Abstract
Dioecious species show a division of labor expressed through the differentiated manifestation of resource acquisition. We hypothesized that the expression of secondary sexual dimorphism (SSD) in the leaf gas exchange of yerba mate would be more intensive in females than in males to permit females the carbon investments necessary to finish the reproductive cycle. This species can present two growth units annually (GU1-fall and GU2-spring) intercalated with two rest periods (R1-summer and R2-winter). The leaf area index (LAI) and the diurnal courses of leaf photosynthesis (Anet), stomatal conductance (gs), leaf transpiration (E), intercellular CO2 concentration (Ci), water use efficiency (WUE), and instantaneous carboxylation efficiency (Anet/Ci) were estimated in female and male plants of yerba mate during four periods of annual rhythmic growth in monoculture (MO) and agroforestry (AFS). Leaf gas exchanges varied over the annual rhythmic growth and were more intensive under MO than under AFS. Anet, Anet/Ci ratios, and WUE were higher in females than in males during the summer (R1) and spring (GU2). Also, gs and E were more intensive in females than males during the summer. Oppositely, higher WUE in males than in females was observed during the fall (GU1) and winter (R2), with males also showing a higher Anet/Ci ratio during the winter and higher E during the spring (GU2). Despite the strong effect of the cultivation system on LAI and leaf gas exchange traits over the diurnal course, SSD expression was rarely modified by the cultivation system, being expressed only in MO for E during the spring (GU2) and WUE during the winter (R2). High WUE in males during the winter would benefit plants during cold and dry periods, improving the balance between carbon acquisition and water loss through transpiration. On the other hand, high Anet during the summer and spring could be considered as a general fitness strategy of female plants to improve photoassimilate supply and support their additional reproduction costs.
1. Introduction
Yerba mate (Ilex paraguariensis A. St.-Hil., family Aquifoliaceae) is an evergreen broadleaf tree species [1] native to South American subtropical humid forests [2], today growing in Brazil, Argentina, Paraguay, and Uruguay [3]. It is widely used for South American tea preparations [4] and, recently, for cosmetics and pharmaceutic industries [2] due to its recognized phytochemical properties [5,6,7,8]. This dioecious tree has rhythmic growth [1,9]. Yerba mate is usually pruned every 18 or 24 months, in ‘plate form’ as a shrub of various geometrical forms [10], and its raw material is used intensively for tea, medicines, and pharmaceuticals. Some rare specimens are grown freely up to a potential height of 15 m, known as female mother trees, with the aim of producing seeds for further propagation [11]. In field conditions, forest clearing of various tree and understory species improves yerba mate production, and complete forest clearing is usual, for cultivating yerba mate in monoculture (MO), in consortium with an annual crop species, or for growing other crop species [12,13].
Decreases in forest biodiversity, as in MO systems, are accompanied by a reduction of soil and water conservation capacity [14] and thermal regulation capacity, increasing the risk of frost, drought, and other environmental stresses [15]. Then, yerba mate plants are more vulnerable to environmental stresses when grown in MO, a scenario that can be more limited under climate change [16]. In fact, higher radiation use efficiency in yerba mate sprouts is obtained in the intercropping system, as compared to MO [17]. Despite the ecological benefits of forest and intercropping systems, MO is the most intensive and productive system for yerba mate. Biomass harvested in MO is about ten times higher compared to anthropized forest understory or agroforestry (AFS) [1,9]. Adult yerba mate plants cultivated in AFS differ from those in MO in leaf-area density up to six times, in plant leaf area (LA) up to seven times, and in leaf area index (LAI) up to twelve times [1,10]. In AFS, yerba mate forms longer internodes and bigger, wider individual leaves than plants cultivated in MO.
More than 70% of tree species have a rhythmic growth [18], meaning that organogenesis and elongation are characterized by periods of active growth and rest [19]. The appearance of growth pauses defines rhythmic growth, which is widely observed in trees [18]. It is characterized by the succession of periods of uninterrupted organ emission and extension and periods of rest. In yerba mate, ideally, two growth flushes are observed when portions of leafy branches, called growth units (GUs), are formed, occurring in spring and fall, interrupted with periods of rest (R) occurring in summer and winter [1,9]. When several successive GUs are formed in the same annual vegetative cycle, they most often present distinctive characteristics, as observed in yerba mate. In the first year after harvest (pruning), the most vigorous individuals do not stop growing during the summer, especially in MO, where such uninterrupted metamer emission is likely due to high light conditions [20]. The useful yerba mate biomass, composed of leaves and fine branches, is harvested according to growth pauses, generally during the winter growth pause (rest) or during the summer rest, and sometimes, the harvest is conducted all year long, depending on industry needs [1]. The end-of-spring flush corresponds to flowering (males and females), while the summer growth pause corresponds to fruit ripening in females [1].
In dioecious species, the differences between the genders in characters not directly related to gamete production are known as secondary sexual dimorphism (SSD) [21]. SSD is generally based on the difference in the use of resources between males and females, which is caused by varying reproductive costs between the genders [22]. While females require larger amounts of resources due to seed and fruit production, males just produce flowers and pollen, which are usually less costly [23]. Sexual reproduction in Sagittaria latifolia imposes asymmetry between the genders, with greater biomass costs for females and greater nitrogen costs for males [24]. Curiously, this difference in nitrogen costs between the genders was not associated with differences in photosynthetic rates [24]. On the other hand, males of Silene latifolia produce small flowers in abundance and have higher stomatal conductance (gs) and leaf transpiration (E) than females, leading to a higher cost of reproduction for males [25]. In some dioecious species and environments, biomass allocation to reproduction is three times higher in female plants than in male ones [22]. In other species, as in Fraxinus mandshurica, there is no biomass difference between the genders during early ontogeny [26]. SSD can be expressed in morphological, physiological, and life-history traits [22,27,28,29] and can promote differential leaf and trunk biomass production [30,31], or variation in leaf chemical composition [7,32] between genders. The complex sexual phenotypic differentiation in angiosperms can be understood through genetic regulation and the spatial and temporal role of plant growth regulators [33]. The single-gene sex-determining genetic systems could be common in dioecious species that evolved via monoecy [34].
