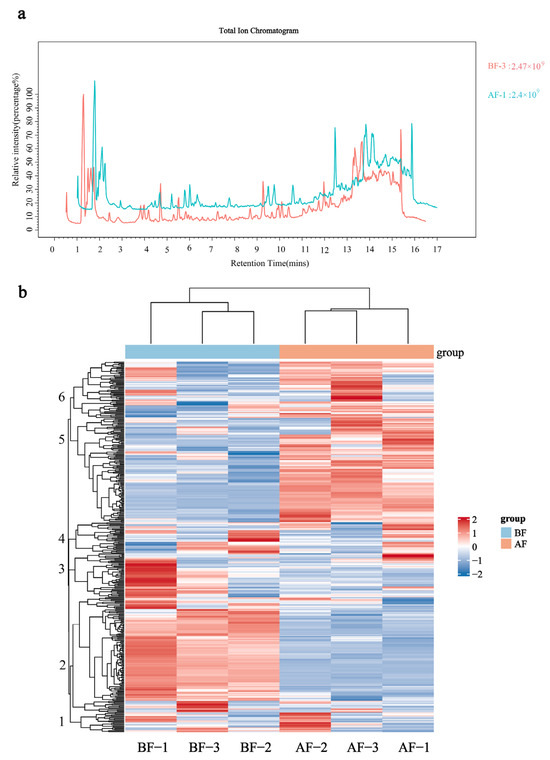
Figure 3.
Quality control of samples: (a) Total Ion Current Overlap Plot in Mass Spectrometry; (b) Metabolite clustering heatmap. BF: prior to figs entry by pollinators; AF: subsequent to figs entry by pollinators. Note: The relative abundance of metabolites depicted in Figure (b) is represented by color intensity, where red signifies increased expression and blue denotes decreased expression. Positive values indicate up-regulated metabolites, while negative values indicate down-regulated metabolites. Metabolites with similar expression patterns are clustered on the left side of the dendrogram, forming a hierarchical tree of differentially expressed metabolites.
The remaining clusters exhibited minimal changes in expression levels. Moreover, the three biological replicates of each treatment were consistently grouped together, indicating good homogeneity among the replicates and ensuring high data reliability. These findings highlight a remarkable diversity in metabolite expression within figs before and after fig wasp pollination occurs (Figure 3b).
3.2.2. Metabolite Composition Analysis
A total of 381 metabolites, classified into 15 categories, were identified in figs before and after pollinators’ entry. These categories primarily encompass organic acids and their derivatives, lipids, cyclic aromatic hydrocarbons derived from benzene, heterocyclic compounds not derived from benzene, organic peroxides, and flavonoids (Figure 4a).
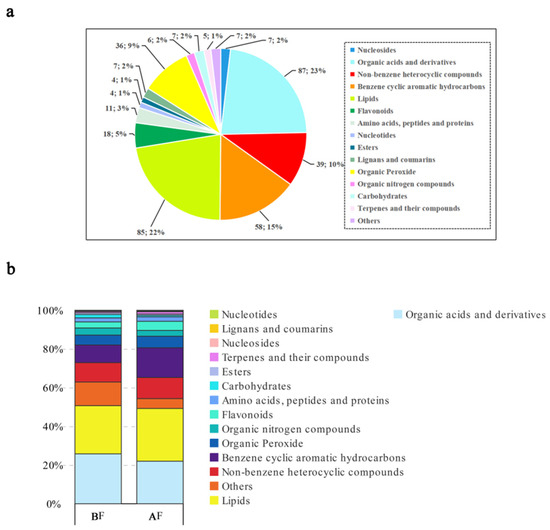
Figure 4.
Metabolite composition analysis: (a) Metabolite categorization pie chart; (b) Metabolite grouping comparison stack; BF: prior to figs entry by pollinators; AF: subsequent to figs entry by pollinators.
The metabolites of figs were classified before and after the wasps entered, and the differences in the expression of different metabolites were compared between the two groups. It was discovered that the presence of pollinators led to significant alterations in metabolite expression levels in figs. Specifically, lipids, benzene cyclic aromatic hydrocarbons, organic peroxides, flavonoids, terpenes, and their compounds exhibited a substantial increase. Conversely, there was a notable decrease in the levels of organic acids and their derivatives, organic nitrogen compounds, carbohydrates, as well as others (in Figure 4, others represent compounds not belonging to primary categories). These findings suggest that the introduction of fig-pollinating wasps elicits a complex metabolic response within figs (Figure 4b).
