Abstract
In line with environmental awareness movements and social concerns, the textile industry is prioritizing sustainability in its strategic planning, product decisions, and brand initiatives. The use of non-biodegradable materials, obtained from non-renewable sources, contributes heavily to environmental pollution throughout the textile production chain. As sustainable alternatives, considerable efforts are being made to incorporate biodegradable biopolymers derived from residual biomass, with reasonable production costs, to replace or reduce the use of synthetic petrochemical-based polymers. However, the commercial deployment of these biopolymers is dependent on high biomass availability and a cost-effective supply. Residual forest biomass, with lignocellulosic composition and seasonably available at low cost, constitutes an attractive renewable resource that might be used as raw material. Thus, this review aims at carrying out a comprehensive analysis of the existing literature on the use of residual forest biomass as a source of new biomaterials for the textile industry, identifying current gaps or problems. Three specific biopolymers are considered: lignin that is recovered from forest biomass, and the bacterial biopolymers poly(hydroxyalkanoates) (PHAs) and bacterial cellulose (BC), which can be produced from sugar-rich hydrolysates derived from the polysaccharide fractions of forest biomass. Lignin, PHA, and BC can find use in textile applications, for example, to develop fibers or technical textiles, thus replacing the currently used synthetic materials. This approach will considerably contribute to improving the sustainability of the textile industry by reducing the amount of non-biodegradable materials upon disposal of textiles, reducing their environmental impact. Moreover, the integration of residual forest biomass as renewable raw material to produce advanced biomaterials for the textile industry is consistent with the principles of the circular economy and the bioeconomy and offers potential for the development of innovative materials for this industry.
1. Introduction
In the textile industry, sustainability continues to be at the forefront of strategic planning, product decisions, and brand initiatives [1]. This industry is being confronted with environmental awareness movements and social concern regarding its environmental impact, as it contributes heavily to environmental pollution and causes serious ecological problems throughout its production chain [2]. The long period required for degradation of non-biodegradable materials produced from non-renewable sources is one of the main reasons for the severe impact on the environment by this industry. To address these environmental impacts, the European Commission published the EU strategy for sustainable and circular textiles in 2022. The strategy aims that, by 2030, textile products placed on the EU market are long-lived and recyclable, largely made of recycled fibers, free of hazardous substances, and produced respecting social rights and the environment [3]. To contribute to such goals, innovative green technologies are needed, as well as the development of innovative and sustainable raw materials as alternatives to petroleum-based counterparts. Therefore, considerable efforts are being made for the development and application of materials based on polymers obtained from natural sources and their derivatives that have low production costs and can be degraded more easily in the environment as alternatives to fossil-based materials [4]. In this context, residual forest biomass has emerged as a promising source of those biomaterials, due to its abundance, renewability, and potential for sustainable production [5,6,7,8]. In fact, forest biomass has already been identified as an excellent raw material for low-carbon building materials, biotextiles, and bioplastics production [9].
This review focuses on a comprehensive analysis of existing literature data while identifying current gaps or issues for the application in the textile industry of three specific biopolymers derived from forest biomass—bacterial cellulose (BC), poly(hydroxyalkanoates) (PHA), and lignin. This need arose because these biomaterials have not been widely presented in the literature and have not been systematically reviewed within the scope of this topic.
2. Forest Biomass as Sustainable Raw Material
Among sustainable resources, biomass is the only natural resource that has an alternative renewal cycle short enough to meet the growing needs of world markets for chemicals, biochemicals, and fuels [10]. The sustainability of using biomass as an alternative raw material is essential for understanding different types and scenarios for applicability in different areas that vary with its origin, characteristics, composition, and disposal methods. In the context of conventional biotechnology, biomass is considered as “any organic matter that arises from the photosynthetic conversion of solar energy”, and the Sun is the main source of energy on Earth [11]. In addition to the forests, which cover about 10% of the total land area, biomass includes all the organic matter available on a renewable or recurring basis, i.e., agricultural crops, wood and wood residues, plants (including aquatic plants), herbaceous species, animal waste, municipal waste, and other wastes, including those derived from industrial processing [12]. Indeed, the growing urban population and activities are responsible for the generation of increasing industrial processing residues, municipal solid waste, and other urban discards, with significant environmental impact, and thus it urges us to valorize this residual biomass.
Forest-based industry is very important for the Portuguese economy, and Eucalyptus globulus is the dominant forestry species, representing more than 2 million acres of forest in 2020, since it constitutes the main wood source for the production of pulp and paper in Portugal (annual pulp production around 2.5 million tons) [13]. Pulp and paper value-chain generates huge amounts of by-products and waste streams. These forest-based residues include branches and tree-tops, leaves and roots from harvesting, together with bark and wood shaving rejects (fines) from industrial wood processing for pulp and paper manufacturing. The residual forest biomass generated in pulp and paper mills assumes particular relevance since it is locally widely available and commonly used for low-value purposes. Part of the harvested wood (logs) is conveyed to the pulp mills still with bark (representing around 8%–18% (dry mass) of the total eucalyptus biomass), which is then removed at the wood yard and used to produce energy. To produce cellulosic pulp, wood logs are cut into small wood chips and fed into a digester. During this operation, chips with smaller dimensions (fines) are rejected, and this biomass is also fed into biomass boilers [13,14]. However, the economic and environmental boost of the pulp and paper industry will rely on the upgrading of all the forest-based residues, which should only be used for direct combustion or be deposited in landfills as a fallback solution. For instance, several studies have demonstrated that E. globulus bark can be a suitable feedstock to produce valuable products, instead of its current undervalued utilization [15,16,17]. Under a circular economy approach, this residual forest biomass can also be an attractive source of biopolymers for textile applications.
Forest biomass, like any plant biomass, exhibits lignocellulosic composition, as most components of biomass do. Indeed, in the cell walls of vascular tissues of all higher land plants, cellulose fibrils are embedded in an amorphous matrix of lignin and hemicellulose (Figure 1). The three types of polymers—cellulose, hemicellulose, and lignin—are strongly associated with each other by physical links as well as by covalent cross-links, forming a composite material known as lignocellulose, which represents more than 90% of the dry weight of a cell plant [12]. The relative proportion and distribution of each biopolymer constituent of lignocellulosic materials vary with species and stage of plant growth and development as well as along the plant itself. On average, lignocellulose consists of 45% cellulose, 30% hemicellulose, and 25% lignin [12].
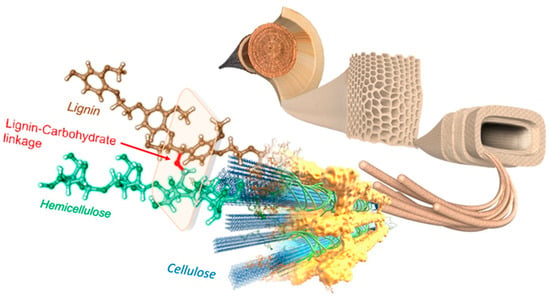
Figure 1.
Lignocellulose matrix, adapted from [18].
The native cellulose polymer is a linear chain of glucose units, joined by glycosidic β-1,4 linkages, possessing a degree of polymerization (DP) up to 15000 glucose units. The polymer chain of cellulose has a flat, ring-shaped, stabilized by strong internal hydrogen bonds, which gives it a high strength, and then it is much used in the form of fibers and films. Although cellulose has a high affinity for water, it is completely insoluble, and it is much more resistant to degradation than other glucose polymers such as starch. The natural cellulose compounds are heterogeneous in structure, possessing both highly ordered regions—crystalline regions—and less ordered—amorphous regions. The fraction of crystalline cellulose varies with the source and how the material is prepared. The degree of crystallinity of cellulose is a very important property, and the crystalline regions are more resistant to enzymatic hydrolysis [12].
While cellulose is a linear homopolymer, somewhat variable in structure from one species to another, naturally occurring hemicelluloses are heteropolysaccharides, highly branched, usually non-crystalline, with a much lower DP [19]. Thereby hemicellulose is more readily hydrolyzed as compared to cellulose. The term hemicellulose encompasses various heterogeneous polysaccharides groups, usually composed of 2–6 different sugar residues with a degree of polymerization of about 200 [19]. The sugar residues found in hemicellulose include pentoses (D-xylose and L-arabinose), hexoses (D-galactose, L-galactose, D-mannose, L-fucose, and L-rhamnose), and uronic acids (D-glucuronic acid). The hemicelluloses are designated according to the main group of sugar present in the backbone: xylans, arabinans, mannans, and galactans. Except for galactose-based hemicelluloses, which are connected via β-1,3, most of the hemicelluloses are based on a β-1,4 linkage between their constituent sugars [19]. The residues appear, variously, modified by acetylation or methylation [12].
Lignin is predominantly located in vascular tissues destined for the transport of liquids. It is not present in mosses, lichens, and algae that have no trachea (tube-shaped cells along the peculiar xylem). The increase in mechanical strength that lignification provides to the tissues of wood allows the large trees, several tens of meters high, to remain standing [12]. Lignin, a complex aromatic polymer, is structurally very different from the other two polymer constituents of wood. It is a copolymer of extremely high molecular weight, synthesized by polymerization from phenylpropane units (C9), having as precursors three alcohols, designated as lignols: coniferyl alcohol, p-sinapyl alcohol, and p-coumaryl alcohol [12]. Lignin is considered difficult to be used as a fermentation substrate because it makes the biomass resistant to chemical and biological degradation [20].
Cellulose is a versatile biomaterial that can be extracted from forest biomass and processed into fibers suitable for textile production [21]. Cellulose fibers offer several advantages for the textile industry, exhibiting high tensile strength, good moisture absorption properties, and can be easily processed into various textile products. The use of cellulose fibers derived from forest biomass provides a sustainable alternative to synthetic fibers, reducing the environmental impact of textile production [21]. However, the applications of forest biomass generating biomaterials for the textile industry extend beyond the use of the cellulose fraction for fiber spinning, and other biomaterials derived from forest biomass also play a significant role in the textile industry. For instance, lignin can be used as a raw material in the production of high-quality fibers and other textile applications.
In addition to utilizing forest biomass directly, researchers have also explored the use of biomaterials derived from forest biomass. Indeed, the high polysaccharide content of lignocellulosic forest-based biomass, which is non-seasonal, highly available at low cost, and renewable, makes it attractive to be upgraded through biochemical conversion processes as a sugar platform for the production of biofuels and biochemicals, replacing traditional fossil raw materials, towards sustainable growth and facing the problems related to environmental and climate changes [16,22,23,24]. Also, the use of residual forest biomass as a sustainable source of new biomaterials presents great potential for the textile industry. Significant advances have currently been achieved by the research community in the development of various biomaterials from forest biomass.
3. Forest Biomass Processing
As previously referred to, a circular economy perspective might be applied to boost the competitiveness of the textile value chain by using residual forest biomass as sustainable raw material for the synthesis of biomaterials for the textile industry by applying biochemical technologies based on the so-called “sugar platform” [25]. This bioconversion requires the prior hydrolysis of its constituent polysaccharides, i.e., cellulose and hemicellulose fractions, in order to obtain the respective monomers (mostly glucose and xylose) [26]. This hydrolysis can be promoted by chemical, thermal, or biological methods. Enzymatic hydrolysis is advantageous due to its greater intrinsic specificity, with the consequent absence of the formation of degradation products (possibly obtained from sugars and lignin) and increased potential yield, reduced energy consumption due to moderate reaction conditions, and its non-corrosive and non-polluting nature [27,28]. However, most sources of lignocellulosic materials are structurally rigid and compact, and, therefore, their polysaccharides are not easily accessible to enzymes. This recalcitrance of lignocellulosic biomass is mainly due to two factors: the low accessibility of microcrystalline cellulose fibers, which prevents the efficient action of cellulases, and the presence of lignin (mainly) and hemicellulose on the cellulose surface, which prevents the effective action of cellulases on the substrate [29,30,31]. Thus, lignocellulosic materials, and in particular residual forest biomass, unlike saccharine and starch-based raw materials (1st generation), need to be subjected to mechanical grinding and/or pre-treatment before applying an enzymatic process [10].
Pretreatment aims to release the cellulose and hemicellulose fractions from the lignin matrix, reduce the crystallinity of cellulose, and increase the porosity of the material [32]. Given the high recalcitrance of lignocellulosic biomass [33], this initial stage of the process is considered one of the key points, being the most complex and costly step involved in the technology for conversion of lignocellulosic biomass into fermentable sugars [34]. Despite the diversity of pre-treatment technologies available, the majority of these are still in the development phase, having not reached a commercial level [35].
Pretreatment technologies can involve biological, physical, chemical, and physico-chemical processes [36]. Ultimately, all pretreatment processes originate cellulose and hemicellulose structures accessible to hydrolysis [37], and thus can also promote an effect of biomass fractionation. Since hemicellulose can be readily hydrolyzed under mild acidic or alkaline conditions, or alternatively by appropriate hemicellulolytic enzymes, this hydrolysis occurs already in the pretreatment step under various process configurations. In turn, the cellulose fraction is more resistant and, therefore, its hydrolysis requires more severe pre-treatment. Lignin is extracted from lignocellulosic raw material to different extents and in different forms by various pretreatment methods. In fact, the separation of lignin from polysaccharides can be promoted by the application of strong alkali conditions (for example, with sodium hydroxide and sodium sulfite at high temperature, as applied in “kraft” or sulphate cooking) or can be hydrolyzed by dilute and concentrated acids, organic solvents (organosolv treatment), or ionic liquids [35].
Biological pre-treatments are based on the use of microorganisms (such as rot fungi) that promote delignification of biomass and degradation of hemicellulose, thus being environmentally benign and low cost, since they use native microorganisms/enzymes and do not require any special equipment. However, this processing is too slow to be applied on an industrial scale, and it also has the disadvantage, in the case of culturing viable microorganisms, of consuming part of the cellulose and hemicellulose in addition to the lignin [38].