In yerba mate, SSD expression is higher under stressful conditions [9,28,30] and depends on phenology, genetic material, and study conditions [1,10,35,36]. In the literature, SSD expressions in yerba mate are still contradictory, and we believe this is partially caused by phenology, as is the case for photosynthetic capacity [35]. In other words, SSD expressions likely change along the annual cycle in field-grown plants. Increasing net photosynthetic rate (Anet) in dioecious Populus spp. during the early summer is related to an increase in the chlorophyll pool during leaf development [37]. However, differences between the genders in terms of photosynthetic gas exchanges are not always observed [37]. The adult female yerba mate trees cultivated in MO presented higher Anet, gs, and E than male trees during the summer rest pause and spring GU emission [36]. However, such sexual segregation is always perceived in sun leaves, not in self-shaded ones, and gs is the most responsive trait to SSD. When evaluating yerba mate progeny, the Anet of fully expanded leaves in female trees grown in AFS was higher than in males, but only during the winter growth pause, while gs did not segregate between genders. In an experiment with clonal yerba mate plants, Anet was higher in females than in males under AFS (but not in MO) over a large part of the diurnal period at the beginning of summer growth pause and beginning of fruit ripening, while SSD was not expressed in terms of carbon gain at the plant scale, irrespective of cultivation system [35]. Then, SSD in yerba mate seems to depend on the environment, phenophase, and cultivation system [10,35].
As the pattern of SSD expression in yerba mate trees varies largely in the literature, we followed the diurnal dynamics of leaf gas exchange during one annual rhythmic growth in males and females. Additionally, we evaluated the SSD expression in terms of leaf water use efficiency [38] and the instantaneous carboxylation efficiency [39], two traits rarely studied in dioecious species [40] and never studied in yerba mate trees in the scope of SSD. Due to SSD, we hypothesized that leaf gas exchange would be more intensive in females than in males to support female carbon investments during the reproductive period.
2. Materials and Methods
2.1. Experimental Area and Cultivation Systems
The experiment was established in an area of the Federal University of Santa Catarina (UFSC), near Curitibanos (27°19′09″ S, 50°42′39″ W, 836 m a.s.l.), Southern Brazil. The climate is defined as subtropical humid (Cfa), according to the Köppen-Geiger classification, with regularly distributed rains and an average air temperature of 20.8 ± 0.3 °C in the warmest month [41].
Two contrasting cultivation systems of yerba mate were established in neighboring plots, with about 150 m distance between plots. One system was the anthropized rainforest in recovery enriched with yerba mate in its understory (agroforestry system, AFS), and the second was the full-sun cultivation (monoculture, MO). Each cultivation system with yerba mate was established at plots of about 75 × 20 m. The rainforest was composed of native tree species (Araucaria angustifolia, Cordia trichotoma, Cordia ecalyculata, Eugenia involucrata, Luehea divaricata, Ocotea spp., Peltophorum dubium, and Syagrus romanzoffiana), non-native tree species (Pinus elliottii and Eucalyptus spp.), and understory vegetation. The MO was established at the initial stage of forest regeneration in AFS. The soil is classified as Dystric Cambisol [35], and lime was applied at a depth of 50 cm only in the planting lines. The fertilization in both cultivation systems was carried out according to soil analyses and species recommendations [42], with mechanical weed control and no pest/disease protection.
In both cultivation systems, young clonal plants issued from vegetative reproduction were planted in June 2018. They were ~10 cm in height, and each clone originated from one plant of the same sex. The planting arrangement was about 3 m distance between rows and 1.5 m between plants in rows. When plants were three years old, six male (♂) and six female (♀) plants were chosen for morpho-physiological evaluations, totaling 24 plants. Plant selection was based on the presence of 5–6 visible growth units (GU), using the chronological age as a reference. In December 2021, at the beginning of the summer rest period, the formation pruning was performed at 40 cm of stem height, leaving about 10%–20% of photosynthesizing leaf area for plant recovery [35], with the regrowth being where the branches of the 1st order tend to grow perpendicular to the supporting structure [10].
2.2. Microclimate, Leaf Area Index, and Physiological Measurements
Microclimate, leaf area, and leaf gas exchange were measured in four seasons, following the plant growth rhythmicity after pruning in December 2021: growth rest 1 (R1, summer—February 2022); growth unit 1 (GU1, fall—May 2022); growth rest 2 (R2, winter—August 2022); and growth unit 2 (GU2, spring—November 2022).
Photosynthetic photon flux density (PPFD, μmol photons m−2 s−1) was measured using silicon pyranometer smart sensors (S-LIx-M003, HOBO, Bourne MA, USA). They captured the sun radiation in a 400–700 nm waveband. Two light sensors were used from summer 2022 to spring 2022 and maintained at two heights from the soil, 0.4 and 2 m (above and below the canopy of representative yerba mate plants), in each cultivation system. PPFD values were recorded with a datalogger (H21-USB Data Logger USB, HOBO, Bourne MA, USA) every 10 s. Here, we used hourly mean values. As we had only one datalogger, the sensors were moved every two to three weeks from one to the other system, AFS and MO, during the experimental period. Simultaneously with PPFD measurements, the air temperature was registered in both systems during the four seasons with the U23-003 HOBO 2x external temperature data logger (HOBO, Bourne MA, USA, Figure S1). The temperature sensor was positioned at 2 m height from the soil, near the upper PPFD sensor. Rainfall was obtained from the local meteorological station.