3.2.3. Screening of Differentially Accumulated Metabolites (DAMs)
To transform the original metabolomics data to conform to a normal distribution, we applied a Log2FC (log2-fold change) transformation. Following this, differential metabolites were identified using screening criteria of VIP value > 1 and p-value < 0.05, resulting in the identification of 155 differential metabolites. Among these, 87 metabolites were up-regulated, while 68 showed down-regulation. Exclusive compounds have not been detected among groups. The major categories encompassed organic acids and their derivatives, non-benzene heterocyclic compounds, lipids, benzene cyclic aromatic hydrocarbons, terpenes, and their derivatives, as well as flavonoids (Figure 5).
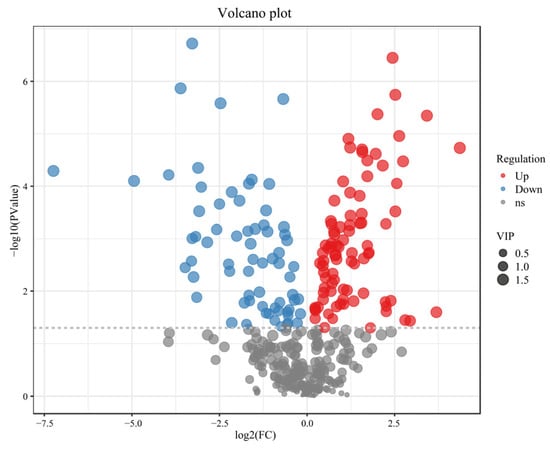
Figure 5.
Volcano plot of the differential expression of the metabolites.
3.2.4. Major Differential Metabolic Pathways in Fig Flower
The accumulated metabolites of figs before and after the entry of pollinators were compared with the KEGG database, leading to the identification of 215 KEGG pathways. The top twenty metabolic pathways are presented based on the screening criteria of p-value < 0.05. Notably, the enriched metabolic pathways included linoleic acid metabolism, alanine, aspartate, and glutamate metabolism, aminoacyl-tRNA biosynthesis, arginine biosynthesis, D-amino acid metabolism, purine metabolism, phenylpropanoid biosynthesis, beta-alanine metabolism, flavonoid biosynthesis, and pyrimidine metabolism (Figure 6). More than 10 out of 215 identified KEGG pathways were significantly enriched both prior to and following pollinator entry, indicating that these pathways may play a pivotal role in fig development.
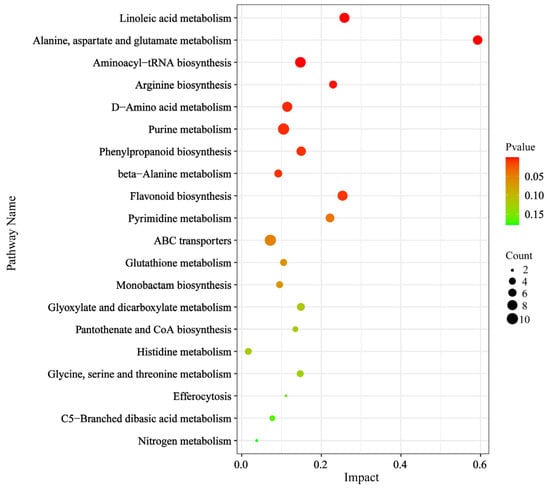
Figure 6.
KEGG enrichment analysis and network pathway analysis.
3.2.5. Linoleic Acid Metabolism Pathway Analysis
To further investigate the response of the fig to the entry of the pollinating fig wasp, we focused on the most significantly KEGG-enriched metabolic pathway for analysis: linoleic acid metabolism. This pathway has been demonstrated to function as a defensive mechanism against attacks and damage in other plants and plays a pivotal role in plant growth, development, and seed energy storage [28]. Subsequently, we evaluated the variations in expression levels of differential metabolites annotated within this pathway. Within the linoleic acid metabolic pathway, six differential metabolites were identified, including four up-regulated differential metabolites and two down-regulated differential metabolites. The linoleic acid metabolic pathway is initiated by the conversion of lecithin to linoleate and can be classified into two distinct pathways. In the first pathway, 13(S)-hydroperoxy linoleic acid (13(S)-HPODE) is down-regulated, resulting in subsequent down-regulation of 13-oxygen-octadecadienoic acid (13-oxo-ODE) and up-regulation of 9,12,13-trihydroxy-10-octadecenoic acid (9,12,13-Trihome). The second pathway begins with the transformation of linoleate into 9(S)-hydroperoxy linoleic acid (9(S)-HPODE), leading to up-regulation of 9,10,13-trihydroxy-11-octadecenoic acid (9,10,13-Trihome), 9(S)-hydroxyoctadecadienoic acid (9(S)-HODE), and 9-hydroxyoctadecadienoic acid (9-OxoODE) (Figure 7).