Physical pre-treatments, which include crushing/grinding operations to reduce the size of biomass particles, make it possible to increase the size of pores and the available surface area, as well as decrease the crystallinity of cellulose and its degree of polymerization. These processes will always have to be combined with other processes, for example, chemical ones, in order to promote effective pre-treatment of the biomass.
Conventional chemical pretreatments consist mainly of acid-catalyzed processes, involving the application of concentrated acid at low temperature or dilute acid at high temperature, normally using sulfuric, hydrochloric, nitric, or trifluoroacetic acid. Concentrated acid processes can promote the hydrolysis of hemicelluloses and cellulose, generally generating low amounts of degradation products by applying low/medium temperatures [26]. With the use of dilute acid at high temperature, hydrolysis of hemicelluloses mainly occurs, producing a solid fraction rich in cellulose, suitable for subsequent enzymatic hydrolysis. Acid hydrolysis requires, however, that the acids are subsequently recycled/neutralized, also leading to losses of hydrolysates, in addition to increased corrosion of equipment and the associated environmental impact [37]. Depending on the operational conditions applied in these processes, degradation products of sugars (furan derivatives and weak acids) and lignin (phenolics) may also be formed, which will inhibit the subsequent biological conversion of sugars [26], which is why they typically present high investment costs, and the environmental impact can be considerable, which will limit its application.
The most developed physico-chemical pretreatment processes, which involve a combination of physical and chemical mechanisms, are hydrothermal processes, which include auto-hydrolysis (“liquid hot water”, LHW) and steam explosion. These methods are based on the use of water or steam, or both, and heat for biomass processing. Under these conditions, hydrolysis of the acetyl groups of hemicelluloses occurs, with partial or total solubilization of them, mostly in oligomeric form [39], while cellulose and lignin remain in the solid phase. While autohydrolysis uses compressed liquid water (pressure above the saturation point), in steam explosion, biomass is treated with saturated water vapor at high pressure (corresponding to saturation temperatures typically of 160–240 °C), promoting the heating and moistening of the material due to steam condensation. Then the pressure is quickly reduced (in a “blow tank”), which causes the material to undergo sudden decompression and consequent explosion, promoting the breakdown of the lignocellulosic matrix as a result of the rupture of inter- and intra-molecular bonds [40]. The efficiency of steam explosion is affected by several factors, such as steam temperature, residence time, particle size of the biomass, and its moisture content [38], but this pretreatment generally allows one to significantly increase the digestibility in subsequent enzymatic hydrolysis, which is a very important asset for increasing the yield of cellulosic biorefineries. Steam explosion operates with higher dry matter contents (solid/liquid ratios) than auto-hydrolysis, making it possible to carry out the enzymatic hydrolysis under higher concentrations of solids, allowing thus to obtain higher concentrations of sugars for the subsequent fermentation, which will also be advantageous for “downstream” processing [38]. Therefore, despite it does not exist a clearly winning technology yet, steam explosion is already one of the most developed pre-treatment technologies, being considered as one of the most efficient [40]. When no chemical catalyst is added, these pre-treatments are even more attractive from an economic and environmental point of view, producing no significant effect on lignin and cellulose and thus generating low levels of inhibitory products [41]. In these processes, as they exhibit a moderate pH, corrosion problems are also very reduced, and the acid and precipitate recycling steps will not be necessary, thus minimizing costs and environmental impact.
Uncatalyzed steam explosion can thus be applied as a sustainable pre-treatment, preceding the enzymatic hydrolysis and fermentation steps, in biorefineries using residual lignocellulosic biomass as raw material. It will significantly increase the enzymatic digestibility of cellulose while minimizing the formation of inhibitors that could adversely affect subsequent microbial cultivation. Thereby, the hydrolysates hereby obtained typically require no detoxification stage before their use for fermentation, as previously demonstrated in several studies [42,43,44] applying uncatalyzed steam explosion.
4. Forest-Derived Biomaterials in the Textile Industry
The sugars obtained by hydrolysis of lignocellulosic materials, such as residual forest biomass, can thus be used as a substrate for bacterial cultivation and production of two types of biodegradable biopolymers: PHAs and BC, as previously reported [44,45]. PHAs are biodegradable bioplastics [46], whose properties can be defined by the selection of bacteria, raw materials, and cultivation conditions. These can be combined with other materials, such as BC, improving their processability in the manufacture of fibers applied in industry. BC is a cellulose of high purity and crystallinity, with nanofibrillar morphology [47]. In addition, cellolignin—the solid fraction remaining after the enzymatic hydrolysis of cellulose and hemicellulose—representing around 20% in Eucalyptus bark, can be recovered and used to obtain biocomposites for several applications. PHA, BC, and lignin are being evaluated for technical applications in the textile industry.
4.1. Poly(hydroxyalkanoates)
PHAs are biodegradable bioplastics whose properties can be modeled by their monomer composition: scl-PHAs, comprising short-chain length monomers (3–5 carbon atoms), are generally highly crystalline, brittle, and rigid thermopolyesters (e.g., poly(hydroxybutyrate), PHB [48]), while mcl-PHAs, which comprise monomers with 6–14 carbon atoms, are flexible elastomers with low crystallinity. There are also scl-mcl-PHAs (e.g., poly(hydroxybutyrate-co-hydroxyvalerate-co-hydroxyhexanoate), PHBHVHHx [49]) that combine characteristics of both types of PHAs [46]. Incorporation of HV and HHx monomers, for example, leads to more flexible biopolymers with lower melting temperatures, which increases their processability window compared to the homopolymer PHB [49]. The possibility of synthesizing PHAs with different monomers with very distinct properties enables the customization of these biopolymers through a wide range of applications, from rigid packaging materials to elastic biomedical devices. All these characteristics make PHAs promising materials for replacing non-biodegradable petrochemical plastics in applications ranging from food packaging, coated cardboard, and film materials to biomedical devices [50,51]. Figure 2 presents the main uses of PHAs within various industries.
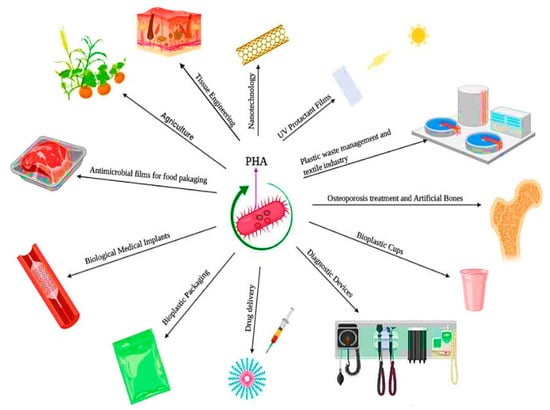
Figure 2.
An overview of the applications of the PHAs [52].
Although representing only 1.4% of the global bioplastics’ market, which, in turn, accounts for 1% of the total annual plastic production [53], PHA production is expected to have significant growth over the next years. In 2023, the PHA market was valued at USD 93 million, being expected to grow at 15.9% CAGR and reach a value of USD 195 million by 2028 (www.marketsandmarkets.com, accessed on 20 November 2024). This trend is driven by the growing awareness of the environmental issues posed by petrochemical counterparts and the need to replace such non-biodegradable plastics with natural-based biodegradable biopolymers, such as PHAs. The significant barrier to the commercialization of polyhydroxyalkanoates (PHAs) is their relatively high production cost, which can be three times higher than that of petroleum-based plastics [54]. Most of such bacteria use sugars as substrates for cell growth and PHA biosynthesis [46]. Therefore, aiming to reduce the production costs, which represent 45-50% of the overall bioprocess cost [51], polysaccharides-rich raw materials, such as lignocellulosic residues, including forest biomass, can be used.
4.1.1. PHA Biosynthesis
PHAs are accumulated intracellularly by various bacteria under growth-limiting conditions concomitant with carbon availability. PHA synthesis begins with the formation of two acetyl-CoA molecules, catalyzed by the enzyme β-ketothiolase. This acetyl-CoA undergoes an NADPH-dependent reaction to form hydroxybutyryl-CoA, which is then polymerized into PHB (poly-3-hydroxybutyrate) granules by the enzyme PHB synthase. The accumulation of PHAs within the cytoplasm occurs as compact granules, coated with phospholipids and phasins (these proteins regulate granule size). The yield of PHAs is directly influenced by the balance between coenzymes NADH and NAD+, which controls the diversion of acetyl-CoA into the PHA pathway rather than the Krebs cycle. In some bacteria, medium-chain-length PHAs (mcl-PHAs) are synthesized from long-chain fatty acids via β-oxidation, forming (R)-3-hydroxyacyl-CoA, which is then polymerized into mcl-PHA by PhaC synthase. Moreover, from simple sugars, fatty acids can be converted into 3-hydroxyacyl precursors via the de novo pathway, which are further processed to form (R)-3-hydroxyfatty acids by CoA transferase (PhaG) that accumulate as mcl-PHAs by PhaC [55,56].
Several bacteria were identified that synthesize PHAs with a diverse monomeric composition using glucose and/or xylose, which are sugars resulting from the hydrolysis of polysaccharides fractions of lignocellulose, such as residual forest biomass. Thus, it is possible to obtain PHAs with different properties by selecting the most appropriate bacterium, raw material, and cultivation conditions, tailoring it to the intended application.
4.1.2. PHA Production from Forest Biomass Residues
Forest biomass residues stand out as valuable feedstocks for sustainable PHA production due to their characteristics as a cost-effective, renewable, and globally abundant carbon source. They represent the most plentiful organic material globally, estimated at 180–200 billion tons per year [57,58]. Moreover, utilizing forest biomass for PHA production avoids competition with the human food chain, further enhancing the sustainability of the overall process [59].
The increasing interest in residual forest biomass as a carbon source for PHA production is evident in the recent publications focused on valorizing this specific type of feedstock, employing cutting-edge technologies to lower the production costs of PHAs (Table 1).

Table 1.
Production of PHAs from hydrolysates obtained from forest biomass by different bacteria.
For most of the PHA-producing bacterial strains, efficient pretreatment and hydrolysis processes are needed to obtain high-sugar-yields streams from the residual forest biomass [67,68,69]. These streams contain mainly glucose, xylose, and arabinose as the primary sugars, along with organic acids (levulinic acid, lactic acid, formic acid, and acetic acid), microbial inhibitors (furfural and hydroxymethylfurfural (HMF)), and lignin derivatives (such as vanillin and phenols). Many of these organic acids, inhibitors, and lignin derivatives hinder PHA-producing strains. Therefore, several pretreatments and detoxification methods have been developed to enable their use as bacterial carbon sources.
Most of the bacterial strains convert the sugars glucose or a glucose–xylose mixture from lignocellulosic biomass into PHA. Bacillus species also showed the ability to metabolize lignin, a complex aromatic polymer abundant in forest biomass [70]. Burkholderia is a genus of bacteria known for its diverse metabolic capabilities, environmental adaptability, and significant contributions to biotechnological applications. Within this genus, B. sacchari has demonstrated the ability to utilize various carbon sources for PHA production. For example, B. sacchari was reported for the conversion of spruce sawdust into PHB [60,71]. The adaptability of Burkholderia strains is further exemplified by B. cepacia, which effectively utilizes spruce sawdust hydrolysate for PHB production. Despite facing inhibitory compounds in the hydrolysate, B. cepacia demonstrated a PHB concentration of 1.05 g/L with a PHB content on biomass of 74.70% in a shake-flask setting [60]. Recently, Rodrigues et al. [44] reported the production of PHB by B. thailandensis DSM 13276, using eucalyptus bark hydrolysate as the sole feedstock, which resulted in the accumulation of PHB in the biomass at a content of 12.3 wt%.
Cupriavidus strains have showcased their ability to thrive in the presence of inhibitory compounds, metabolizing them during the fermentation process. Dilute acid pretreatment of sugar maple wood chips, for instance, yielded a PHB concentration of 8.72 g/L using C. necator in a bioreactor [64]. This approach not only enhances sugar recovery but also minimizes the concentration of inhibitory compounds, revealing the detoxification competence of Cupriavidus sp. in lignocellulosic-based biopolymer production. In the context of forest biomass, Pseudomonas strains have displayed competence in fermenting hydrolysates from various sources. Steam explosion and enzymatic hydrolysis of trembling aspen, for instance, produced a xylose-rich feedstock. Subsequent fermentation with P. cepacia resulted in a poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxypropionate) (PHBVP) concentration of 1.56 g/L with a significant PHA mass fraction in biomass. Furthermore, P. putida KT2440 tolerated high concentrations of lignin and aromatic compounds and converted them into mcl-PHA, up to 57% of CDW [72]. A new isolate Pseudomonas sp. was shown to be able to grow on eucalyptus bark as the sole feedstock and to produce up to 7.70 g/L PHB, corresponding to a polymer content in the biomass of 31wt% [44].
Other examples include Brevundimonas vesicularis and Sphingopyxis macrogoltabida, which demonstrated the ability to accumulate a co-polymer named poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxypropionate) (PHBVP) from Pinus radiata sawdust hydrolysate. However, owing to limited cell growth, the concentrations of PHA reached only 0.162-0.231 g/L [64,73]. Metabolic engineering strains may help to grow in the presence of toxic compounds. E. coli was engineered from beechwood xylan to produce poly(3-hydroxybutyrate-co-lactate) (P(3HB-co-LA)). This was achieved by introducing genes for xylan utilization and PHA synthesis, resulting in P(3HB-co-LA) production using xylose and beechwood xylan as carbon sources [74].