Leaf area index (LAI) was measured with an LAI–2000 plant canopy analyzer (LICOR, Lincoln, NE, USA). The procedure comprised a set of six readings: the first one was taken above the yerba mate plants; and the next four below the plants at 0.2 m from the trunk and oriented to the four cardinal points; the sixth reading was again taken above the yerba mate plants. In AFS, the 1st and 6th readings were taken inside the forest, capturing the canopy above the yerba mate plants. The LAI estimated in AFS was expected to be higher than in reality because the reference readings were not taken under a clear sky, but rather under the forest upper canopy layer.
The responses of leaf gas exchange to light in yerba mate were measured over four seasons. Twenty-three PPFD levels were used: 1500, 1200, 800, 500, 200, 100, 90, 80, 70, and every 5 μmol m−2 s−1 between 70 and 0 μmol m−2 s−1. The 6-cm2 leaf cuvette was fitted with a red–blue light source (6400-02B, LICOR, Lincoln NE, USA, Figure S1). For respiration in the light (RL), we used the Kok method [43], computing the y-axis intercept of a first-order linear regression fitted to Anet/PPFD when PPFD varied between 25 and 65 μmol m−2 s−1 [44]. All gas exchange data were corrected for the increase in intercellular CO2 concentrations (Ci) when decreasing PPFD [44].
By using the response curves of leaf CO2 assimilation to PPFD and hourly PPFD values (Figure S1), we estimated diurnal variation of leaf CO2 assimilation (Anet, μmol m−2 s−1) in MO and AFS. As variables from light response curves [45], we had the apparent quantum efficiency of CO2 assimilation (Φ, μmol CO2 μmol photons−1, calculated from the initial linear slope of the modelled light response curves for PPFD < 30 μmol m−2 s−1), the maximum net photosynthesis (Amax, μmol m−2 s−1, a proxy of photosynthetic capacity), RL, and the convexity factor of the curve (θ), as shown in Equation (1) [46]:
The stomatal conductance (gs, mol m−2 s−1), leaf transpiration rate (E, mmol m−2 s−1), and intercellular CO2 concentration (Ci, μL L−1) dependences on PPFD were also estimated by using a second-order polynomial equation, which tended to a linear regression in the case of a null quadratic coefficient. The instantaneous leaf water use efficiency (WUE, μmol mmol−1) was calculated as Anet/E. Additionally, the Anet/Ci ratio (μmol m−2 s−1 Pa−1), as a proxy of the instantaneous carboxylation efficiency, was also calculated. The last two parameters were calculated for diurnal hours when Anet > 0.
2.3. Experimental Design and Statistical Analysis
A complete randomized design was used, which considered two genders (female and male) grown in two cultivation systems (AFS and MO) with six replications (plants) over four seasons. Two-way ANOVA was applied to analyze effects of factors, such as genders and cultivation systems (separately for each season) on LAI. Three-way ANOVA was applied to analyze effects of factors, such as genders and cultivation systems observed over the diurnal period (separately for each season) on leaf gas exchange. ANOVAs considered a mixed linear model (lme function from the ‘nlme’ package) and maximum likelihood to test the significance of studied variables. The Bartlett homogeneity test and the Shapiro normality test were performed for each variable in each season. Genders, cultivation systems and evaluation time were considered as fixed factor effects, while plant number (repetitions) was considered a random effect observed separately for each season/growth period. If interaction was non-significant, the model reduction was applied, starting from the interaction of two or three factors. After that, the model was fitted again. For comparison of variables among the four seasons, one-way ANOVA was performed, in which the seasons were considered as fixed factor effect, while total plant number as a random effect. All ANOVAs were performed at 95% confidence, followed by a Tukey Honestly Significant Difference test, where p < 0.05 for mean comparisons was considered significant. P-values, estimated means, and standard errors (SE) are shown in figures. All statistical analyses were performed using R. 4.1.0 software [45], with ‘nlme’, ‘emmeans’, and ‘multcomp’ packages for ANOVA analyses.
3. Results
3.1. Climate Conditions
At midday, the mean PPFD values coming to the top of the yerba mate canopy in MO (2 m from the soil) were 1120, 850, 790, and 950 μmol m−2 s−1 during the summer, fall, winter, and spring, respectively (Figure 1A–D). The maximum hourly PPFD values ranged between 1510 and 2000 μmol m−2 s−1 in MO at midday (from 10:45 h to 14:45 h) during the experimental period. In MO and at 40 cm from the soil surface (below plant canopies), the mean PPFD values at midday were about 160, 70, 85, and 45 μmol m−2 s−1, and represented 14.3%, 8.2%, 10.8%, and 4.7% of the upcoming PPFD in the summer, fall, winter, and spring, respectively. The decreased light transmission in MO was primarily related to high absorbance and reflectance of light by increased leaf area of yerba mate plants (Figure 2) and less to seasonal variation of PPFD. In AFS, the mean PPFD coming at 2 m of height from the soil (above plant canopy) was about 46, 39, 53, and 75 μmol m−2 s−1, being reduced by 96%, 95%, 93%, and 92% when compared to PPFD coming to 2 m of height from the soil in MO (Figure 1A–D). The values of PPFD coming at 2 m of height from the soil in AFS were additionally reduced by 43%, 57%, 57%, and 63% at a height of 40 cm (below the plant canopy). Compared to MO, lower PPFD absorbance in AFS was certainly related to low LAI (Figure 2).
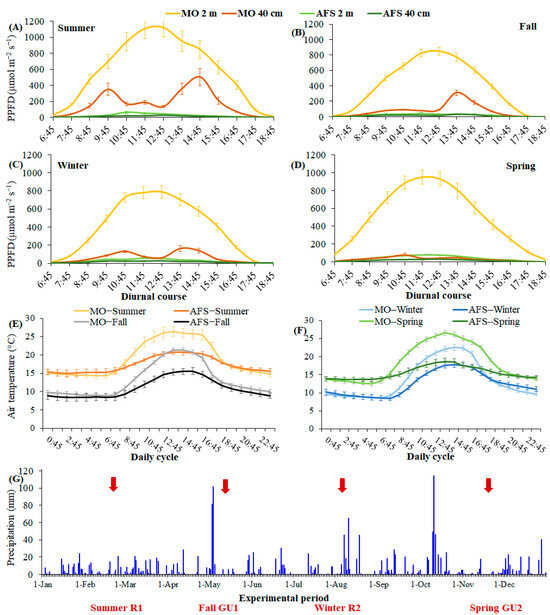
Figure 1.