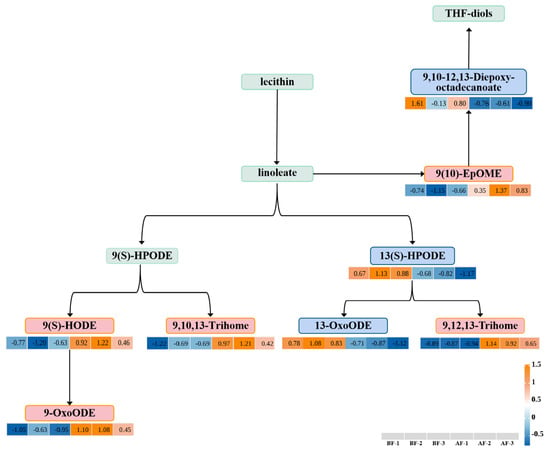
Figure 7.
Linoleic acid metabolism pathway. BF: prior to figs entry by pollinators; AF: subsequent to figs entry by pollinators. Note: Blue and red indicate down-regulation and up-regulation, respectively. Note: The relative abundance of metabolites depicted is represented by color intensity, where red signifies increased expression and blue denotes decreased expression. Positive values indicate up-regulated metabolites, while negative values indicate down-regulated metabolites.
3.2.6. Phenylpropanoid Biosynthesis and Flavonoid Biosynthesis Pathway Analysis
Population genomic analysis of the resequenced genomes of the Sycomorus subgenus in Ficus has revealed that phenylpropanoid biosynthesis and flavonoid biosynthesis are likely key metabolic pathways influencing fig-wasp mutualism and species differentiation [29]. In this study, these two pathways were also identified in the KEGG enrichment analysis of the metabolomics data. To investigate the obligate mutualistic relationship between figs and their pollinating wasps from a metabolomics perspective, we analyzed the significantly expressed metabolite intermediates in the phenylpropanoid and flavonoid biosynthesis pathways and further investigated the metabolic alterations in figs before and after wasp entry. The flavonoids and phenylpropanoids are crucial constituents of the plant cell wall, playing a defensive role in response to external stimuli [30]. Our study investigated the synthetic pathways of these compounds, revealing six differentially expressed metabolites in the phenylpropanoid biosynthesis pathway, including two up-regulated and four down-regulated metabolites. Additionally, we identified four differentially expressed metabolites in the flavonoid biosynthesis pathway, all of which were up-regulated. Both pathways are initiated via the enzymatic conversion of tyrosine to cinnamoyl-CoA, subsequently leading to the production of p-coumaroyl-CoA, thus providing precursors for distinct downstream pathways. The phenylpropanoid biosynthesis pathway comprises three branches. The first branch involves the down-regulation of tyrosine, which catalyzes the production of cinnamoyl-CoA, subsequently leading to the down-regulated expression of cinnamaldehyde. The second branch yields up-regulated caffeic acid through the interaction between cinnamoyl-CoA and tyrosine, ultimately resulting in a non-significantly expressed sinapoylmalate. The third branch, initiated by the conversion of cinnamoyl-CoA to p-coumaroyl-CoA, leads to the down-regulation of p-coumaraldehyde and coniferylaldehyde expression while up-regulating isoeugenol production, thereby resulting in a non-significant expression of isomethyleugenol. Moreover, the flavonoid biosynthesis pathway comprises three branches. The first branch is catalyzed by cinnamoyl-CoA and promotes increased pinocembrin levels, subsequently facilitating elevated chrysin production. The second branch utilizes p-coumaroyl-CoA as a precursor metabolite and results in up-regulated naringenin chalcone alongside down-regulated naringenin and sakuranetin. Additionally, the inhibitory effect of naringenin contributes to the down-regulation of eriodictyol while promoting up-regulation of luteolin and (-)-epigallocatechin (Figure 8).
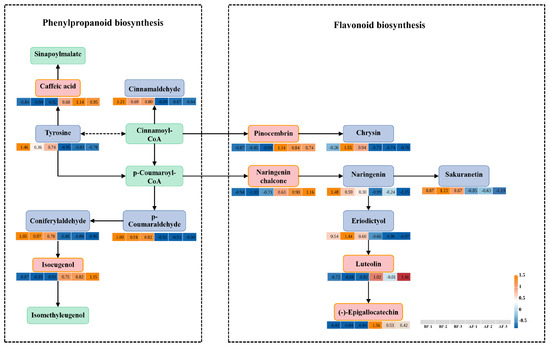
Figure 8.