These studies highlight the versatility of microbial PHA production from forest biomass, addressing the importance of tailored strategies for different types of residual materials and microbial strains. The integration of pretreatment, detoxification (if required), and metabolic engineering approaches contributes to the efficient utilization of lignocellulosic resources for sustainable PHA production. The main sugars present in hydrolysates from forest residues and hardwoods, specifically xylose and arabinose, follow a less efficient metabolic pathway compared to glucose metabolism. Nonetheless, significant improvements can be achieved through the development of enhanced microorganisms, either through genetic modification or the isolation of organisms proficient in consuming C5 sugars.
4.1.3. Application of PHA in the Textile Industry
The use of PHAs for the manufacture of fibers has been investigated to develop materials for several areas, including textiles, where these biopolymers are mainly used for medical products [75]. Although PHAs can be used as the sole material in such applications, they still underperform mechanically and have no antimicrobial activity. Moreover, PHAs’ processability is limited due to their crystallinity and low degradation temperature. To overcome such limitations, PHAs can be combined with other materials, namely, other biopolymers (e.g., bacterial cellulose, BC), to form biocomposites with broader functionality [75,76]. BC nanocrystals [77] have been reported for reinforcing PHA-based materials.
The thermoplastic nature of PHB renders it suitable for continuous filament fabrication via melt-spinning, the most effective method for producing fibers, enabling its diverse use in textile applications [78]. However, challenges arise from its brittleness and restricted processing range, presenting hurdles in the fiber melt-spinning process [79]. The low melt processing window in PHB stems from the nonradical, random chain scission (cis elimination) reaction, leading to PHB instability beyond 160 °C and resulting in a substantial decline in molecular weight during melt processing [79,80]. The brittleness is attributed to the large size of spherulites and secondary crystallization [79,81]. Consequently, large-scale melt-spinning of PHB into fibers has not been viable, prompting focus primarily on laboratory-scale.
PHAs find utility within the textile industry due to their high tensile strength, biodegradability, and molecular weight. The polymer’s conversion into fibers mirrors processes akin to nylon treatment, presenting an avenue where the shortcomings associated with static electricity and reduced air permeability, characteristic of polyester materials, can be mitigated through the integration of bioplastic fibers [79]. Even though PHAs exhibit numerous desirable characteristics, they also present certain drawbacks, including high manufacturing costs, typical brittleness with a low elongation break, low resistance to thermal deterioration due to the proximity of their melting temperature and degradation temperature, consequently yielding a tiny processing window [79]. However, their high crystallinity (>50%, often 60%–80%) and narrow processing window pose significant challenges in proper fibers from PHAs. Hence, it is imperative to control crystallinity when processing PHAs.
Thus, blends of polymers and copolymers have demonstrated an enhancement in the mechanical characteristics of PHAs. Blending PHA presents a cost-effective and relatively simple approach to modifying the polymer’s final properties. Nonetheless, this adjustment could potentially decrease the degradation time, rendering the blend less suitable for specific applications [75,82]. The properties of the blend vary according to the specific polymer combination and its ratio. Typically, alterations occur in thermal transitions such as the glass transition and melt temperature [75,82], crystallinity, and mechanical properties [75,83]. Tanaka et al. [84] exemplified this by developing robust fibers using a P(3HB-co-3HV) copolymer, achieving high strength without requiring nucleation agents or additional additives. Moreover, elevating the HV content resulted in decreased polymer crystallinity while concurrently improving the copolymer’s flexibility. Alagoz et al. [85] used syringe wet spinning to create coated fibers, while Kundrat et al. [86] engineered porous wet-spun P3HB fibers using a simplified syringe setup.
The most researched blend to date is PHA/PLA. PLA has been accepted as the most potentially sustainable and biodegradable polymer that can be melt-spun into textile fibers on a big scale and replace traditional PET fiber in textile goods. Kervran et al. [87] recently explored PLA/PHB blends, concluding that incorporating PLA enhances the thermal stability of PHB. Simultaneously, the selection of additives significantly influences the thermal stability of these blends. Huang et al. [88] effectively produced PLA/PHB blend fibers, demonstrating notably enhanced thermal stability. In their prior research, it was observed that PHAs functioned effectively as plasticizing and nucleating agents, thereby facilitating the crystallization process in PLA. As a result, the PLA/PHA blend fibers displayed improved heat resistance [89].
4.2. Bacterial Cellulose
Cellulose is the most prevalent renewable biopolymer on Earth and the primary constituent of the cell walls of higher plants. It is also biosynthesized by various types of microorganisms. In the textile industry, the application of materials with polymers from natural or bio-based sources and derivatives that can be more easily degraded and with lower production costs compared to petroleum-based materials has emerged in response to more sustainable approaches. This industry has faced environmental awareness movements and social concerns, hence the choice of cellulose because it has high biodegradability and is derived from a renewable source.
BC possesses a structure akin to that of vegetable cellulose, comprising a linear glucan with monomers interconnected by β-1,4 glycosidic bonds, with the chemical formula (C6H10O5)n [90,91,92]. The primary distinction between the two lies in their appearance and water content: while vegetable cellulose exhibits a fibrous structure, BC takes on a gel-like form, i.e., BC is composed of microfibrils (up to 130 nm in width) and nanofibrils (up to 4 nm in diameter) that are arranged in a three-dimensional network. In comparison to vegetable cellulose, BC is regarded as a highly pure variant of cellulose, devoid of lignin, hemicellulose, or pectin in its composition [47,93]. This characteristic is particularly advantageous within the textile industry, as it negates the necessity for conventional pre-treatment processes aimed at removing these contaminants from plant-based fibers—processes that typically require elevated temperatures, significant water consumption, and the application of harmful chemicals [94]. In terms of its properties, BC is distinguished by several important attributes, in addition to the characteristics acquired from vegetable cellulose, including dimensional stability, mechanical strength, crystallinity, and water-holding capacity [90,95,96,97]. Furthermore, BC is recognized as a valuable biomaterial that can be synthesized from a variety of biomass sources. Numerous studies have investigated diverse methods and sources for the production of BC.
The global market for BC has been on an upward trajectory, demonstrating robust growth patterns. In 2016, the market was valued at approximately USD 207 million, which increased to around USD 250 million by 2019. Projections indicate that by the end of 2022, the market could reach USD 500 million, reflecting a compound annual growth rate (CAGR) of 16% over the subsequent years. Future estimates suggest that the BC market will surpass USD 570 million by 2024, achieve USD 680 million by late 2025, and potentially reach USD 700 million by 2026, culminating in an anticipated value of USD 780 million by 2027 [98]. A notable trend in this sector is the substantial rise in research and patent registrations, particularly since the 1980s. By 2019, approximately 6300 patents had been filed, predominantly in nations such as China, the United States, and Japan. This figure surged to 7800 in 2020 and exceeded 14,400 by 2021. The fields of chemistry, polymer sciences, and microbiology, particularly in relation to biotechnology, have been particularly prominent among these patents [98].
The estimated cost for producing around 500 tons of BC utilizing the Komagataeibacter xylinus strain is approximately USD 13 million. It is crucial to consider the total capital investment required for the industrialization process, which encompasses equipment, facilities, and construction costs. The annual production cost of BC is estimated at USD 7.4 million, which includes fixed expenses and direct operational costs, accounting for 58% of the total production cost. Based on a market price of USD 25 per kilogram for the final packaged product, the projected net profitability is estimated at USD 3.3 million per year [98].
In light of growing environmental concerns, industries are increasingly motivated to develop sustainable bioprocesses that mitigate ecological impacts and risks. Life cycle assessments of the BC production process, particularly through static culture methods, have indicated that water is the primary resource utilized, with a consumption of 36 tons per kilogram of BC produced. This is followed by raw material production (18 tons/kg), carton packaging (14 tons/kg), and the fermentation process (4 tons/kg). Notably, approximately 98% of the total resources are recovered post-treatment, and minimal environmental impacts have been identified, especially when industrial waste is repurposed as raw material [98].
4.2.1. Biosynthesis of BC
A variety of traditional carbon sources have been identified as viable substrates for the production of BC. These include glucose, fructose, lactose, acetate, ethanol, glycerol, mannitol, sucrose, maltitol, sucralose, and xylitol. Research has predominantly concentrated on utilizing glucose as the primary substrate for BC biosynthesis. The metabolic pathways and fluxes involved in BC production vary depending on the carbon source employed. The initial stage of this biosynthetic process involves the conversion of the chosen carbon source into hexoses, specifically glucose or fructose [98].
The BC synthesis process is orchestrated by the cellulose synthase (CS) complex. This complex initiates its activity in the bacterial cytoplasm upon activation by uridine diphosphate (UDP). The CS complex then systematically constructs BC from glucose molecules. The resulting product consists of fibrils made up of d-glucose units connected via β-1,4-glycosidic bonds. These fibrils are subsequently extruded through transmembrane pores to the exterior of the cell. Once outside the cell, the linear chains of glucose units align both unidirectionally and laterally. This alignment is facilitated by hydrogen bonding, both within individual chains (intrachain) and between different chains (interchain), occurring at the free hydroxyl groups. These aligned chains form nanofibrils, which can further aggregate into larger fibrils. These larger fibrils can reach widths of up to 100 nanometers and are responsible for the primary structural characteristics of bacterial cellulose. The schematic representation depicted in Figure 3 showcases the initial studies that concentrated on the biosynthesis of BC, which were conducted using a strain of K. xylinus. This strain, previously classified as Acetobacter xylinum and Gluconacetobacter xylinus, is now recognized as a model microorganism for research related to BC production [98].
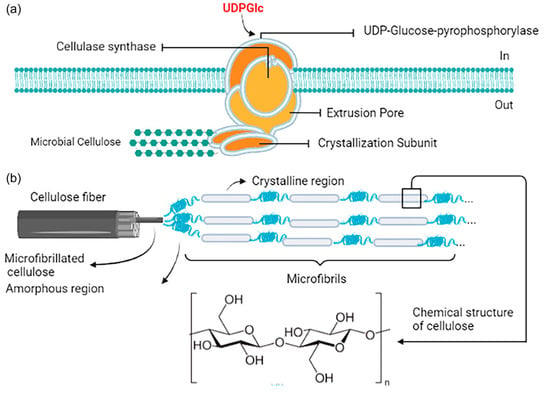
Figure 3.
Schematic representation of the CS Complex. (a) The CS complex synthesizes BC from glucose in a progressive manner after being activated by UDP within the bacterial cytoplasm, and (b) fibrils made up of D-glucose units connected by β-1,4-glycosidic bonds are transported through transmembrane pores out of the cell, where linear chains are aligned both unidirectionally and laterally through intra- and interchain hydrogen bonds at free hydroxyl groups [98].
Numerous investigations have been conducted to optimize BC production on an industrial scale, focusing on the utilization of low-cost raw materials that generate minimal by-products. In this context, various carbon sources, additive compounds, and types of agro-industrial waste are employed throughout the process. The carbon sources selected for BC biosynthesis possess distinct characteristics, including bioavailability, molecular mass, and diverse chemical structures, which consequently influence both the rate of BC production and its structural properties [98].
The biosynthesis of BC is a metabolically intensive process, consuming approximately 10% of the total adenosine triphosphate (ATP) produced during bacterial metabolism.
The metabolic flux for BC production from different carbon sources involves the synthesis of UDP-glucose through three specific steps when exogenous glucose serves as the carbon source. The enzymatic reactions involved in this pathway are facilitated by glucokinase, which phosphorylates glucose; phosphoglucomutase, which is responsible for the isomerization of glucose-6-phosphate to glucose-1-phosphate; and glucose-1-phosphate-uridylyltransferase, which catalyzes the formation of UDP-glucose. Additionally, fructose and glycerol are recognized as significant carbon sources for BC production. Subsequently, the synthase complex synthesizes BC from UDP-glucose, independent of the carbon source utilized (Figure 4) [98].
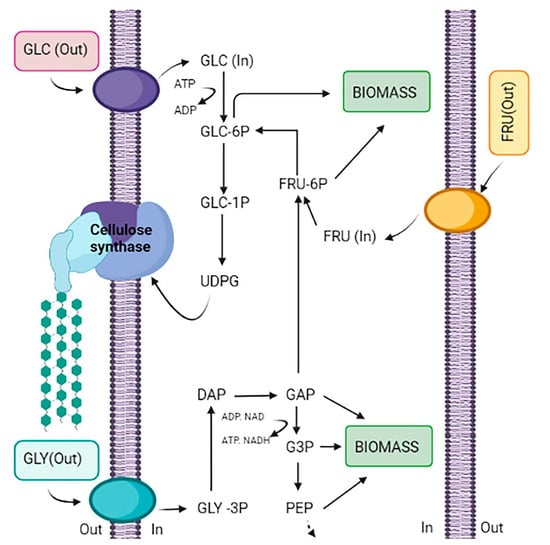
Figure 4.
Schematic representation of the metabolic flux for BC production from different carbon sources [98].
4.2.2. Production of BC by Microorganisms
BC is a glucan that can be synthesized extracellularly by various species of Gram-negative bacteria, including those from the genera Acetobacter, Agrobacterium, Alcaligenes, Komagataeibacter, Pseudomonas, Rhizobium, Salmonella, and Sarcina, which is the sole Gram-positive bacterial genus [99,100,101]. BC is characterized by high crystallinity, high water-holding capacity, high tensile strength, and a fine web-like network structure [95].
The production of BC can be achieved in diverse sizes and shapes, with specific properties that are contingent upon the cultivation methodology employed. In static fermentation, BC is generated as a three-dimensional interconnected reticular pellicle exhibiting a uniform texture. Conversely, during the process of agitated fermentation, the formation of small circular pellets occurs, characterized by their irregular shapes [93,102,103].