Microclimate in the monoculture (MO) and agroforestry (AFS) of yerba mate. Mean ± SE (n = 18–72) for photosynthetic photon flux density (PPFD) measured over the diurnal cycle at two heights from the soil surface (2 and 0.4 m) during the summer (A), fall (B), winter (C), and spring (D), and air temperature measured over the daily cycle at 2 m height from the soil surface during the summer and fall (E) and during the winter and spring (F). In (G), daily precipitation during the experimental period (year 2022) is shown. Red arrows indicate when physiological measurements were taken during seasons and growth periods [(rests 1 and 2 (R1 and R2) and growth unit formations (GU1 and GU2)].
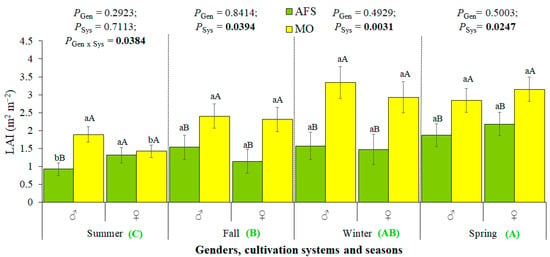
Figure 2.
Yerba mate leaf area index (LAI) estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS) for two genders (female—♀ and male—♂) in four seasons [summer—rest 1 (R1); fall—growth unit 1 (GU1); winter—rest 2 (R2); spring—growth unit 2 (GU2)]. Estimated mean ± SE and P-values (bold when significant) are shown (n = 6). Lowercase black letters compare genders (Gen) in each cultivation system (Sys) and each season, whereas uppercase black letters compare cultivation systems for each gender in each season. The uppercase green bold letters at the bottom position compare seasons (n = 24, P < 0.0001).
During the summer, the mean daily temperatures were 18.9 °C in MO and 17.3 °C in AFS, while the lowest/highest mean hourly temperatures were 14.4/26.4 °C and 15.0/20.8 °C in MO and AFS, respectively (Figure 1E). The absolute minimum/maximum hourly temperatures during the experimental period were 6.8/35.7 °C and 4.2/24.2 °C in MO or AFS, respectively. During the fall, the mean daily temperatures were 13.2 °C in MO and 11.0 °C in AFS, while the lowest/highest mean hourly temperatures were 8.8/21.3 °C and 8.4/15.6 °C in MO and AFS, respectively (Figure 1E). The absolute minimum/maximum hourly temperatures during the fall were −3.6/35.4 °C and −0.4/26.4 °C in MO and AFS, respectively. During the winter, the mean daily temperatures were 13.6 °C in MO and 12.4 °C in AFS, while the lowest/highest mean hourly temperatures were 8.6/22.5 °C and 8.5/17.8 °C, occurring early in the morning and at midday in MO and AFS, respectively (Figure 1F). The absolute minimum/maximum hourly temperatures of −4.3/33.4 °C and −2.1/27.1 °C were registered during the winter in MO and AFS, respectively. During the spring, the mean daily temperatures were 18.3 °C in MO and 15.5 °C in AFS, while the lowest/highest mean hourly temperatures of 12.4/26.6 °C and 13.6/18.6 °C occurred early in the morning and at midday in MO or AFS, respectively (Figure 1F). The absolute minimum/maximum hourly temperatures during the spring were −0.9/38.5 °C and 9.7/26.3 °C in MO and AFS, respectively.
The precipitation summed 1957 mm in 2022, with 364 mm in summer, 491 mm in fall, 494 mm in winter, and 608 mm in spring. Precipitations were relatively well-distributed over the year, except for May 3 and 4 and October 10, when rainfall was excessive, i.e., 82, 102, and 115 mm, respectively.
3.2. Expression of SSD in LAI
LAI increased during the 1st year after pruning, from season to season, and it was much higher in MO than in AFS (Figure 2). LAI difference between cultivation systems could be even higher than shown here (about 70%–100%, depending on the season), because, in AFS, the sensors also captured a fraction of the forest canopy, including not only the LAI of yerba mate plants.
The gender effects on LAI had been significant only in the summer, after formation pruning, with males showing higher LAI than females in MO. The opposite was detected in AFS, with LAI being higher in females than in males in the summer (Figure 2). Such a situation was highly dependent on the remaining leaf area after pruning, as a new leaf area was not formed between the R1 (summer) and GU1 (fall). From the fall, SSD in LAI was not expressed anymore.
3.3. Expression of SSD in Diurnal Leaf Gas Exchanges
The estimated leaf CO2 assimilation rate (Anet, leaf photosynthesis) was the highest during the fall and winter and the lowest during the spring, with intermediate values during the summer (Figure 3). Anet was impacted by the cultivation system over all seasons, showing higher values in MO than AFS, except for early morning and late afternoon hours in all seasons (interactions System × Hour). The difference between the two systems for Anet increased over the diurnal hours, being the most expressive at the midday period, from 10:45 h to 14:45 h. The SSD in Anet was expressed during the summer R1 and spring GU2 periods, expressed in higher values of females than in males (Figure 3A,D).