Phenylpropanoid biosynthesis pathway and flavonoid biosynthesis pathway; BF: prior to figs entry by pollinators; AF: subsequent to figs entry by pollinators. Note: Blue and red indicate down-regulation and up-regulation, respectively. Note: The relative abundance of metabolites depicted is represented by color intensity, where red signifies increased expression and blue denotes decreased expression. Positive values indicate up-regulated metabolites, while negative values indicate down-regulated metabolites.
4. Discussion
The fig–fig-wasp mutualistic system serves as an exemplary model for investigating the cooperative and competitive relationships between species in nature, as it exemplifies both mutualism and competition for reproductive resources [31]. The findings from controlled experiments demonstrate that upon entering figs, pollinating fig wasps promptly initiate egg-laying and pollination activities within the fig. Subsequently, the receptive phase of the fig terminates within 1–2 days, rapidly transitioning into the interflower phase. The loss of attraction to fig wasp indicates a rapid transition from the nutrient growth stage to the reproductive stage in the fig. This suggests that foundresses promptly initiate egg-laying and pollination upon entering the figs and that figs react as promptly to either pollination or oviposition in their ovules by terminating the receptivity period. However, if no wasps enter, there is an extended receptive phase lasting over a week. This implies that the fig tree may actively extend its receptive phase in order to attract pollinators, as observed in previous studies on Ficus sermicordata [11]. The phenomenon of figs “awaiting” the arrival of pollinators can enhance their reproductive success rate and provide increased reproductive opportunities for fig wasps. Our findings indicate that the receptive phase of figs is primarily governed by wasp behavior. Specifically, the pollination and oviposition (galling) activities of wasps appear to “trigger” a critical threshold of ovule development, which in turn determines the termination of the receptive phase.
The fig-wasp symbiosis has undergone a long evolutionary history, resulting in mutual adaptation and cooperation [32]. However, limited research has been conducted to investigate whether the behavior of pollinating fig wasps mediates fig development. Our findings revealed a significant accumulation of organic acids and derivatives, organic nitrogen compounds, and carbohydrates during the receptive phase prior to wasp entry. Previous studies have demonstrated that these metabolites play a crucial role in facilitating energy generation and amino acid synthesis, providing sugars, regulating metabolism, and ultimately promoting plant growth and development [33,34,35]. Once the pollinator enters the fig, there is a significant increase in the expression of metabolites such as lipids, benzene cyclic aromatic hydrocarbons, organic peroxides, flavonoids, terpenes, and their compounds in female flowers. These substances provide essential energy and metabolic resources for the plant organism and, thus, for the symbiotic larvae in the galls of the ovules [30,36,37,38]. The metabolic expression patterns of figs display substantial discrepancies pre- and post-pollinator entry. The metabolic regulation prior to pollinator entry primarily aims to facilitate the growth and development of figs. Subsequent to the pollinator entry, in addition to maintaining fig growth, a substantial number of eggs are deposited by the fig wasps within the ovary of the female flower. Therefore, considering the stability of the mutualism system, monoecious figs may have evolved metabolic regulation mechanisms to “facilitate” the occupation of reproductive resources by pollinators while simultaneously developing strategies to “mitigate” their overutilization.
Further analysis of the metabolic pathways of figs at different stages revealed a significant enrichment of important metabolites in the linolenic acid pathway. Linolenic acid, being the predominant polyunsaturated fatty acid in plant oils, serves as a crucial component for both defensive responses against external interferences and energy provision for plant growth and development [28,39]. After wasp entry, the expression of 13-OxoODE in the linoleic acid metabolic pathway is down-regulated in figs. This compound can function as a peroxisome proliferator-activated receptor (PPAR) ligand to regulate lipid metabolism in plants [40], suggesting that lipid metabolism may be inhibited, causing lipid accumulation within figs, and providing resources for subsequent seed development and gall formation. Furthermore, previous studies have demonstrated that 9,12,13-Trihome and 9,10,13-Trihome possess the ability to induce defense mechanisms in plants [41]. Their expression is up-regulated after wasp entry, indicating their establishment of a defense mechanism against wasp interference. Alternatively, the plant may support the egg or larva of the pollinator by acting as a signaling molecule to activate or prime the plant’s immune system for faster and stronger responses. This may particularly promote gall development, which protects the developing larva of the pollinator. In the study investigating the response of mint leaves to Phenanthrene stress, it was confirmed that 9-OxoODE activates early tolerance in mint leaves toward Phenanthrene stress [42]. Furthermore, our findings demonstrate a significant up-regulation of 9-OxoODE expression upon wasp entry, suggesting its potential role in activating tolerance against wasp interference. The metabolites identified within the linolenic acid metabolic pathway not only serve as effective lipid mediators but also play a pivotal role in the expression of defense immunity.