A comprehensive review conducted by Wang et al. [104] examined three prevalent methods for BC production: static culture, agitated culture, and bioreactor systems. In the same way, more recently, Girard et. al. [90]. The authors discussed the properties and potential applications of BC produced using these methods. Furthermore, the authors highlighted the challenges associated with various BC production methods and offered appropriate culture strategies for its application across diverse fields.
Another study by Stepanov & Efremenko [105] focused on the production of BC utilizing concentrated immobilized cells. The researchers successfully applied various media containing sugars and glycerol, based on hydrolysates of renewable biomass sources such as aspen, Jerusalem artichoke, rice straw, and microalgae. The level of BC accumulation using immobilized cells was found to be 1.3–1.8 times greater than that produced by suspended cells, thereby demonstrating the potential of using renewable biomass sources for BC production.
In addition to these studies, Sa’Adah et al. [106] explored a range of methods for producing BC microparticles, including mechanical methods using a high-pressure homogenizer, acid hydrolysis, microbial hydrolysis, hydrogel fiber cultivation, microfluidic processes, and ultrasonication. These methods offer alternative approaches for producing BC from biomass sources.
Furthermore, Amarasekara et al. [107] examined the potential of using the kombucha pellicle, a waste product from the kombucha beverage industry, as a source of BC. Their study compared different purification methods for the BC derived from the kombucha pellicle. This research highlights the potential of utilizing residual biomass sources for BC production.
Mohammadkazemi et al. [108] investigated the production of BC using different carbon sources, such as mannitol, sucrose, and glucose, as well as different culture media. They found that the carbon source and culture media significantly influenced the yield and properties of BC. This study demonstrates the flexibility in carbon source and culture media selection for BC production [108].
On the other hand, other authors [109] focused on the production of BC by Gluconacetobacter hansenii CGMCC 3917 using waste beer yeast as the sole nutrient source. They optimized the fermentation conditions and found that waste beer yeast could effectively support BC production. This study highlights the potential of utilizing residual materials as nutrient sources for BC production [109].
Lupașcu et al. [110] provided an overview of microbial aspects of BC production and its applications. They discussed the different bacterial species capable of producing cellulose and the factors influencing cellulose production. This review article provides a comprehensive understanding of the microbial aspects of BC production [110].
These references collectively demonstrate the diverse approaches and sources that can be used for the production of BC. By exploring different carbon sources, culture media, and microbial species, researchers are expanding the possibilities for BC production and its applications, namely in the fields of food technology, paper, cosmetics, pharmaceuticals, electronics, and medicine [111,112,113].
Given the features of PHA and BC, and the fact that they can be produced from lignocellulosic residues, such as forest biomass, in line with the circular economy concept, it is a worthy challenge to combine them into biodegradable composite materials with improved properties for several applications, including the textile sector.
4.2.3. Application of BC in the Textile Industry
The industrial applications of BC span a diverse array of industries, each leveraging its unique properties for various purposes. In this review, we identified four main categories for which BC has been used: the medical industry, the cosmetics industry, the textile industry, and the foodstuff industry, only focusing on more detail in the textile industry. An overview of these applications is available in Figure 5 [90].
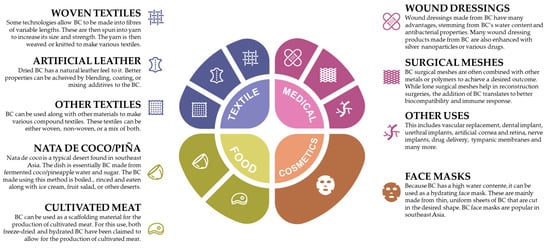
Figure 5.
Main uses of bacterial cellulose within various industries [90].
The textile industry faces increasing pressure to accelerate its green transition while simultaneously addressing the rising demand for textile products. This situation necessitates the exploration of new and more sustainable raw materials to replace non-renewable sources. BC has emerged in the literature as a promising biocompatible and biodegradable sustainable material for the textile industry. In addition to its sustainability credentials, the textile industry’s interest in BC stems from its unique properties compared to conventional vegetable cellulose. As previously referred to, BC boasts higher purity, greater crystallinity, improved dimensional stability, and enhanced mechanical strength, making it an attractive alternative for textile applications. However, despite these advantages, consolidated applications of BC in textiles represent a niche market, with only a limited number of solutions currently available, but BC as a biomaterial has been gaining attention in the textile industry. Provin et al. [114] discuss the use of BC and the challenge of wettability in textile applications [114]. BC also offers advantages in terms of hydrophobicity, which is desirable for textile applications [112,114], and it has been further characterized as a biomaterial for apparel products [112]. Its development contributes to a greener and more sustainable production of textiles.
In order to generate BC in various colours, several studies have investigated the use of different fermentation media and modifications to their components. Nonetheless, the colours pallettes produced did not meet the standards required by the fashion industry. As a result, various strategies have been suggested, including the incorporation of a colouring component directly into the fermentation medium (in situ) or applying them post-production and purification as a dyebath (ex situ) [113,115,116,117,118].
In the realm of biocomposites for textile applications, existing literature reports several innovative approaches. For instance, research conducted by Mizuno et al. was focused on creating BC biocomposites by depositing BC onto natural fibers that had been included in the fermentation medium [119]. Additionally, the development of nanocomposites involved depositing nanosized BC onto the surfaces of banana peel (BP) fibers, which were also added to the fermentation medium. This approach significantly improved the thermal stability and tensile strength of the resulting materials [120].
Several investigations have been focused on enhancing the mechanical characteristics of BC composites. In their research, Fernandes et al. [113] formulated BC biocomposites that show promise for applications within the textile and footwear industries. These composites demonstrated significantly improved properties, including a tensile strength of 12.1 Mpa, a water vapor permeability rate of 65.1 g·m−2·24 h−1, and enhanced thermal stability when the emulsified acrylated epoxidized soybean oil (AESO) was polymerized prior to the exhaustion dyeing process. Conversely, when polymerization occurred after the exhaustion phase, the composites exhibited increased hydrophobicity and a greater elongation of 19.1%. It is noteworthy that while the tensile strength of the BC composites was lower than that of pure BC, they nonetheless displayed superior elongation properties [90,113]. Malleable BC composites were developed by incorporating commercial hydrophobic products commonly used in textile finishing. This approach yielded a notable elongation at break of 7.97% and an enhancement in tensile strength (48.35 Mpa) [121]. Additionally, glycerol and polyethylene glycol have been employed as plasticizers in BC membranes intended for wound dressing applications. However, an increase in surface roughness was noted with higher plasticizer concentrations [122].
Another application of BC in textiles is illustrated by the production of yarn supercapacitors (YSC), where cotton yarn was soaked in a BC nanofiber suspension. This study substantiated the viability of the engineered YSC for applications in wearable electronics while also highlighting the potential of BC as an alternative cost-effective biomaterial to carbon nanotubes or reduced graphene oxide. The YSC exhibited impressive characteristics, including high capacitance, flexibility, and energy density, which further supports its application in this domain [123].
The Biocouture and SOYA C(O)U(L)TURE projects represent pioneering examples of the application of BC in the textile industry, as proofs of concept. The Biocouture project successfully created a collection of jackets and shoes made from BC using conventional manufacturing methods [124]. In contrast, the SOYA C(O)U(L)TURE project focused on developing dyeing and coating processes for BC sheets, resulting in clothing items and craft materials [125].
Innovative cultivation techniques have also been explored to produce BC textiles. One such method is the tailor-shaped cultivation technique, which allows for the production of different components of a t-shirt directly within fermentation containers designed in the desired shapes and sizes. The BC material produced was dyed black and sewn using traditional sewing techniques, resulting in a lightweight garment with a leather-like appearance. This approach not only demonstrates the suitability of BC as a raw material for textile production but also supports the reduction in textile waste by eliminating the cutting step typically required in garment manufacturing [103]. Recent evaluations have indicated that while BC sheets have lower tensile strength compared to animal leather, they outperform non-woven fabrics in this regard. Moreover, BC exhibits superior abrasion resistance. However, a critical drawback for apparel applications is its low breathability and high moisture retention, which can affect comfort [126].
Notwithstanding several investigations showcasing the potential of BC in textile applications, only a limited number of BC-based products have made it to market. Among the most notable successes is Nanollose, which has developed viscose-rayon like fibers from cellulose derived from BC, branded as nullarbor™ [90,98,127]. The startup ScobyTec has created a BC leather alternative. Known as ScobyTec BNC, which boasts high mechanical strength and non-flammability. This material is utilized in the production of children’s shoes, gloves, and business bags [90,98,128]. Other startups, the Make Grow Lab, have manufactured “leather” and packaging using BC and agro-industrial waste [98]. Bolt Threads company made sneakers using the microbial weaving technique [98]. Additionally, Malai markets BC materials (biocomposites formed by a matrix comprising BC and banana leaf fibers) itself in various colours, offering small bags and other accessories collections (including fanny packs, wallets, sandals, and bracelets) made from the material [98,113]. Polybion™ has established the first industrial-scale facility for producing BC material, including luxury handbags [90,129], with prototypes like small bags and a blazer produced in collaboration with fashion brand Ganni. In the same way, Ganni has also developed a handbag using a BC-based material from Modern Synthesis, a biotechnology company engaged in BC production [90,130,131].
The primary challenge hindering the market entry of BC products is the economic feasibility of producing BC on an industrial scale. High production costs and low productivity have been identified as the key issues impacting the commercialization of BC-based materials [132,133,134,135]. Dourado et al. conducted a technical and economic analysis of BC production using Super-Pro Designer software, version 9.0, for Windows operating system, highlighting these [136].
To address these issues, several studies have explored the use of industrial wastes and low-cost substrates as nutrients and carbon sources in BC production. This approach not only aims to reduce production costs but also emphasizes environmental sustainability. Examples of such substrates include corncob and sugarcane bagasse [98,137], grape bagasse [98,138], corn steep liquor [98,139], orange peel waste [140], olive mill [141], liquid tapioca waste [142], and tofu factories [125] wastewater. Companies like Nanollose [143], Polybion [129], and Modern Synthesis [131] have successfully adopted these strategies by utilizing agro-industrial fruit wastes in their BC production processes. This not only helps in minimizing production costs but also contributes to waste reduction and sustainable practices in the textile industry. By leveraging these low-cost materials, there is potential for improving the economic viability of BC production and expanding its applications in various markets.
4.3. Lignin
As referred to in Section 2, lignin is a highly branched, irregular, aromatic, natural polymer, a macromolecular structural component of all vascular plants, second only in abundance to cellulose [144]. Lignin isolated from industrial streams of chemical processing of plant biomass is called technical lignin, being a large-scale byproduct. About 50 million tons of lignin are obtained annually by the pulp-and-paper industry and several million tons from the hydrolysis mills [144,145]. Nowadays, more than 95% of industrially produced lignin is burned to produce energy. However, it is well recognized that lignin represents a promising renewable resource of aromatic hydrocarbons. Lignin can be used in its polymeric form in adhesives and new polymeric materials or can be upgraded into a series of value-added low-molecular weight chemicals (e.g., vanillin, benzene, toluene, xylene, quinones, eugenol, etc.) able to be used in the chemical, pharmaceutical, cosmetic, and textile industries [144,145,146].
Technical lignins are usually classified according to their production methods and can vary substantially in relation to their structure, functional group composition, and molecular weight. Thus, kraft lignin is isolated from spent liquors (black liquors) of sulphate pulping carried out in (NaOH + Na2S) solution at 160–180 °C [147]. Soda lignin is isolated from black liquors of soda pulping carried out in NaOH solutions at 150–170 °C. The sulphite lignin (lignosulphonate) is removed from wood to spent liquor (SSL) during sulphite pulping, which uses SO2 as a principal reagent combined with suitable bases (CaO, MgO, Na2O, or NH3) at 130–145 °C [147]. The hydrolysis lignin is a byproduct from the hydrolysis industry, which uses percolation hydrolysis with diluted sulphuric acid at high temperatures (160–180 °C). The hydrolysis lignin is a solid residue containing significant amounts of concomitant cellulose (20%–30%), and it is thus typically nominated as cellolignin [148]. Among referred technical lignins, only lignosulphonates (LS) are used on a large scale for different technical needs. In the last 50 years, the development of new pulping methods and plant biomass processing revealed new types of technical lignins. Thus, organosolv lignin is isolated from spent liquors of organosolv cooking carried out in aqueous organic solvent media [149]. The enzymatic upgrading of plant material leads to the formation of so-called enzymatic lignin. One of the particular cases of enzymatic lignin, consisting of cellolignin, is the residue remaining after enzymatic hydrolysis of plant biomass following steam explosion as a pre-treatment [45]. These relatively new types of technical lignins (organosolv and enzymatic lignins) urge further utilization to meet environmental and sustainable development. For most lignin applications, its purity and the absence of potentially hazardous elements (e.g., sulphur or heavy metals) are essential pre-requisites. In this sense, the organosolv lignin is the most attractive, and hydrolysis and enzymatic lignins are usually less acceptable for the potential applications. Therefore, the latter need to be refined before their use in non-fuel applications [45]. This is certainly a challenge for future developments. Table 2 briefly summarizes some basic characteristics of conventional and perspective technical lignins that are produced at industrial or pilot scale.

Table 2.
Basic characteristics of technical lignins [145,147,149].
Application of Lignin in the Textile Industry
Although technical lignins have widely been used in various technical fields for almost a century, their use in the textile industry is relatively recent and was stimulated by the desire to replace fossil materials with natural ones, also within the concept of biorefinery and circular economy. The different applications of lignin in the textile area can be conventionally divided into three main groups: (i) modification of man-made, synthetic, and natural fibers for their use in the production of fabrics or nonwovens to improve their consumer properties; (ii) modification of ready-made textile fabrics or nonwovens to confer them special advanced properties; and (iii) as an integral part of adhesives used for bonding or finishing non-woven fabrics and composites. The approaches and degree of lignin involvement can vary significantly depending on the objectives. The most common functions brought by lignin in textile materials are increased hydrophobicity, dyeing, UV blocking, antioxidant and antibacterial properties, flame retardance, and crease resistance, among others [150,151,152]. Figure 6 summarizes the main lignin applications in textiles and the methods used thereof.