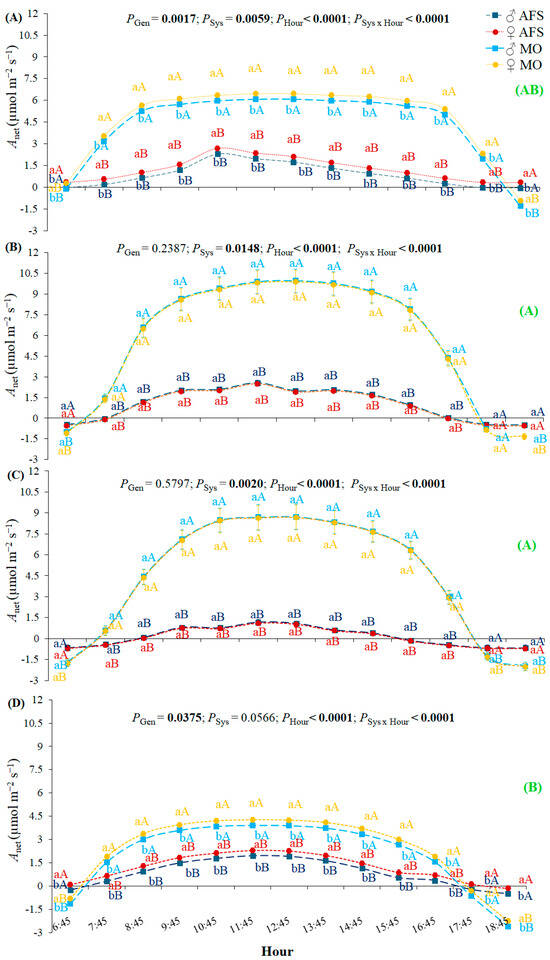
Figure 3.
Yerba mate leaf net CO2 assimilation rate (Anet) estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS) for two genders (female—♀ and male—♂) during four seasons: (A) summer—rest 1 (R1), (B) fall—growth unit 1 (GU1), (C) winter—rest 2 (R2), and (D) spring—growth unit 2 (GU2)] along the diurnal period. Estimated mean ± SE and P-values (bold when significant) are shown (n = 6). Lowercase, colored letters compare genders (Gen) under each cultivation system (Sys) for each hour and each season; uppercase, colored letters compare cultivation systems for each gender for each hour and for each season. The uppercase bold green letters in parenthesis on the right side compare general Anet seasonal responses (P < 0.0001).
The estimated stomatal conductance (gs) was the highest in the winter, followed by generally similar gs in fall and spring (two periods of growth unit formations, GU1 and GU2), and the lowest values were found in the summer R1 period (Figure S2). The gs was higher in MO than in AFS, except during the fall of GU1, when it was similar between the two cultivation systems (Figure S2B). The gs showed low variations during the diurnal course in AFS, while in MO, such diurnal variation was significant. At midday, gs was up to 4–5 times higher than one registered at the beginning or end of the day during R1 and R2 (Figure S2A,C). The SSD in gs was expressed only during summer R1, being higher in females than in males (Figure S2A) and allowing higher Anet in females than in males (Figure 3A).
The estimated leaf transpiration rate (E) was the highest in spring GU2 as compared to all three previous growth periods (Figure 4). It was impacted by the cultivation system over all four annual growth periods, being higher in MO than in AFS. Stronger variations over the diurnal course were observed in MO when compared to AFS. Hourly E variations in AFS were non-significant during GU1 and GU2 periods—interaction Sys × Hour (Figure 4B,D). The SSD was expressed for E and females presented higher values than males during the summer R1 period (Figure 4A). The opposite situation in SSD expression was observed during the spring GU2, when males had higher E than females in MO, without any gender segregation in AFS (Figure 4D). In addition, no gender differentiation in E was observed during the fall (GU1) and winter (R2) seasons (Figure 4B,C).
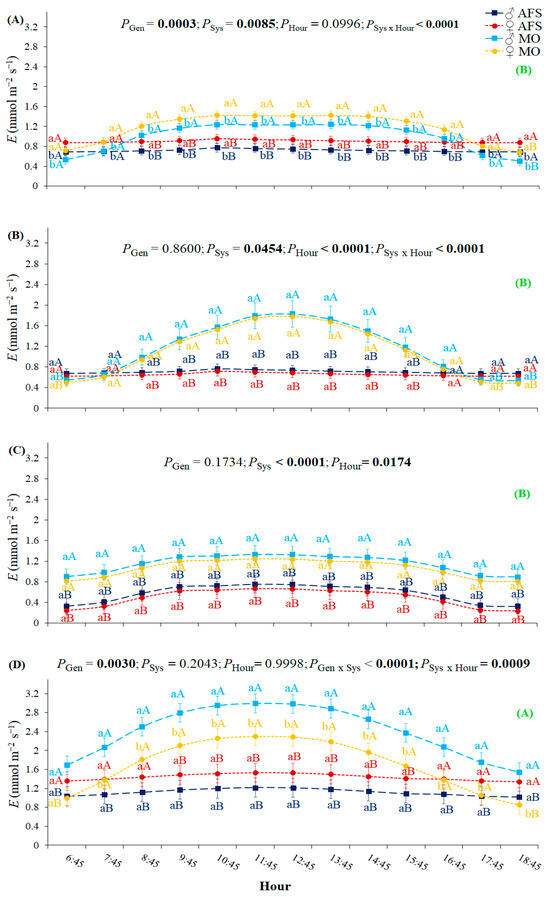
Figure 4.
Yerba mate leaf transpiration rate (E) estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS) for two genders (female—♀ and male—♂) during four seasons: (A) summer—rest 1 (R1), (B) fall—growth unit 1 (GU1), (C) winter—rest 2 (R2), and (D) spring—growth unit 2 (GU2)] for each hour. Estimated mean ± SE and P-values (bold when significant) are shown (n = 6). Lowercase colored letters compare gender (Gen) responses under each cultivation system (Sys) for each hour and each season; uppercase colored letters compare cultivation systems for each gender, hour, and season. The uppercase bold green letters in parenthesis on the right side compare general E seasonal responses (P < 0.0001).