Flavonoids and phenolic compounds exist in various plant organs and tissues, playing a crucial role in promoting plant growth and development [38,43]. Simultaneously, they serve as vital components of the plant’s defense mechanism against biotic or abiotic stress [30]. In this study, tyrosine, an intermediate in the phenylpropanoid biosynthesis pathway, exhibited up-regulation prior to wasp entry and down-regulation after wasp entry. Tyrosine serves as a precursor for various antioxidants, attractants, and defense compounds [44]. We propose that the dynamic change in tyrosine levels might be one of the mechanisms governing the alteration in the fig’s attraction to its pollinator. Additionally, isoeugenol, a metabolite with defensive interference properties [33], showed up-regulation following pollinator entry. This alteration may represent one of the strategies employed by figs to respond to pollinating fig wasps’ egg-laying behavior [33,44]. Additionally, in the phenylpropanoid biosynthesis pathway, caffeic acid, a precursor of lignin, is up-regulated in figs following wasp entry. Lignin serves as a crucial metabolic substance for plant physical defense [45]. In the flavonoid biosynthesis pathway, pinocembrin, naringenin chalcone, luteolin, and (-)-epigallocatechin are all up-regulated in the figs after wasp entry. These compounds play defensive roles when plants encounter biotic or abiotic stressors [46]. Therefore, we propose that these defense metabolites activate the fig’s defense mechanism in response to wasp oviposition behavior [30]. In the benzaldehyde biosynthesis pathway, cinnamaldehyde, p-coumaraldehyde, and coniferylaldehyde exhibit down-regulation after wasps enter the fig. Cinnamaldehyde has been shown to facilitate the activation of plant defense mechanisms and enhance tolerance against external interference [47]. On the other hand, p-coumaraldehyde and coniferylaldehyde play crucial roles as precursors and enzymes involved in lignin formation [48,49]. Accumulation of p-coumaraldehyde has been demonstrated to confer stress tolerance in cucumbers by promoting rapid lignification of injured tissues [50], while coniferylaldehyde contributes to preventing toppling over in Carex [51]. In the flavonoid biosynthesis pathway, sakuranetin and eriodictyol are also down-regulated after pollinator entry. Sakuranetin has been demonstrated to attenuate endocytosis in plant cells and activate the plant’s responsiveness to external disturbances [52,53], while eriodictyol exhibits antioxidant properties and enhances resistance against oxidative stress [54]. Based on the aforementioned metabolic regulation results, it can be inferred that the dynamic expression of diverse defense metabolites in both pathways achieves a balance of this mutualistic system. Fig trees achieve both the production of seeds and pollen vectors within the same fig by providing resources for the growth of the seeds and the growth of the pollinator larva and its gall. The entry of fig wasps into the fig can be considered a pivotal or a triggering factor in regulating this dynamic balance.
Our study investigated the balance of resource allocation in the mutualistic fig–fig wasp system, focusing on the interplay between competition and cooperation from an ecological and metabolic perspective. The results suggest that fig wasp entry plays a mediating role in fig development. Specifically, we observed a shift in the metabolic regulation of figs after fig wasps entered, with a transition from nutrient growth to primarily responding to wasp-induced stress. This dynamic balance is achieved through up-regulation and down-regulation of metabolites in different metabolic pathways. The results partially explain why pollinating wasps do not convert all fig flowers into wasp galls from a metabolic perspective, thereby providing support for the “unbeatable seeds hypothesis”. This hypothesis posits the existence of a mechanism that confers partial flowers are immune to wasp galling [9]. However, it is important to note that these mechanisms may differ among species, and multiple mechanisms may work together to prevent excessive gall formation in figs [9].
Our study provides interesting insights into understanding the stable mechanism underlying fig–fig-wasp mutualism and can serve as a reference for other studies investigating insect-plant mutualisms. However, Our study did not examine the metabolic differences between galled and pollinated flowers. Further research on metabolic differences in different styled flowers would be valuable research.
5. Conclusions
This study employed controlled experiments and LC-MS methods to investigate the impact of pollinating fig wasps on fig development and metabolic regulation. A total of 381 metabolites, including 155 differential metabolites, were identified. Prior to wasp entry, metabolic regulation primarily relied on carbohydrates and amino acids to facilitate fig growth and development. Following wasp entry, metabolic regulation shifted toward coping with wasp stress and providing nutrition for the subsequent reproduction of fig-wasp offspring. Furthermore, we annotated and enriched the differential metabolites, focusing on the linoleic acid metabolism pathway, phenylpropanoid biosynthesis pathway, and flavonoid biosynthesis pathway. Our findings demonstrate that figs maintain the stability of the fig-wasp symbiotic system through the coordinated regulation of multiple metabolic pathways, thereby providing support for the “unbeatable seeds hypothesis” to a certain extent. This study provides scientific evidence elucidating the mechanism underlying stability in the fig-wasp system.