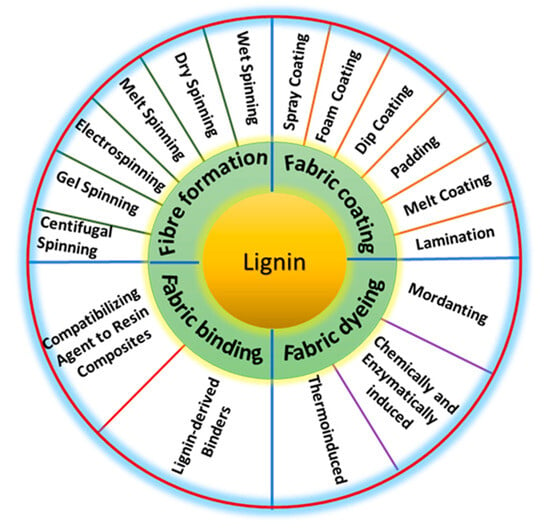
Figure 6.
Applications of lignin in textiles and methodology involved thereof.
The modification of man-made fibers, mainly regenerated cellulose fibers, called for increased attention in the last decade. This is related to its growing importance as a substitute for natural fibers whose production is limited for agricultural capabilities and environmental concerns [153]. In particular, lignin can be introduced in the spinning dope containing cellulose dissolved in direct solvents (e.g., NMMO in the Lyocell® process [154] or [DBNH][oAc] in the IonCell-F® process [155]). In this case, lignin is retained on the regenerated fibers proportionally to its content in the dope, thus increasing fibers hydrophobicity. Although the introduction of more than 10% of lignin led to the compromised tensile strength, these modified fibers are still appropriate for textile applications [154,156,157]. The same approach is proposed to introduce as high as 30–50% of lignin to produce lignin-based carbon fibers [157]. Regarding lignin-based carbon fibers, besides conventional wet spinning from dope solution, other spinning techniques can be applied for these purposes, including melt spinning of lignin blends with synthetic polymers, dry spinning, electrospinning, gel-spinning, and centrifugal spinning [152]. The lignins of high molecular weight without mineral contaminants and sulfur are preferable for most of these applications [152]. Both pure and modified lignins can be used for this type of application, depending on the methodology for incorporating them into the fibers. However, the issues of regenerating solvents containing significant lignin content have also not yet been resolved.
The common way of lignin introduction into synthetic fibers is a blending with a polymeric matrix in melt or dissolution in a polymer solution followed by the fiber formation by one of the suitable techniques (e.g., melt-spinning, dry-spinning, or electrospinning) [151,152]. Among matrix polymers, polyvinyl alcohol (PVOH), poly(acrylonitrile) (PAN), poly(ethylene oxide) (PEO), poly(methyl methacrylate) (PMMA), poly(caprolactone) (PCL), poly(lactic acid) (PLA), thermoplastic polyurethane (TPU), polyesters, and different poly(ethylene) (PE) and poly(propylene) formulations were reported [151]. However, these modified lignin synthetic fibers are used only for nonwoven applications. For example, hybrid lignin-PEO fibers were obtained by electrospinning from dimethylformamide (DMF) solution using different technical lignins (kraft, lignosulphonate, and organosolv lignins) and PEO of 60 kDa [158]. Up to 30-50% of lignin can be introduced in fibers, and the obtained nonwoven mesh can be used for various purposes, including water and air filtration or the production of carbonized nonwovens. Another example of lignin blending with a polymer’s matrix is lignin-PLA hybrid fibers produced from kraft lignin and PLA by melt spinning [159]. Up to 20% of lignin was introduced into the PLA matrix, resulting in spinnable fibers. Although lignin blending with PLA led to the decrease in hybrid fibers’ tenacity, the lignin together with ammonium polyphosphate conferred increased heat resistance and flame retardance properties to fabrics. Lignin introduction into a heat—sensible TPU provides an antioxidant effect, thus improving the processability of the formulation produced thereof [160].
The modification of ready-made textile fabrics with lignin and its derivatives is another way to give them specific properties. This can be done, for example, while using lignin as a filler in textile fabrics made of different natural (cotton, wool, etc.) or synthetic fibers, also in combination with conventional inorganic fillers (ZnO, TiO2, etc.) or as organic–inorganic hybrid formulations [150,151]. The application of lignin as is or as nanoparticles or in the composition of bio-nanocomposites in textile fibers can be carried out using different techniques, including conventional spray coating, foam coating, dip coating, and padding (also using suitable binders), but also employing calendaring, melt coating, electrostatic spraying, meltblowing, and lamination, among others [161]. Superhydrophobic properties were imparted to mercerized cotton fabric by dip-coating the fabric surface with a lignin-silica hybrid formulation [162]. The resulting coating was resistant to washing and demonstrated acceptable comfort and sensorial properties, advanced thermal stability, and UV-blocking properties. However, imparting strong hydrophobic properties to textile materials using lignin without significantly modifying it seems to be a difficult task. Lignin was also considered as a bio-based flame-retardant agent. Thus, the cotton fabric alternately impregnated in aqueous solutions of chitosan and LS (layer-by-layer approach) showed a flame-retardancy effect [163]. This effect was assigned to stabilizing lignin-induced char formation on the fiber surface, leading to the decrease in heat release rate and total heat release values. LS can also be introduced solely to a large extent into cotton fabrics by the hot padding method, showing the increased flame retardancy and the UV blocking at the same time [164]. The combination of lignin with graphene, carbon nanotubes, and graphene nanoplatelets leads to the hybrids (composite materials) with enhanced flame-retardant lignin characteristics [150]. Another field of use of lignin in textile applications is related to its antimicrobial functions. This effect is mainly related to the ability of the polyphenolic groups of lignin to damage the cell membrane of microorganisms and cause lysis of the bacteria followed by the release of their cellular contents. Both biocidal and biostatic lignin effects were reviewed, also when applied to textile materials [165]. The most common way to enhance the antimicrobial properties of lignin is to combine it with gold, silver, or transition metal oxide nanoparticles (CuO, ZnO, TiO2, etc.) [150,151,165]. The use of lignin in the form of nanoparticles is another steady trend of lignin application for antimicrobial purposes [165,166,167]. Furthermore, lignin nanoparticles can be used alone or as part of different nanocomposites. Several recent reviews on this topic are available [165,166]. The antimicrobial lignin properties were used to diminish the biodegradation of cotton fabrics while using them as natural fiber geotextiles [168]. A thermoformable kraft lignin composite was applied on the cotton mesh by melt coating, and the burial tests were carried out for 160 days. The lignin-coated cotton yarn showed a significantly longer durability against the microorganisms in the soil compared to uncoated yarns, being suitable for geotextile applications.
Fabric dyeing is another topic related to the use of lignin in the textile field. This is an attractive alternative to conventional synthetic dyes within the context of sustainable textile production looking for non-toxic, safe, biocompatible, and biodegradable perspectives [169]. In addition to the dull brown color with tonal variations, lignin gives textile fiber additional antioxidant, antibacterial, stain resistance, and UV-blocking properties. Typically, the water-soluble technical lignin, lignosulfonate, has been reported for these purposes [169,170]. However, kraft lignin can also be used for textile dyeing [171] or lignins isolated by other less conventional methods [172]. The most common way of textile dyeing with lignin is mordanting with suitable mordant agents under appropriate dyeing conditions to improve the colorfastness or better UV blocking properties [169,171]. Thus, wool fabrics were dyed at 98 °C for 60 min with various concentrations of water-soluble H2O2-upgraded sulphonated lignin using several sustainable mordanting agents, such as calcium (Ca), aluminum (Al), iron (Fe), and copper (Cu) salts, to improve the color yield and also the colorfastness to washing along with introducing new functional properties [169]. Similarly, cationic-treated cotton fabrics were dyed at 90 °C for 90 min in weak alkali kraft lignin solutions followed by a mordanting stage with Fe, Cu, Mg, and Al salts at 60 °C for 30 min [171]. It is unclear, however, how the mordanting approach fits with textile fabric safety and environmental concerns. Flax fabric dyeing ability and colorfastness were significantly improved while applying a laccase-promoted lignosulphonate oxidation [170]. A synergistic effect between lignin-based polymers formed and the lignin groups at the surface of flax fabrics was suggested in that work. These kinds of effects were also considered while using polyoxometalates as mediators in laccase-mediated oxidation systems [173].
Lignin can also be used as a part of binding formulation in knitted fabrics or nonwovens [174]. In this case, both neat and modified technical lignins may be applied as a compatibilizer in natural fiber-epoxy resin composites or, after the functionalization, be used as a proper binder agent. Several composites involving natural fibers (hemp, flax, oil palm empty fruit bunch, etc.) using lignin-derived binders were reviewed [174]. Lignin-based coating formulations were also reported to be an interesting option for reinforcement of textile mesh and to confer some special properties. Thus, the developed waterborne lignin-based polyurethane binder formulation was applied to the surface of cotton fabric by combined dip coating/padder squeezing followed by thermal curing at 150 °C for 5 min [174]. The applied adhesive has been shown to bind the fibers together to form a cohesive film that provides advanced antibacterial and UV protection properties [175].
5. Conclusions
A circular economy perspective might be applied to boost the competitiveness of the textile value chain. Under this approach, residual forest biomass can be used as sustainable raw material for the implementation of biomaterials and bioproducts in the textile industry, for example, through the application of biochemical technologies based on the so-called “sugar platform”.
Overall, the utilization of forest biomass as a source of biomaterials presents great potential for the textile industry, and researchers have been achieving significant advances in developing biomaterials from forest biomass, as demonstrated in the present review for three specific biomaterials—BC, PHAs, and lignin.
Despite the demonstrated potential and interest associated with PHAs, the developed fibers still exhibit inferior performance compared to most petrochemical-derived products concerning thermal and mechanical properties, restricting their applicability in a broader range of textile products. In this context, it becomes important to develop new solutions and methodologies based on PHAs that enable the production of new biodegradable fibers derived from natural sources and with adequate performance for applications in textile products.
BC exhibits exceptional mechanical characteristics that render it suitable for a diverse array of applications. The textile industry, in particular, has garnered significant interest from researchers in this context. When BC is subjected to drying, it reveals impressive tensile strength and can be processed into either non-woven or nano-spun fabrics, which enhances its moldability and biodegradability. Furthermore, the remarkable thinness of BC positions it as a viable alternative to traditional leather, especially in lightweight applications. However, the practical application of BC within the fashion and textile industries is influenced by several critical factors. Among these, the challenges associated with wettability/hydrophobicity, wear resistance, elasticity, breathability, dyability, and the overall comfort of the product are pivotal in assessing the feasibility of BC’s integration into these sectors.
Also, despite the rather significant successes in the use of lignin in textiles, this approach is not yet widespread. One of the reasons for this is that the proposed solutions are not always feasible in practice, and lignin is not always able to replace less “green” reagents of fossil origin with the same efficiency and without loss of quality indicators of textile materials.
In conclusion, future trends in the use of forest biomass as a source of new biomaterials for the textile industry are promising and rely on the sustainable recovery and valorization of renewable resources by using diverse biomass sources. The integration of residual forest biomass into biomaterial production, namely PHAs and BC, as well as the recovery of lignin from residual forest biomass, aligns with these principles of sustainability and offers potential for the development of advanced materials for the textile industry.
6. Future Directions
The potential of forest biomass as a source of new biomaterials for the textile industry is indeed promising and still under-explored. Forest biomass, being a renewable carbon-rich material due to its lignocellulosic nature, holds potential for the production of a wide variety of industrial and commodity products, including advanced materials for the textile industry. However, although residual forest biomass offers an alternative and sustainable source for the production of biomaterials, providing a renewable and eco-friendly option, they are still not widely used in the textile industry.
Indeed, the sustainable utilization of residual forest biomass for the production of biomaterials aligns with the principles of bioeconomy. However, it is essential to consider the overall sustainability of utilizing forest biomass for biomaterial recovery/production. The sourcing of biomass for textile production has been associated with unsustainable forest management practices, illegal logging, and sourcing from endangered forests and sensitive sites, highlighting the need for responsible and sustainable practices in biomass sourcing. This drawback may be overcome by the use of integrated biomass technologies, which not only offer opportunities for biomaterial recovery/production but also contribute to improving forest health by collaborating with the pulp and paper industry to remove less-desirable biomass and offset the costs associated with restoring damaged ecosystems.
The primary challenge hindering the industrial utilization of BC is the substantial expense associated with the cultivation medium and carbon sources. Nevertheless, current research is predominantly directed towards identifying alternative fermentation sources, including by-products from various industrial sectors, as well as optimizing and regulating biosynthetic pathways. BC derived from alternative sources exhibits physicochemical, mechanical, and structural properties that closely resemble those of cellulose produced through more traditional and costly methods. Approaches in genetic and metabolic engineering allow for the modification of BC-producing bacteria, thereby altering the structural characteristics of the cellulose generated. For instance, adjustments in the crystallinity or tensile strength can broaden the industrial applications of BC and enhance its effectiveness in existing sectors. The application of genomic-scale metabolic models for BC-producing bacteria may significantly advance genetic engineering efforts aimed at discovering strains that yield high amounts of BC while being resilient to the specific conditions of large-scale production environments. However, achieving success in this area requires a collaborative approach involving experts from various fields related to biomaterial production. Ultimately, significant progress in the production and application of BC could lead to the emergence of a diverse sector with considerable economic implications.