During fall R1, the intercellular CO2 concentration (Ci) was the lowest among the four growth periods (Figure S3). Ci was modified by the cultivation system over all seasons, generally being higher in AFS than in MO, with the exception of early morning and late afternoon hours (Figure S3). Significant variations in Ci were observed over the diurnal cycle, especially in MO, and very low variations were registered in AFS. Ci showed gender segregation during the four rhythmic growth periods. Ci was higher in males than in females in the summer R1 and spring GU2 periods (Figure S3A,D), while it was higher in females than in males during the fall GU1 and winter R2 periods (Figure S3B,C).
The estimated Anet/Ci ratio was the highest during the fall GU1 and diminished gradually during the winter and spring GU2 (Figure 5). Anet/Ci ratio was always higher in MO than in AFS, and it varied significantly over the four rhythmic growth periods in MO, but only slightly in AFS. In the summer R1 and spring GU2 periods, the Anet/Ci ratio was statistically higher in females than in males in both cultivation systems (Figure 5A,D), as happened with SSD in Ci (Figure S3A,D). During fall GU1, no significant SSD expression in the Anet/Ci ratio was observed (Figure 5B), while Anet/Ci was higher in males than in females during the winter R2 period (Figure 5C).
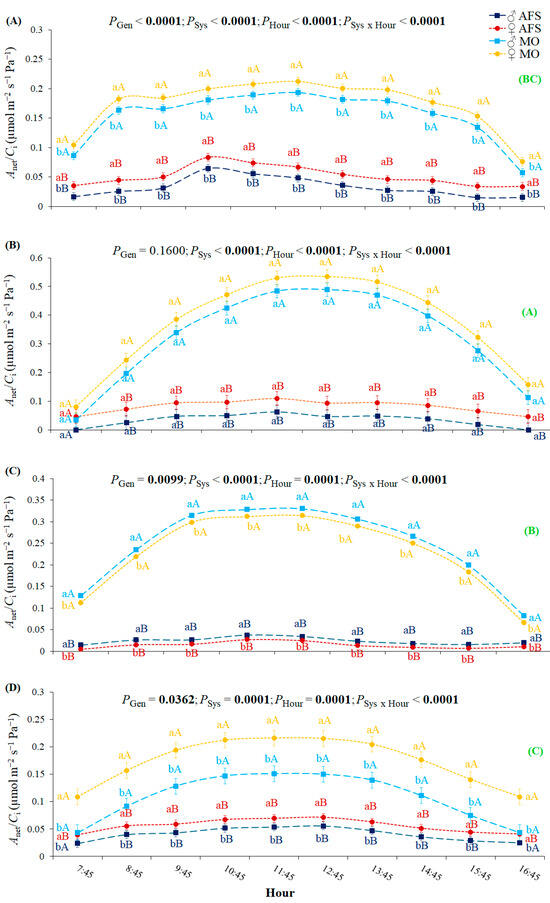
Figure 5.
Yerba mate Anet/Ci ratio estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS) for two genders (female—♀ and male—♂) during four seasons: (A) summer—rest 1 (R1), (B) fall—growth unit 1 (GU1), (C) winter—rest 2 (R2), and (D) spring—growth unit 2 (GU2)] for each hour. Estimated mean ± SE and P-values (bold when significant) are shown (n = 6). Lowercase colored letters compare gender (Gen) responses under each cultivation system (Sys) for each hour and season; uppercase, colored letters compare cultivation systems for each gender, hour, and season. The uppercase, bold, green letters in parenthesis on the right side compare general Anet/Ci seasonal responses (P < 0.0001). Different y-axis scales for seasons are used to observe the gender segregations.
During the spring, the estimated instantaneous water use efficiency (WUE) was the lowest among the four seasons (Figure 6). WUE was higher in MO than in AFS during the summer R1 and the fall GU1 periods (Figure 6A,B). During winter R2, WUE was higher in MO than in AFS for males but not for females (Figure 6C). However, no differences among the two cultivation systems were observed during the spring GU2 period (Figure 6D). The SSD was expressed in WUE in all growth periods, with females showing higher values than males in both systems during the summer R1 and spring GU2 periods (Figure 6A,D). Males showed higher WUE than females during the fall GU1 period in both cultivation systems (Figure 6B), while such gender segregation was observed only in the MO during the winter R2 period (Figure 6C).
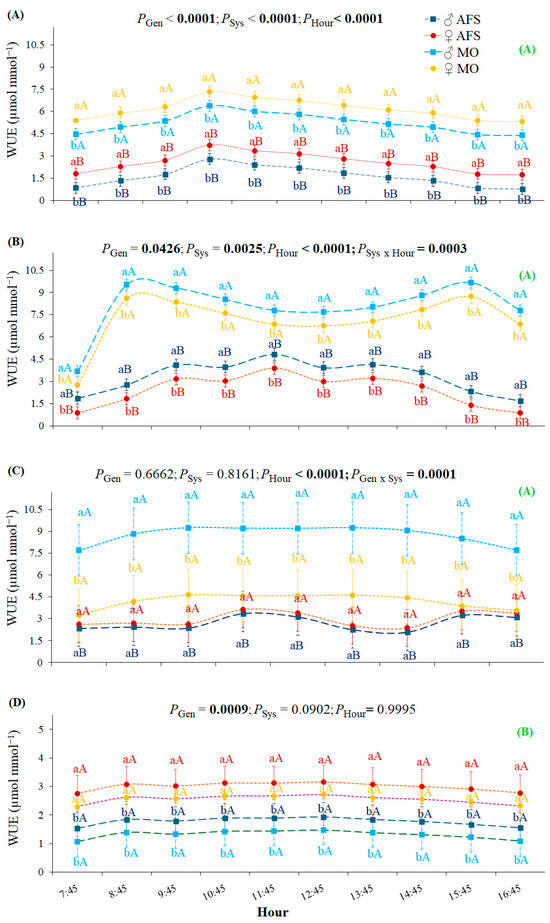
Figure 6.