Author Contributions
Conceived and designed the experiments, Y.Z. (Ying Zhang); Conceptualization, Y.Z. (Ying Zhang), Y.G. and Z.L.; writing—original draft preparation, Y.Z. (Ying Zhang) and Y.G.; provided the plant materials, Y.Z. (Ying Zhang), Y.W., C.C., and X.Y.; performed the experiments, Y.Z. (Ying Zhang), Y.W., C.C., and X.Y.; analyzed the data, and prepared the figures and tables, Y.Z. (Ying Zhang) and Y.Z. (Yuan Zhang); writing—review and editing, Y.Z. (Yuan Zhang) and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China (32160296, 32260719, 31560116), the Fundamental Research Program of Yunnan Province, China (202401AT070265, 202401BD070001-111), the Young Top-Notch Talent of Yunnan Outstanding Talent Program (XDYCQNRC-2022-0207), and the First Class Forestry Academic Subject in Yunnan Province (523003).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, H.; Wang, S.; Chen, J.; Gui, P. Molecular phylogeny of Ficus section Ficus in China based on four DNA regions. J. Syst. Evol. 2012, 50, 422–432. [Google Scholar] [CrossRef]
- Francis, J.K. Tropical Ecosystems|Ficus spp. (and other important Moraceae). In Encyclopedia of Forest Sciences; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1699–1704. [Google Scholar]
- Stover, E.; Aradhya, M.; Ferguson, L.; Crisosto, C.H. The Fig: Overview of an Ancient Fruit. HortScience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R. Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef]
- Rhett, D.H. Figs and the Diversity of Tropical Rainforests. BioScience 2005, 55, 1053–1064. [Google Scholar]
- Di Giusto, B.; Bain, A. Local ecological factors, not interference competition, drive the foundress number of two species of fig wasp sharing Ficus septica figs. PLoS ONE 2024, 19, e0290439. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.J.; Bain, A.; Chou, L.S.; Cruaud, A.; Gonzales, R.; Hossaert-McKey, M.; Rasplus, J.Y.; Tzeng, H.Y.; Kjellberg, F. Diversification and spatial structuring in the mutualism between Ficus septica and its pollinating wasps in insular South East Asia. BMC Evol. Biol. 2017, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Cruaud, A.; Ronsted, N.; Chantarasuwan, B.; Chou, L.S.; Clement, W.L.; Couloux, A.; Cousins, B.; Genson, G.; Harrison, R.D.; Hanson, P.E.; et al. An extreme case of plant-insect codiversification: Figs and fig-pollinating wasps. Syst. Biol. 2012, 61, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.W. Stability in fig tree–fig wasp mutualisms: How to be a cooperative fig wasp. Biol. J. Linn. Soc. Lond. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Weiblen, G.D. How to be a fig wasp. Annu. Rev. Entomol. 2002, 47, 299–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.R.; Peng, Y.Q.; Compton, S.G. Costs of inflorescence longevity for an Asian fig tree and its pollinator. Evol. Ecol. 2012, 26, 513–527. [Google Scholar] [CrossRef]
- Borges, M. Interactions Between Figs and Gall-Inducing Fig Wasps: Adaptations, Constraints, and Unanswered Questions. Front. Ecol. Evol. 2021, 9, 685542. [Google Scholar] [CrossRef]
- Proffit, M.; Bessière, J.; Schatz, B.; Hossaert-McKey, M. Can fine-scale post-pollination variation of fig volatile compounds explain some steps of the temporal succession of fig wasps associated with Ficus racemosa? Acta Oecol. 2017, 90, 81–90. [Google Scholar] [CrossRef]
- Li, Z.T.; Peng, Y.Q.; Wen, X.L.; Jandér, K.C. Selective resource allocation may promote a sex ratio in pollinator fig wasps more beneficial for the host tree. Sci. Rep. 2016, 6, 35159. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.W.; Shi, A.N.; Zhang, X.W.; Liu, M.; Jandér, K.C.; Dunn, W. Asymmetric and uncertain interactions within mutualisms. J. Plant Ecol. 2024, 17, rtad042. [Google Scholar] [CrossRef]
- Hall, C.R.; Robertson, L.P.; Carroll, A.R.; Kitchinget, R. The effect of Psephodiplosis rubi (Diptera: Cecidomyiidae) leaf galls on the secondary metabolite profiles of two congeneric host plants. Austral Entomol. 2018, 57, 228–237. [Google Scholar] [CrossRef]