In short, to unlock the full potential of forest biomass, an integrated approach must be developed and implemented. Envisaging circularity for application in the textile industry, the valorization of the polysaccharide fractions of residual forest biomass into value-added biopolymers (e.g., PHA and BC) should be pursued through fermentation following pre-treatment and enzymatic hydrolysis. As a complement, lignin from cellolignin, which corresponds to the residue remaining in the forest biomass after the enzymatic hydrolysis of its polysaccharides, should also be applied in the textile industry. The applicability in the textile industry of these forest-derived biomaterials and bioproducts, although promising, is still not validated nor implemented.
Author Contributions
Conceptualization, J.C.D.; Methodology, J.C.D.; Investigation, S.M., F.F., P.C.B., C.A.V.T., T.R., D.V.E.; writing—original draft preparation, J.C.D.; writing—review and editing, J.C.D., S.M., F.F., D.V.E.; supervision, J.C.D.; project administration, C.J.S.; funding acquisition, C.J.S., F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Integrated Project be@t—Textile Bioeconomy, to strengthen the National Bioeconomy, financed by the Environmental Fund through Component 12—Promotion of Sustainable Bioeconomy (Investment TC-C12-i01—Sustainable Bioeconomy No. 02/C12- i01.01/2022) and Carla J. Silva is responsible for this fund. This work was financed by national funds from FCT/MCTES—Fundação para a Ciência e a Tecnologia, I.P., Ministério da Ciência, Tecnologia e Ensino Superior, in the scope of the projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO, project LA/P/0140/202019 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
Acknowledgments
Thomas Rodrigues acknowledge FCT I.P. for PhD Grant 2023.03035.BD. The financial support by project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 (DOI 10.54499/UIDB/50011/2020), UIDP/50011/2020 (DOI 10.54499/UIDP/50011/2020) & LA/P/0006/2020 (DOI 10.54499/LA/P/0006/2020), financed by national funds through the FCT/MCTES (PIDDAC) is greatly acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferreira, P.; Apolinário, A.; Forman, G. Optimising Textile Biomaterial Selection for Sustainable Product and Circular Design: Practical Guidelines for a Greener Future. Mater. Circ. Econ. 2023, 5, 14. [Google Scholar] [CrossRef]
- Desore, A.; Narula, S. An Overview on Corporate Response towards Sustainability Issues in Textile Industry. Environ. Dev. Sustain. 2018, 20, 1439–1459. [Google Scholar] [CrossRef]
- European Comission EU Strategy for Sustainable and Circular Textiles. Available online: https://environment.ec.europa.eu/strategy/textiles-strategy_en (accessed on 30 October 2023).
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Adamopoulos, S.; Jones, D.; Amiandamhen, S.O. Forest Biomass Availability and Utilization Potential in Sweden: A Review. Waste Biomass Valorization 2021, 12, 65–80. [Google Scholar] [CrossRef]
- Tian, W.; Huang, K.; Zhu, C.; Sun, Z.; Shao, L.; Hu, M.; Feng, X. Recent Progress in Biobased Synthetic Textile Fibers. Front. Mater. 2022, 9, 1098590. [Google Scholar] [CrossRef]
- Zhou, Y. The Importance and Applications of Forest Biomaterials. J. For. Res. 2023, 12, 1–2. [Google Scholar]
- Martini, S.; Kharismadewi, D.; Mardwita; Ginting, Y.R. Biomass Potential as an Alternative Resource for Valuable Products in the Perspective of Environmental Sustainability and a Circular Economy System. IOP Conf. Ser. Earth Environ. Sci. 2023, 1175, 012012. [Google Scholar] [CrossRef]
- Leitão, A.; Rebelo, F.; Pintado, M.; Ribeiro, T.B. AgroForest Biomass and Circular Bioeconomy. In Research Anthology on Ecosystem Conservation and Preserving Biodiversity; IGI Global: Hershey, PA, USA, 2022; pp. 1052–1097. [Google Scholar]
- van Wyk, J.P.H. Biotechnology and the Utilization of Biowaste as a Resource for Bioproduct Development. Trends Biotechnol. 2001, 19, 172–177. [Google Scholar] [CrossRef]
- Wiseman, A. (Ed.) Handbook of Enzyme Biotechnology; John Wiley and Sons Inc.: New York, NY, USA, 1975. [Google Scholar]
- Kennedy, J.F.; Phillips, J.F.; Williams, G.O. Wood and Cellulosics: Industrial Utilisation, Biotechnology, Structure and Properties, 1st ed.; Ellis Horwood Ltd.: Chichester, UK, 1987; Volume 71. [Google Scholar]
- Celpa: Boletim Estatístico 2020. Available online: https://www.agroportal.pt/celpa-boletim-estatistico-2020/ (accessed on 23 November 2023).
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.d.C.; Pereira, H. Potential of Eucalyptus Globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jang, S.-K.; Kim, J.-H.; Park, S.-Y.; Kim, J.-C.; Jeong, H.; Kim, H.-Y.; Choi, I.-G. Simultaneous Production of Glucose, Furfural, and Ethanol Organosolv Lignin for Total Utilization of High Recalcitrant Biomass by Organosolv Pretreatment. Renew. Energy 2019, 130, 952–960. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade Utilization of Lignocellulosic Biomass to High-Value Products. Green. Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv Fractionating Pre-treatment of Lignocellulosic Biomass for Efficient Enzymatic Saccharification: Chemistry, Kinetics, and Substrate Structures. Biofuels Bioprod. Biorefining 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Nishimura, H.; Kamiya, A.; Nagata, T.; Katahira, M.; Watanabe, T. Direct Evidence for α Ether Linkage between Lignin and Carbohydrates in Wood Cell Walls. Sci. Rep. 2018, 8, 6538. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose Bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Enzyme-Based Hydrolysis Processes for Ethanol from Lignocellulosic Materials: A Review. Bioresources 2007, 2, 707–738. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Van Le, Q. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Araque, E.; Parra, C.; Freer, J.; Contreras, D.; Rodríguez, J.; Mendonça, R.; Baeza, J. Evaluation of Organosolv Pretreatment for the Conversion of Pinus Radiata D. Don to Ethanol. Enzym. Microb. Technol. 2008, 43, 214–219. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent Advances in Organosolv Fractionation: Towards Biomass Fractionation Technology of the Future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Romaní, A.; Larramendi, A.; Yáñez, R.; Cancela, Á.; Sánchez, Á.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus Nitens Bark by Organosolv Pretreatment for the Production of Advanced Biofuels. Ind. Crops Prod. 2019, 132, 327–335. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for Fuel Ethanol: A Review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A. Microbial Enzyme Production Using Lignocellulosic Food Industry Wastes as Feedstock: A Review. Bioengineering 2016, 3, 30. [Google Scholar] [CrossRef]
- Yankov, D. Fermentative Lactic Acid Production From Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Reviving the Carbohydrate Economy via Multi-Product Lignocellulose Biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Cheng, J.J.; Timilsina, G.R. Status and Barriers of Advanced Biofuel Technologies: A Review. Renew. Energy 2011, 36, 3541–3549. [Google Scholar] [CrossRef]
- Geddes, C.C.; Nieves, I.U.; Ingram, L.O. Advances in Ethanol Production. Curr. Opin. Biotechnol. 2011, 22, 312–319. [Google Scholar] [CrossRef][Green Version]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.d.S.; Woiciechowski, A.L.; Soccol, C.R. Current Advances in On-Site Cellulase Production and Application on Lignocellulosic Biomass Conversion to Biofuels: A Review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Bacovsky, D.; Ludwiczek, N.; Ognissanto, M.; Wörgetter, M. Status of Advanced Biofuels Demonstration Facilities in 2012. A Report to IEA Bioenergy Task 39; IEA: Paris, France, 2013; Volume 39, pp. 1–209. [Google Scholar]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Taylor, R.; Nattrass, L.; Alberts, G.; Robson, P.; Chudziak, C.; Bauen, A.; Libelli, I.M.; Lotti, G.; Prussi, M.; Nistri, R.; et al. From the Sugar Platform to Biofuels and Biochemicals: Final Report for the European Commission Directorate-General Energy, Contract No. ENER/C2/423-2012/SI2.673791. Available online: https://ec.europa.eu/energy/content/sugar-platform-biofuels-and-biochemicals_en (accessed on 8 November 2023).
- Jatoi, A.S.; Abbasi, S.A.; Hashmi, Z.; Shah, A.K.; Alam, M.S.; Bhatti, Z.A.; Maitlo, G.; Hussain, S.; Khandro, G.A.; Usto, M.A.; et al. Recent Trends and Future Perspectives of Lignocellulose Biomass for Biofuel Production: A Comprehensive Review. Biomass Convers. Biorefin 2023, 13, 6457–6469. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Medeiros, R.; Gírio, F.M. Optimization of Brewery’s Spent Grain Dilute-Acid Hydrolysis for the Production of Pentose-Rich Culture Media. Appl. Biochem. Biotechnol. 2004, 115, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Glasser, W.G.; Wright, R.S. Steam-Assisted Biomass Fractionation. II. Fractionation Behavior of Various Biomass Resources. Biomass Bioenergy 1998, 14, 219–235. [Google Scholar] [CrossRef]
- Dias, B.; Lopes, M.; Fernandes, H.; Marques, S.; Gírio, F.; Belo, I. Biomass and Microbial Lipids Production by Yarrowia lipolytica W29 from Eucalyptus Bark Hydrolysate. Renew. Energy 2024, 224, 120173. [Google Scholar] [CrossRef]
- Francisco, M.; Aguiar, T.Q.; Abreu, G.; Marques, S.; Gírio, F.; Domingues, L. Single-Cell Oil Production by Engineered Ashbya gossypii from Non-Detoxified Lignocellulosic Biomass Hydrolysate. Fermentation 2023, 9, 791. [Google Scholar] [CrossRef]
- Rodrigues, T.; Torres, C.A.V.; Marques, S.; Gírio, F.; Freitas, F.; Reis, M.A.M. Polyhydroxyalkanoate Production from Eucalyptus Bark’s Enzymatic Hydrolysate. Materials 2024, 17, 1773. [Google Scholar] [CrossRef]
- Magina, S.; Marques, S.; Gírio, F.; Lourenço, A.; Barros-Timmons, A.; Evtuguin, D.V. Chemical Composition and Structural Features of Cellolignin from Steam Explosion Followed by Enzymatic Hydrolysis of Eucalyptus Globulus Bark. Ind. Crops Prod. 2024, 211, 118217. [Google Scholar] [CrossRef]
- Silva, J.B.; Pereira, J.R.; Marreiros, B.C.; Reis, M.A.M.; Freitas, F. Microbial Production of Medium-Chain Length Polyhydroxyalkanoates. Process Biochem. 2021, 102, 393–407. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef]
- Esmail, A.; Pereira, J.R.; Sevrin, C.; Grandfils, C.; Menda, U.D.; Fortunato, E.; Oliva, A.; Freitas, F. Preparation and Characterization of Porous Scaffolds Based on Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Life 2021, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Rodríguez, B.; Torres-Giner, S.; Reis, M.A.M.; Silva, F.; Matos, M.; Cabedo, L.; Lagarón, J.M. Blends of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Fruit Pulp Biowaste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate-Co-3-Hydroxyhexanoate) for Organic Recycling Food Packaging. Polymers 2021, 13, 1155. [Google Scholar] [CrossRef] [PubMed]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of Polyhydroxyalkanoate (PHA) Polymers Family as Substitutes of Petroleum Based Polymers for Packaging Applications and Solutions Brought by Their Composites to Form Barrier Materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Gautam, S.; Gautam, A.; Pawaday, J.; Kanzariya, R.K.; Yao, Z. Current Status and Challenges in the Commercial Production of Polyhydroxyalkanoate-Based Bioplastic: A Review. Processes 2024, 12, 1720. [Google Scholar] [CrossRef]
- Vicente, D.; Proença, D.N.; Morais, P.V. The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. Int. J. Environ. Res. Public Health 2023, 20, 2959. [Google Scholar] [CrossRef]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef]
- Tortajada, M.; da Silva, L.F.; Prieto, M.A. Second-Generation Functionalized Medium-Chain-Length Polyhydroxyalkanoates: The Gateway to High-Value Bioplastic Applications. Int. Microbiol. 2013, 16, 1–15. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Al-Battashi, H.; Annamalai, N.; Al-Kindi, S.; Nair, A.S.; Al-Bahry, S.; Verma, J.P.; Sivakumar, N. Production of Bioplastic (Poly-3-Hydroxybutyrate) Using Waste Paper as a Feedstock: Optimization of Enzymatic Hydrolysis and Fermentation Employing Burkholderia sacchari. J. Clean. Prod. 2019, 214, 236–247. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Pérez-Rivero, C.; Uwaezuoke, O.J.; Ngwuluka, N.C. From Waste to Wealth: Upcycling of Plastic and Lignocellulosic Wastes to PHAs. J. Chem. Technol. Biotechnol. 2022, 97, 3217–3240. [Google Scholar] [CrossRef]