Yerba mate instantaneous water use efficiency (WUE) estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS) for two genders (female—♀ and male—♂) during four seasons: (A) summer—rest 1 (R1), (B) fall—growth unit 1 (GU1), (C) winter—rest 2 (R2), and (D) spring—growth unit 2 (GU2)] for each hour. Estimated mean ± SE and P-values (bold when significant) are shown (n = 6). Lowercase, colored letters compare gender (Gen) responses under each cultivation system (Sys) for each hour and season; uppercase, colored letters compare cultivation systems for each gender, hour, and season. The uppercase, bold, green letters in parenthesis on the right side compare general WUE seasonal responses (P < 0.0001). Different y-axis scales for seasons are used to observe the gender segregations.
4. Discussion
The leaf gas exchanges of yerba mate were more intense in MO than in AFS for all traits, with the exception of Ci. The SSD in yerba mate was expressed over all four annual periods of rhythmic growth for physiological traits such as Ci and WUE, while it was less frequent in other traits. SSD was modified by the cultivation system only in two cases, being expressed in E only in MO during the spring (Figure 4D) and in WUE in MO during the winter (Figure 6C). In higher dioecious species, females have larger reproductive efforts compared with males, especially during the summer R1 and spring GU2 growth periods. Over the phenology and seasonal variations in environmental conditions, males and females would express different strategies to finish the phenophases and cope with any limiting condition. Females were more intensive in Anet (R1 and GU2) and gs (R1), while the superiority of male or female individuals was expressed in different growth periods in other traits studied here (Figure 7). Such great variation in SSD altering the gender dominance over time and seasons was observed previously for photosynthetic capacity of yerba mate and for physiological responses in various other dioecious trees [29,40,47,48] and grasses [49].
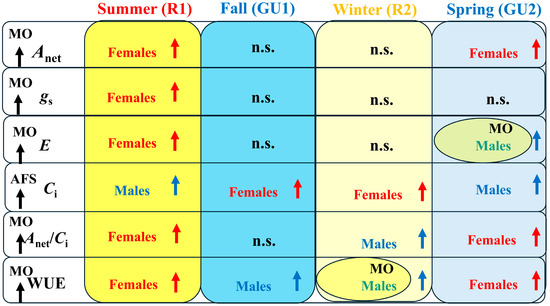
Figure 7.
Summary of the gender dominance in yerba mate leaf gas exchange (net CO2 assimilation rate—Anet, stomatal conductance—gs, transpiration rate—E, intercellular CO2 concentration—Ci, instantaneous carboxylation efficiency—Anet/Ci ratio, and instantaneous leaf water use efficiency—WUE) during the annual rhythmic growth [summer—rest 1 (R1), fall—growth unit 1 (GU1), winter—rest 2 (R2), and spring—growth unit 2 (GU2)] under two contrasting cultivation systems (monoculture—MO and agroforestry—AFS). The black upper arrows indicate the cultivation system with higher values for each of leaf gas exchange parameters. The dominance of females (red) or males (blue) is marked with upper arrows, while ‘n.s.’ means no significant gender dominance. Two cases when SSD expression was modified by the cultivation system, being expressed only in MO with male dominance in E and WUE, are tagged with yellow ovals.
4.1. Expression of SSD in Yerba Mate Leaf Gas Exchanges During the Annual Rhythmic Growth Under Contrasting Cultivation Systems
Adult yerba mate growth strongly responds to open areas and high light conditions, showing intensive flushing in MO [9]. In AFS, they form longer internodes and bigger, wider individual leaves than plants cultivated in MO. Both the cultivation system and the developmental stage act on the sex expression of various architectural parameters in yerba mate, as in LA distribution over the plant profile, light interception, leaf, and canopy photosynthesis [10,35]. When cultivated in AFS, yerba mate plants reduce leaf area density up to six times, total leaf area up to seven times, and, in LAI, up to twelve times compared to those in MO [10]. Such a difference was less pronounced herein (Figure 2), which is likely due to the methods and equipment used for evaluating plants, the genetic background of populations/clones, and environmental conditions.
Yerba mate can be classified as a “less shade-tolerant species” [50] because it clearly responds to decreased light intensity, as seen here under the low light availability of AFS (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6), a kind of environmental pressure. On the other hand, high light conditions in MO, especially in the early stages after planting and after pruning, can be equally considered as a stressful factor due to species origin in the forest shade [2]. A higher degree of environmental degradation is indicated by a higher leaf C/N ratio in MO than in AFS, with females showing leaves with a lower C/N ratio and higher photosynthesis than males in both systems [35]. Herein, the two cases of gender segregation for E and WUE occurred only in MO conditions (Figure 4D and Figure 6C), suggesting the MO as the system of higher environmental pressure for yerba mate and then causing higher gender segregation than AFS.