- Escudero, N.; Marhuenda-Egea, F.C.; Ibanco-Cañete, R.; Zavala-Gonzalez, E.A.; Lopez-Llorca, L.V. A metabolomic approach to study the rhizodeposition in the tritrophic interaction: Tomato, Pochonia chlamydosporia and Meloidogyne javanica. Metabolomics 2014, 10, 788–804. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Mamirova, A.; Ashimuly, K.; Vibe, Y.P.; Krekova, Y.A. Anatomical and Metabolome Features of Haloxylon aphyllum and Haloxylon persicum Elucidate the Resilience against Gall-Forming Insects. Int. J. Mol. Sci. 2024, 25, 4738. [Google Scholar] [CrossRef]
- Miao, B.G.; Liu, M.X.; Wang, B.; Peng, Y.Q.; Lesne, A.; Kjellberg, F.; Jandér, K.C. Active pollination in a functionally dioecious Ficus species: An interplay between pollinator behaviour and floral morphology. Flora 2023, 302, 152274. [Google Scholar] [CrossRef]
- Jousselin, E.; Hossaert-Mckey, M.; Vernet, D.; Kjellberg, F. Egg deposition patterns of fig pollinating wasps: Implications for studies on the stability of the mutualism. Ecol. Entomol. 2001, 26, 602–608. [Google Scholar] [CrossRef]
- Jandér, K.C.; Herre, E.A. Host sanctions in Panamanian Ficus are likely based on selective resource allocation. Am. J. Bot. 2016, 103, 1753–1762. [Google Scholar] [CrossRef]
- Zhang, X.W.; Dunn, W.; Wang, R.W. Egg load is a cue for offspring sex ratio adjustment in a fig-pollinating wasp with male-eggs-first sex allocation. J. Evol. Biol. 2020, 33, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zidi, K.; Kati, D.E.; Bachir-bey, M.; Genva, M.; Fauconnier, M.L. Comparative Study of Fig Volatile Compounds Using Headspace Solid-Phase Microextraction-Gas Chromatography/Mass Spectrometry: Effects of Cultivars and Ripening Stages. Front. Plant Sci. 2021, 12, 667809. [Google Scholar] [CrossRef] [PubMed]
- Martinson, E.O.; Hackett, J.D.; Machado, C.A.; Arnold, A.E. Metatranscriptome Analysis of Fig Flowers Provides Insights into Potential Mechanisms for Mutualism Stability and Gall Induction. PLoS ONE 2015, 10, e0130745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, B.; Buyantuev, A.; He, X.; Gao, W.; Wang, Y.; Dawazhaxi; Yang, Z. Urban agglomeration of Kunming and Yuxi cities in Yunnan, China: The relative importance of government policy drivers and environmental constraints. Landsc. Ecol. 2019, 34, 663–679. [Google Scholar] [CrossRef]
- Wei, G.; Zheng, R.; Yang, X. Extraction and the Chemical Composition Analysis of the Essential Oil Flowers of Ficus hookeriana Corner. Adv. Mater. Res. 2012, 581–582, 94–99. [Google Scholar] [CrossRef]
- Cruaud, A.; Jabbour-Zahab, R.; Genson, G.; Cruaud, C.; Couloux, A.; Kjellberg, F.; Van Noort, S.; Rasplus, J.Y. Laying the foundations for a new classification of Agaonidae (Hymenoptera: Chalcidoidea), a multilocus phylogenetic approach. Cladistics 2010, 26, 359–387. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Luque, F.; Jiménez-Ruiz, J.; Padilla1, M.N.; Fierro-Risco, J.; Valderrama, R.; Fernández-Ocaña, A.; Corpas, F.J.; et al. Transcriptomic profiling of linolenic acid-responsive genes in ROS signaling from RNA-seq data in Arabidopsis. Front. Plant Sci. 2015, 6, 122. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Zhang, S.; Chen, S.; Wang, Y.; Wen, P.; Ma, X.; Shi, Y.; Qi, R.; Yang, Y.; et al. Genomes of the Banyan Tree and Pollinator Wasp Provide Insights into Fig-Wasp Coevolution. Cell 2020, 183, 875–889.e17. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.B.; Li, Y.H.; Shu, X.C.; Pu, Y.T.; Wang, X.J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef]