- Kucera, D.; Benesova, P.; Ladicky, P.; Pekar, M.; Sedlacek, P.; Obruca, S. Production of Polyhydroxyalkanoates Using Hydrolyzates of Spruce Sawdust: Comparison of Hydrolyzates Detoxification by Application of Overliming, Active Carbon, and Lignite. Bioengineering 2017, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Dias, M.; Rodríguez-Contreras, A.; Kunaver, M.; Žagar, E.; Kržan, A.; Braunegg, G. Liquefied Wood as Inexpensive Precursor-Feedstock for Bio-Mediated Incorporation of (R)-3-Hydroxyvalerate into Polyhydroxyalkanoates. Materials 2015, 8, 6543–6557. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Cho, D.-H.; Jung, H.J.; Kim, B.; Kim, S.H.; Bhatia, S.K.; Gurav, R.; Jeon, J.-M.; Yoon, J.-J.; Kim, W.; et al. Finding of Novel Polyhydroxybutyrate Producer Loktanella sp. SM43 Capable of Balanced Utilization of Glucose and Xylose from Lignocellulosic Biomass. Int. J. Biol. Macromol. 2022, 208, 809–818. [Google Scholar] [CrossRef]
- Bowers, T.; Vaidya, A.; Smith, D.A.; Lloyd-Jones, G. Softwood Hydrolysate as a Carbon Source for Polyhydroxyalkanoate Production. J. Chem. Technol. Biotechnol. 2014, 89, 1030–1037. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Sustainable PHA Production in Integrated Lignocellulose Biorefineries. N. Biotechnol. 2019, 49, 161–168. [Google Scholar] [CrossRef]
- Dai, J.; Gliniewicz, K.; Settles, M.L.; Coats, E.R.; McDonald, A.G. Influence of Organic Loading Rate and Solid Retention Time on Polyhydroxybutyrate Production from Hybrid Poplar Hydrolysates Using Mixed Microbial Cultures. Bioresour. Technol. 2015, 175, 23–33. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, X.; Li, Q.; Wang, X.; Liu, M.; Xie, S.; Chai, L.; Yuan, J. Directed Bioconversion of Kraft Lignin to Polyhydroxyalkanoate by Cupriavidus basilensis B-8 without Any Pretreatment. Process Biochem. 2017, 52, 238–242. [Google Scholar] [CrossRef]
- Li, M.; Wilkins, M.R. Recent Advances in Polyhydroxyalkanoate Production: Feedstocks, Strains and Process Developments. Int. J. Biol. Macromol. 2020, 156, 691–703. [Google Scholar] [CrossRef]
- Frederick, N.; Li, M.; Carrier, D.J.; Buser, M.D.; Wilkins, M.R. Switchgrass Storage Effects on the Recovery of Carbohydrates after Liquid Hot Water Pretreatment and Enzymatic Hydrolysis. AIMS Bioeng. 2016, 3, 389–399. [Google Scholar] [CrossRef]
- Ding, C.; Li, M.; Hu, Y. High-Activity Production of Xylanase by Pichia Stipitis: Purification, Characterization, Kinetic Evaluation and Xylooligosaccharides Production. Int. J. Biol. Macromol. 2018, 117, 72–77. [Google Scholar] [CrossRef]
- Sa, K.; Ravi, Y.K.; Ma, G.; Chattopadhyay, I.; Palani, S.; Kumar, V.; Kumar, G.; Jeyakumar, R.B. Development of an Integrated Biorefinery System for Bioconversion of Lignocellulosic Biomass to Polyhydroxyalkanoates and Biohydrogen. ACS Sustain. Chem. Eng. 2023, 11, 4606–4622. [Google Scholar] [CrossRef]
- Cerrone, F.; Davis, R.; Kenny, S.T.; Woods, T.; O’Donovan, A.; Gupta, V.K.; Tuohy, M.; Babu, R.P.; O’Kiely, P.; O’Connor, K. Use of a Mannitol Rich Ensiled Grass Press Juice (EGPJ) as a Sole Carbon Source for Polyhydroxyalkanoates (PHAs) Production through High Cell Density Cultivation. Bioresour. Technol. 2015, 191, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.H.; Bhukya, B. Biotransformation of Toxic Lignin and Aromatic Compounds of Lignocellulosic Feedstock into Eco-Friendly Biopolymers by Pseudomonas putida KT2440. Bioresour. Technol. 2022, 363, 128001. [Google Scholar] [CrossRef]
- Silva, L.F.; Taciro, M.K.; Raicher, G.; Piccoli, R.A.M.; Mendonça, T.T.; Lopes, M.S.G.; Gomez, J.G.C. Perspectives on the Production of Polyhydroxyalkanoates in Biorefineries Associated with the Production of Sugar and Ethanol. Int. J. Biol. Macromol. 2014, 71, 2–7. [Google Scholar] [CrossRef]
- Salamanca-Cardona, L.; Scheel, R.A.; Bergey, N.S.; Stipanovic, A.J.; Matsumoto, K.; Taguchi, S.; Nomura, C.T. Consolidated Bioprocessing of Poly(Lactate-Co-3-Hydroxybutyrate) from Xylan as a Sole Feedstock by Genetically-Engineered Escherichia coli. J. Biosci. Bioeng. 2016, 122, 406–414. [Google Scholar] [CrossRef]
- Kopf, S.; Åkesson, D.; Skrifvars, M. Textile Fiber Production of Biopolymers—A Review of Spinning Techniques for Polyhydroxyalkanoates in Biomedical Applications. Polym. Rev. 2023, 63, 200–245. [Google Scholar] [CrossRef]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef]
- Usurelu, C.D.; Badila, S.; Frone, A.N.; Panaitescu, D.M. Poly(3-Hydroxybutyrate) Nanocomposites with Cellulose Nanocrystals. Polymers 2022, 14, 1974. [Google Scholar] [CrossRef]
- Hinuber, C.; Haussler, L.; Vogel, R.; Brunig, H.; Heinrich, G.; Werner, C. Hollow Fibers Made from a Poly(3-Hydroxybutyrate)/Poly-ε-Caprolactone Blend. Express Polym. Lett. 2011, 5, 643–652. [Google Scholar] [CrossRef]
- Uddin, M.K.; Novembre, L.; Greco, A.; Sannino, A. Polyhydroxyalkanoates, A Prospective Solution in the Textile Industry—A Review. Polym. Degrad. Stab. 2024, 219, 110619. [Google Scholar] [CrossRef]
- Liu, Q.; Shyr, T.-W.; Tung, C.-H.; Liu, Z.; Shan, G.; Zhu, M.; Deng, B. Particular Thermal Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Oligomers. J. Polym. Res. 2012, 19, 9756. [Google Scholar] [CrossRef]
- Vogel, R.; Tändler, B.; Voigt, D.; Jehnichen, D.; Häußler, L.; Peitzsch, L.; Brünig, H. Melt Spinning of Bacterial Aliphatic Polyester Using Reactive Extrusion for Improvement of Crystallization. Macromol. Biosci. 2007, 7, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent Progress in Development and Chemical Modification of Poly(Hydroxybutyrate)-Based Blends for Potential Medical Applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Mármol, G.; Gauss, C.; Fangueiro, R. Potential of Cellulose Microfibers for PHA and PLA Biopolymers Reinforcement. Molecules 2020, 25, 4653. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fujita, M.; Takeuchi, A.; Suzuki, Y.; Uesugi, K.; Ito, K.; Fujisawa, T.; Doi, Y.; Iwata, T. Formation of Highly Ordered Structure in Poly[(R)-3-Hydroxybutyrate- Co -(R)-3-Hydroxyvalerate] High-Strength Fibers. Macromolecules 2006, 39, 2940–2946. [Google Scholar] [CrossRef]
- Alagoz, A.S.; Rodriguez-Cabello, J.C.; Hasirci, V. PHBV Wet-Spun Scaffold Coated with ELR-REDV Improves Vascularization for Bone Tissue Engineering. Biomed. Mater. 2018, 13, 055010. [Google Scholar] [CrossRef]
- Kundrat, V.; Matouskova, P.; Marova, I. Facile Preparation of Porous Microfiber from Poly-3-(R)-Hydroxybutyrate and Its Application. Materials 2019, 13, 86. [Google Scholar] [CrossRef]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Wang, W.; Sheng, Y.; Guo, Y.; Li, Y.; Yang, Q.; Chen, P. Polylactide/Poly[(R)-3-hydroxybutyrate] (PHB) Blend Fibers with Superior Heat-resistance: Effect of PHB on Crystallization. Polym. Adv. Technol. 2023, 34, 1479–1491. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; Li, Y.; Wang, W.; Sui, M.; Yang, Q.; Tong, Y.; Yang, K.; Chen, P. Toward Heat Resistant Polylactide Blend Fibers via Incorporation of Low Poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate] Content. J. Appl. Polym. Sci. 2022, 139, e52652. [Google Scholar] [CrossRef]
- Girard, V.; Chaussé, J.; Vermette, P. Bacterial Cellulose: A Comprehensive Review. J. Appl. Polym. Sci. 2024, 141, 55163. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent Developments in the Production and Applications of Bacterial Cellulose Fibers and Nanocrystals. Crit. Rev. Biotechnol. 2017, 37, 510–524. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, A.; Patra, A.K.; Mahajan, R. A Sustainable and Green Process for Scouring of Cotton Fabrics Using Xylano-Pectinolytic Synergism: Switching from Noxious Chemicals to Eco-Friendly Catalysts. 3 Biotech 2018, 8, 184. [Google Scholar] [CrossRef]
- Esmail, A.; Rebocho, A.T.; Marques, A.C.; Silvestre, S.; Gonçalves, A.; Fortunato, E.; Torres, C.A.V.; Reis, M.A.M.; Freitas, F. Bioconversion of Terephthalic Acid and Ethylene Glycol Into Bacterial Cellulose by Komagataeibacter xylinus DSM 2004 and DSM 46604. Front Bioeng Biotechnol 2022, 10, 853322. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Tudoroiu, E.-E.; Ionescu, D.-G.; Dinu-Pîrvu, C.-E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef]
- da Silva Rocha, A.R.; Venturim, B.C.; Ellwanger, E.R.A.; Pagnan, C.S.; da Silveira, W.B.; Martin, J.G.P. Bacterial Cellulose: Strategies for Its Production in the Context of Bioeconomy. J. Basic. Microbiol. 2023, 63, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Dourado, F.; Ryngajllo, M.; Jedrzejczak-Krzepkowska, M.; Bielecki, S.; Gama, M. Taxonomic Review and Microbial Ecology in Bacterial Nanocellulose Fermentation. In Bacterial Nanocellulose from Biotechnology to Bio-Economy; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amesterdam, The Netherlands, 2016; pp. 1–17. [Google Scholar]
- Thongwai, N.; Futui, W.; Ladpala, N.; Sirichai, B.; Weechan, A.; Kanklai, J.; Rungsirivanich, P. Characterization of Bacterial Cellulose Produced by Komagataeibacter maltaceti P285 Isolated from Contaminated Honey Wine. Microorganisms 2022, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological Production of Cellulose by Acetic Acid Bacteria: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for Cost-Effective and Enhanced Production of Bacterial Cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef]
- Chan, C.K.; Shin, J.; Jiang, S.X.K. Development of Tailor-Shaped Bacterial Cellulose Textile Cultivation Techniques for Zero-Waste Design. Cloth. Text. Res. J. 2018, 36, 33–44. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef]
- Sa’adah, A.N.; Milyawan, G.N.A.; Nadya, T.; Silviana, S. A Mini-Review of Bio-Scrubber Derived from Bacterial Cellulose Impregnated by Flavonoid of Moringa Leaves. IOP Conf. Ser. Earth Environ. Sci. 2022, 963, 012022. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wang, D.; Grady, T.L. A Comparison of Kombucha SCOBY Bacterial Cellulose Purification Methods. SN Appl. Sci. 2020, 2, 240. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of Bacterial Cellulose by Gluconacetobacter hansenii CGMCC 3917 Using Only Waste Beer Yeast as Nutrient Source. Bioresour Technol 2014, 151, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lupașcu, R.E.; Ghica, M.V.; Dinu-Pîrvu, C.-E.; Popa, L.; Ștefan Velescu, B.; Arsene, A.L. An Overview Regarding Microbial Aspects of Production and Applications of Bacterial Cellulose. Materials 2022, 15, 676. [Google Scholar] [CrossRef] [PubMed]
- Bianchet, R.T.; Vieira Cubas, A.L.; Machado, M.M.; Siegel Moecke, E.H. Applicability of Bacterial Cellulose in Cosmetics—Bibliometric Review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.D.S.; Rocha, M.A.V.; Fenrnandes, L.M.A.; Queiroz, J.A.; Agra, A.C.M.G.; Amorim, J.D.P.; Sarubbo, L.A.; Sarubbo, L.A. Bacterial Cellulose: Characterization of a Biomaterial for Apparel Products Application. Res. J. Text. Appar. 2022, 26, 532–545. [Google Scholar] [CrossRef]
- Fernandes, M.; Souto, A.P.; Dourado, F.; Gama, M. Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites. Polysaccharides 2021, 2, 566–581. [Google Scholar] [CrossRef]
- Provin, A.P.; dos Reis, V.O.; Hilesheim, S.E.; Bianchet, R.T.; de Aguiar Dutra, A.R.; Cubas, A.L.V. Use of Bacterial Cellulose in the Textile Industry and the Wettability Challenge—A Review. Cellulose 2021, 28, 8255–8274. [Google Scholar] [CrossRef]
- Han, J.; Shim, E.; Kim, H.R. Effects of Cultivation, Washing, and Bleaching Conditions on Bacterial Cellulose Fabric Production. Text. Res. J. 2019, 89, 1094–1104. [Google Scholar] [CrossRef]
- Zhong, C. Colored Bacteria Cellulose Product and Preparation Method. Thereof. Patent CN102127576A, 24 April 2013. [Google Scholar]
- Shim, E.; Kim, H.R. Coloration of Bacterial Cellulose Using In Situ and Ex Situ Methods. Text. Res. J. 2019, 89, 1297–1310. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.R. Production of Coffee-Dyed Bacterial Cellulose as a Bio-Leather and Using It as a Dye Adsorbent. PLoS ONE 2022, 17, e0265743. [Google Scholar] [CrossRef]
- Mizuno, M.; Kamiya, Y.; Katsuta, T.; Oshima, N.; Nozaki, K.; Amano, Y. Creation of Bacterial Cellulose-Fabric Complexed Material. Sen’i Gakkaishi 2012, 68, 42–47. [Google Scholar] [CrossRef]
- Naeem, M.A.; Siddiqui, Q.; Khan, M.R.; Mushtaq, M.; Wasim, M.; Farooq, A.; Naveed, T.; Wei, Q. Bacterial Cellulose-Natural Fiber Composites Produced by Fibers Extracted from Banana Peel Waste. J. Ind. Text. 2022, 51, 990S–1006S. [Google Scholar] [CrossRef]
- Fernandes, M.; Gama, M.; Dourado, F.; Souto, A.P. Development of Novel Bacterial Cellulose Composites for the Textile and Shoe Industry. Microb. Biotechnol. 2019, 12, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Meng, C.; Zheng, Y.; Xie, Y.; He, W.; Wang, Y.; Qiao, K.; Yue, L. The Effects of Two Biocompatible Plasticizers on the Performance of Dry Bacterial Cellulose Membrane: A Comparative Study. Cellulose 2018, 25, 5893–5908. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, L.; Yuan, Y.; Wei, C.; Bai, Z.; Xu, J. All-Solid-State Yarn Supercapacitors Based on Hierarchically Structured Bacterial Cellulose Nanofiber-Coated Cotton Yarns. Cellulose 2016, 23, 3987–3997. [Google Scholar] [CrossRef]
- Catherine Jewell Designing with Life: Biofabricate’s Suzanne Lee Envisions a “New Material World”. Available online: https://www.wipo.int/web/wipo-magazine/articles/designing-with-life-biofabricates-suzanne-lee-envisions-a-new-material-world-56276 (accessed on 29 December 2023).
- SOYA C(O)U(L)TURE—Useful Things Arise out of Waste. Available online: https://ars.electronica.art/aeblog/en/2015/09/30/soya-coulture/ (accessed on 30 September 2023).
- Wood, J.; Verran, J.; Redfern, J. Bacterial Cellulose Grown from Kombucha: Assessment of Textile Performance Properties Using Fashion Apparel Tests. Text. Res. J. 2023, 93, 3094–3108. [Google Scholar] [CrossRef]
- Nanollose. Available online: https://nanollose.com/Technology/Our-Technology/ (accessed on 11 December 2023).
- Leather-Free Handbag Made of Bacterial Cellulose. Available online: https://materialdistrict.com/article/handbag-bacterial-cellulose/ (accessed on 11 December 2023).
- Polybion. Available online: https://www.polybion.bio/ (accessed on 11 December 2023).
- Hahn, J. Ganni Unveils Handbag Made from Bacterial Leather at LDF. Available online: https://www.dezeen.com/2023/07/04/ganni-bacterial-cellulose-leather-jacket-polybion/ (accessed on 11 December 2023).
- Carlson, C. Modern Synthesis Uses Bacteria to Create Biomaterial Fabric. Available online: https://www.dezeen.com/2023/04/03/modern-synthesis-bacteria-biomaterial-fabric/ (accessed on 11 December 2023).
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of Bacterial Cellulose Production from Prickly Pear Peels and Its Ex Situ Impregnation with Fruit Byproducts for Antimicrobial and Strawberry Packaging Applications. Carbohydr. Polym. 2023, 302, 120383. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Kiruba, T. Bacterial Cellulose—A Potential Material for Sustainable Eco-Friendly Fashion Products. J. Nat. Fibers 2022, 19, 3275–3287. [Google Scholar] [CrossRef]
- Pedroso-Roussado, C. The Fashion Industry Needs Microbiology: Opportunities and Challenges. mSphere 2023, 8, e0068122. [Google Scholar] [CrossRef]
- da Silva, C.J.G.; de Medeiros, A.D.M.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial Cellulose Biotextiles for the Future of Sustainable Fashion: A Review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Dourado, F.; Fontão, A.; Leal, M.; Cristina Rodrigues, A.; Gama, M. Process Modeling and Techno-Economic Evaluation of an Industrial Bacterial NanoCellulose Fermentation Process. In Bacterial Nanocellulose: From Biotechnology to Bio-Economy; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 199–214. [Google Scholar]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from Agricultural Residues by Two Komagataeibacter sp. Strains. Bioengineered 2022, 13, 10010–10025. [Google Scholar] [CrossRef]
- Ogrizek, L.; Lamovšek, J.; Čuš, F.; Leskovšek, M.; Gorjanc, M. Properties of Bacterial Cellulose Produced Using White and Red Grape Bagasse as a Nutrient Source. Processes 2021, 9, 1088. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of Bacterial Cellulose by Gluconacetobacter hansenii Using Corn Steep Liquor As Nutrient Sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Maina, S.; Ladakis, D.; Kookos, I.K.; Koutinas, A. Integrated Biorefinery Development for the Extraction of Value-Added Components and Bacterial Cellulose Production from Orange Peel Waste Streams. Renew. Energy 2020, 160, 944–954. [Google Scholar] [CrossRef]
- Sar, T.; Yesilcimen Akbas, M. Potential Use of Olive Oil Mill Wastewater for Bacterial Cellulose Production. Bioengineered 2022, 13, 7659–7669. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and Characterization of Bacterial Cellulose by Acetobacter xylinum Using Liquid Tapioca Waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Bioenergy International. Available online: https://bioenergyinternational.com/wbm/ (accessed on 11 December 2023).
- Glasser, W.G.; Kelly, S.S. Lignin. In Encyclopedia of Polymer Science and Engineering, 1st ed.; Mark, H.B., Bikales, N.M., Overberger, C.C., Menges, G., Eds.; WileyInterscience: New York, NY, USA, 1987; Volume 8. [Google Scholar]
- Blažej, A.; Košík, M. Phytomass: A Raw Material for Chemistry and Biotechnology; Ellis Horwood: New York, NY, USA, 1993. [Google Scholar]
- Agarwal, S.L.; Russo, S. (Eds.) Comprehensive Polymer Science; Pergamon: Oxford, UK, 1992. [Google Scholar]
- Belgacem, N.; Pizzi, A. (Eds.) Lignocellulosic Fibers and Wood Handbook: Renewable Materials for Today’s Environment; Wiley-Scrivener Publ. LLC.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rabinovich, M.L. Lignin By-Products of Soviet Hydrolysis Industry: Resources Characteristics and Utilization as a Fuel. Cellul. Chem. Technol. 2014, 48, 613–631. [Google Scholar]
- Tofani, G.; Jasiukaitytė-Grojzdek, E.; Grilc, M.; Likozar, B. Organosolv Biorefinery: Resource-Based Process Optimisation, Pilot Technology Scale-up and Economics. Green Chem. 2024, 26, 186–201. [Google Scholar] [CrossRef]
- Raman, A.; Sankar, A.; Abhirami, S.D.; Abhirami, A.; Saritha, A. Insights into the Sustainable Development of Lignin-Based Textiles for Functional Applications. Macromol. Mater. Eng. 2022, 307, 202200114. [Google Scholar] [CrossRef]
- Gaynor, J.G.; Szlek, D.B.; Kwon, S.; Tiller, P.S.; Byington, M.S.; Argyropoulos, D.S. Lignin Use in Nonwovens: A Review. Bioresources 2022, 17, 3445–3488. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, J.; Cheng, Y.; Lu, C. Lignin-Based High-Performance Fibers by Textile Spinning Techniques. Materials 2021, 14, 3378. [Google Scholar] [CrossRef]
- Mendes, I.S.F.; Prates, A.; Evtuguin, D.V. Production of Rayon Fibres from Cellulosic Pulps: State of the Art and Current Developments. Carbohydr. Polym. 2021, 273, 118466. [Google Scholar] [CrossRef] [PubMed]
- Protz, R.; Lehmann, A.; Ganster, J.; Fink, H.-P. Solubility and Spinnability of Cellulose-Lignin Blends in Aqueous NMMO. Carbohydr. Polym. 2021, 251, 117027. [Google Scholar] [CrossRef] [PubMed]
- Hummel, M.; Michud, A.; Tanttu, M.; Asaadi, S.; Ma, Y.; Hauru, L.K.J.; Parviainen, A.; King, A.W.T.; Kilpeläinen, I.; Sixta, H. Ionic Liquids for the Production of Man-Made Cellulosic Fibers: Opportunities and Challenges. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Advances in Polymer Science; Rojas, O., Ed.; Springer: Cham, Switzerland, 2015; Volume 271, pp. 133–168. [Google Scholar]
- Byrne, N.; De Silva, R.; Ma, Y.; Sixta, H.; Hummel, M. Enhanced Stabilization of Cellulose-Lignin Hybrid Filaments for Carbon Fiber Production. Cellulose 2018, 25, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Trogen, M.; Le, N.-D.; Sawada, D.; Guizani, C.; Lourençon, T.V.; Pitkänen, L.; Sixta, H.; Shah, R.; O’Neill, H.; Balakshin, M.; et al. Cellulose-Lignin Composite Fibres as Precursors for Carbon Fibres. Part 1—Manufacturing and Properties of Precursor Fibres. Carbohydr. Polym. 2021, 252, 117133. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of Technical Lignins for the Production of Fibrous Networks. J. Wood Chem. Technol. 2010, 30, 315–329. [Google Scholar] [CrossRef]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Fierro, V.; Celzard, A. PLA with Intumescent System Containing Lignin and Ammonium Polyphosphate for Flame Retardant Textile. Polymers 2016, 8, 331. [Google Scholar] [CrossRef]
- Gama, N.; Evtyugin, D.D.; Lourenço, A.; Lopes, C.; Evtuguin, D.V. Ellagic Acid as Stabilizer in the Thermo-Oxidative Degradation of Thermoplastic Polyurethane. Polym. Degrad. Stab. 2023, 215, 110456. [Google Scholar] [CrossRef]
- Amid, H.; Mazé, B.; Flickinger, M.C.; Pourdeyhimi, B. Hybrid Adsorbent Nonwoven Structures: A Review of Current Technologies. J. Mater. Sci. 2016, 51, 4173–4200. [Google Scholar] [CrossRef]
- Nisar, S.; Raza, Z.A. Corn Straw Lignin—A Sustainable Bioinspired Finish for Superhydrophobic and UV-Protective Cellulose Fabric. Int. J. Biol. Macromol. 2024, 257, 128393. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Xu, Y.-J.; Jiang, Z.-M.; Liu, Y.; Zhu, P. Novel and Eco-Friendly Flame-Retardant Cotton Fabrics with Lignosulfonate and Chitosan through LbL: Flame Retardancy, Smoke Suppression and Flame-Retardant Mechanism. Polym. Degrad. Stab. 2020, 181, 109302. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, V.; Basak, S.; Ali, S.W. Sodium Lignin Sulfonate: A Bio-Macromolecule for Making Fire Retardant Cotton Fabric. Cellulose 2019, 26, 8191–8208. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.-S.; Zhong, X.-Q.; Guo, Y.; Xie, X.-B. High Value Valorization of Lignin as Environmental Benign Antimicrobial. Mater. Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S. Lignin Nanoparticles: Eco-Friendly and Versatile Tool for New Era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Ali, M.A.-S.; Abdel-Moein, N.M.; Owis, A.S.; Ahmed, S.E.; Hanafy, E.A. Eco-Friendly Lignin Nanoparticles as Antioxidant and Antimicrobial Material for Enhanced Textile Production. Sci. Rep. 2024, 14, 17470. [Google Scholar] [CrossRef]
- Kaya, C.; Stegmaier, T.; Gresser, G.T. Investigation of the Protective Function of a Lignin Coating of Natural Fiber Geotextiles against Biodegradation. Materials 2023, 16, 4849. [Google Scholar] [CrossRef]
- Hassan, M.M. Valorisation of Sulphonated Lignin as a Dye for the Sustainable Colouration of Wool Fabric Using Sustainable Mordanting Agents: Enhanced Colour Yield, Colourfastness, and Functional Properties. RSC Sustain. 2024, 2, 676–685. [Google Scholar] [CrossRef]
- Kim, S.; Silva, C.; Zille, A.; Lopez, C.; Evtuguin, D.V.; Cavaco-Paulo, A. Characterisation of Enzymatically Oxidised Lignosulfonates and Their Application on Lignocellulosic Fabrics. Polym. Int. 2009, 58, 863–868. [Google Scholar] [CrossRef]
- Mun, J.S.; Mun, S.P. Potential of Hardwood Kraft Lignin as a Bio-Based Dye for Cotton Fabrics. Bioresources 2023, 19, 973–984. [Google Scholar] [CrossRef]
- Bhushan, S.; Kumar, A.; Singh, N.; Sheikh, J. Functionalization of Wool Fabric Using Lignin Biomolecules Extracted from Groundnut Shells. Int. J. Biol. Macromol. 2020, 142, 559–563. [Google Scholar] [CrossRef]
- Kim, S.; Silva, C.; Evtuguin, D.V.; Gamelas, J.A.F.; Cavaco-Paulo, A. Polyoxometalate/Laccase-Mediated Oxidative Polymerization of Catechol for Textile Dyeing. Appl. Microbiol. Biotechnol. 2011, 89, 981–987. [Google Scholar] [CrossRef]
- Khan, T.A.; Lee, J.-H.; Kim, H.-J. Lignin-Based Adhesives and Coatings. In Lignocellulose for Future Bioeconomy; Ariffin, H., Sapuan, S.M., Hassan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–206. [Google Scholar]
- Lin, L.; Tu, Y.; Li, Z.; Wu, H.; Mao, H.; Wang, C. Synthesis and Application of Multifunctional Lignin-Modified Cationic Waterborne Polyurethane in Textiles. Int. J. Biol. Macromol. 2024, 262, 130063. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).