- Wang, G.; Compton, S.G.; Chen, J. The mechanism of pollinator specificity between two sympatric fig varieties: A combination of olfactory signals and contact cues. Ann. Bot. 2012, 111, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Souto-Vilarós, D.; Machac, A.; Michalek, J.; Darwell, C.T.; Sisol, M.; Kuyaiva, T.; Isua, B.; Weiblen, G.D.; Novotný, V.; Segar, T. Faster speciation of fig wasps than their host figs leads to decoupled speciation dynamics: Snapshots across the speciation continuum. Mol. Ecol. 2019, 28, 3958–3976. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.K.; Muhlemann, J.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Singer, D.; Chen, G. Protein interactomes for plant lipid biosynthesis and their biotechnological applications. Plant Biotechnol. J. 2014, 21, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Tanashvi, S.; Sejal, A.; Shahid, U.; Ravi, G. The intricate role of lipids in orchestrating plant defense responses. Plant Sci. 2024, 338, 111904. [Google Scholar]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant compounds for plant defence and for a healthy human diet. Not. Bot. Horti Agrobot. Cluj Napoca. 2018, 46, 14–21. [Google Scholar] [CrossRef]
- Marilyn, S.S.; Duck-Kee, K.; Sa-Youl, G. Linoleic acid-induced expression of defense genes and enzymes in tobacco. J. Plant Physiol. 2014, 171, 1757–1762. [Google Scholar]
- Takahashi, H.; Kamakari, K.; Suzuki, H.; Mohri, S.; Goto, T.; Takahashi, N.; Matsumura, Y.; Shibata, D.; Kawada, T. Localization of 9- and 13-oxo-octadecadienoic acids in tomato fruit. Biosci. Biotechnol. Biochem. 2014, 78, 1761–1764. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, X.; Du, X.; Liu, H.; An, T.; Zhao, Y.; Yu, H.; Chen, Y.; Wen, J. Comparative metabolomics reveal the participation of soybean unique rhizosphere metabolites in susceptibility and resistance of host soybean to Phytophthora sojae. Plant Soil 2022, 480, 185–199. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Yang, X.; Ren, L.; Wang, Y.; Ma, D.; Fan, P.; Wang, H.; Liu, L.; Dong, B.; et al. Effects of phenanthrene on the essential oil composition and leaf metabolome in peppermint plants (Mentha piperita L.). Ind. Crops. Prod. 2022, 187, 115383. [Google Scholar] [CrossRef]
- Yao, T.; Feng, K.; Xie, M.; Barros, J.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Phylogenetic Occurrence of the Phenylpropanoid Pathway and Lignin Biosynthesis in Plants. Front. Plant Sci. 2021, 12, 704697. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- Mughal, A.; Jabeen, N.; Ashraf, K.; Sultan, K.; Farhan, M.; Hussain, M.I.; Deng, G.; Alsudays, I.M.; Saleh, M.A.; Tariq, S.; et al. Exploring the role of caffeic acid in mitigating abiotic stresses in plants: A review. Plant Stress 2024, 12, 100487. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, N.; Liu, R.; Bai, H.; Tao, W.; Chen, J.; Shi, Z. Cinnamaldehyde Facilitates Cadmium Tolerance by Modulating Ca2+ in Brassica rapa. Water Air Soil Pollut. 2021, 232, 19. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, T.; Kontturi, E.; Vuorinen, T. Distribution of lignin and its coniferyl alcohol and coniferyl aldehyde groups in Picea abies and Pinus sylvestris as observed by Raman imaging. Phytochemistry 2011, 72, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Varbanova, M.; Porter, K.; Lu, F.; Ralph, J.; Hammerschmidt, R.; Jones, A.D.; Day, B. Molecular and Biochemical Basis for Stress-Induced Accumulation of Free and Bound p-Coumaraldehyde in Cucumber. Plant Physiol. 2011, 157, 1056–1066. [Google Scholar] [CrossRef]
- Diehn, S.; Kirby, N.; Ben-Zeev, S.; Saranga, Y.; Elbaum, R. Raman developmental markers in root cell walls are associated with lodging tendency in tef. Planta 2024, 259, 54. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Schuster, S.; Leibman, M.; Ezer, R.; Avni, A. The function of EHD2 in endocytosis and defense signaling is affected by SUMO. Plant Mol. Biol. 2014, 84, 509–518. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Zhao, Y.; Zhu, H.; Fu, Q.; Lu, X.; Huang, W.; Yang, X.; Zhou, X.; Wu, L.; et al. Phytoalexin sakuranetin attenuates endocytosis and enhances resistance to rice blast. Nat. Commun. 2024, 15, 3437. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Farooq, A.; Qudsia, T. A Centum of Valuable Plant Bioactives; Academic Press: Cambridge, MA, USA, 2021; pp. 467–489. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).