Gas exchange of two genders of Silene latifolia do not respond differently to low resource availability (light, water, nitrogen, phosphorus, and potassium), and higher female reproductive effort relative to males does not differentially affect their ability to assimilate carbon, despite male Anet and gs being slightly, but consistently, higher than those of females [51]. Varying gender acclimation to low light can be observed in some dioecious species, as in Amaranthus palmeri females [52]. They respond to shading by stem elongation, whereas the male plants respond by increasing specific leaf area. A. palmeri genders showed a differential response to stressful conditions because of differences in their ontogeny and physiology, and possibly due to the cost of reproduction. In yerba mate, males are more sensitive to environmental changes than females, especially in MO. Females optimize foliage structure and phenology in MO, which is determined intrinsically by high fruit loading and modified by night length [11]. Fruit load varies tremendously among yerba mate genotypes [1]. On average, the fruit yield is 1.6 kg tree−1, varying from 0.57 kg tree−1 in a Brazilian progeny to 14.6 kg in the M35 genotype from breeding experiments in Argentina. As a matter of fact, the demand for photosynthetic products will be different in males as compared with females because of sink investments. Even with optimized foliage structure and physiology in females compensating for greater reproductive costs in early developmental stages, females and males are similar in terms of canopy photosynthesis after 2 years of regrowth [10]. Sexual specialization in leaf and plant photosynthesis is related to early vegetative and reproductive stages in yerba mate, which is when females have shown higher carbon assimilation than males [10,35]. Interestingly, leaves in MO have higher stomatal density in females than in male plants, and this trait shows higher values when leaves are formed during the vegetative growth period compared to the flowering period [53]. Herein, we noticed higher Anet in females than in males at early vegetative (R1) and early reproductive (GU2) stages (Figure 2 and Figure 7). Despite that genetic variation could have a prominent role in sex expression in dioecious species [21,35], the findings from three independent experiments with varying genetic material indicate a generalized SSD expression in yerba mate, and this could be associated with a fitness strategy of female plants in their additional reproduction efforts.
Values of Anet, gs, and E were relatively low in yerba mate in some experiments that did not study seasonal variation or SSD [54,55]. The Anet estimated in females cultivated in MO is higher than in males, differing only at the fruit ripening stage during the first year after pruning [10]. The opposite situation in SSD is found under AFS, with the Anet of females being lower than that of males at spring flush and fruit ripening [10]. Similarly, the greater photosynthetic capacity of female plants compared with male ones was noticed under high light conditions (compared here to MO) in Amaranthus palmeri, and such differential photosynthetic performance diminished in both genders with progressed phenology [56]. Even the quality of light can contribute to gender differentiation in a number of plants (gender ratio), as observed in Drynaria roosii ferns [57]. The red light promoted more males than blue light did in D. roosii, with the last contributing to a female-biased trend. Carbon gain of entire female plants in MO is higher than that of male ones during fruit ripening, while this sexual differentiation occurred during spring flushing in AFS [10]. In our study, the cultivation systems modified the expression of SSD, and MO was the system where males had more intensive E and WUE during spring GU2 and winter R2, respectively (Figure 7). Such rare cases, when compared to other case studies, could be related to leaf ontogeny and evaluated traits.
4.2. Expression of SSD in Yerba Mate Leaf Gas Exchanges Under Environmental Pressures During Ontogeny
Sexual specialization in yerba mate biomass production is more expressed under environmental pressures [31]. The ontogeny, together with environmental stressors (e.g., drought and light stresses), play a role in the SSD expression of Pistacia lentiscus [40]. When environmental conditions are optimal under controlled environments (e.g., growth chambers), just a few consistent SSD expressions in ecophysiological responses are found at the leaf level. In older communities, the ecological advantage of male plants is due to higher competition for water uptake, while in the youngest open areas, it is the higher WUE of female plants that confers an ecological advantage. In our experiment, WUE was higher in females than in males during the early vegetative (summer R1) and early reproductive (spring GU2) periods (Figure 6), conferring an ecological advantage of female yerba mate plants. On the other hand, higher WUE was observed in males in fall (GU1) and winter (R2), conferring the ecological advantage of male plants due to higher competition for water uptake during colder and drier periods (Figure 7). Longer observations, i.e., at least during 10–20 years, could reveal whether the ontogeny changes the male or female dominance in leaf gas exchange expressions in yerba mate, while hormonal balance and genetic markers could help to elucidate the pathways of gender dominance.
Females of Taxus baccata have similar photosynthetic capacity to males, and the higher reproductive efforts of females do not happen at the expense of their photosynthetic capacity [58]. In such species, SSD fluctuates seasonally and is remarkable under nutrient deficiency, being related to gender’s ability to protect the photosynthetic apparatus against photoinhibition through antioxidants. Under stresses caused by heavy metal (lead) and low water availability, Populus cathayana males exhibit greater plasticity in the photosynthetic capacity than females [59], with females allocating more carbon and nitrogen to leaf defense chemical components than males after long-term severe defoliation under nitrogen deficiency [60]. Even the recently described phenomenon of leaf water uptake by P. euphratica is significantly greater in female than in male plants, and it increases from the initial to the final stages of one vegetative period [61]. The Anet and gs in P. cathayana females responded more gradually in water-related traits than males under drought and heat stress, with growth and photosynthesis being mainly driven by soil moisture in females, while male performance is mainly related to temperature [62]. Female roots of P. cathayana release diverse phenolic allelochemicals into the soil environment, resulting in growth inhibition of same-sex neighbors when grown in sex monoculture, but their growth with males is consistently enhanced, especially root growth [63]. However, the dioecious species of the genus Populus do not always present SSD and the gender superiority is not the same [64]. Such discrepancies in literature could be associated with the experimental design, varying species and genotypes, stress conditions, and sampling.
In field-grown Pistacia lentiscus, SSD observed in Anet and gs can be related to low water availability during the summer [40]. A similar trend was observed in Anet and gs of yerba mate (Figure 3A and Figure S1A). In Acer negundo, canopy gs is higher in females during the spring but higher in males during the summer [29], with seasonal fluctuations comparable to those of E for yerba mate (Figure 4). Ci of Pistacia lentiscus is lower in females than males during the summer [40], as observed in yerba mate leaves during the summer and spring (Figure S3A,D and Figure 7). Summer water stress significantly reduces the growth of Ilex aquifolium female trees in the following growing season, revealing greater vulnerability of female trees to increasing drought [65]. Salix glauca females have lower drought tolerance than males under years of extreme aridity, while males grew at a consistent rate regardless of habitat aridity, which was observed during a ten-year period [66]. Such responses indicate that one annual cycle probably would not be enough for a full generalization about yerba mate. The spatial gender segregation could shift under global warming, and female plants of various dioecious species would lose their dominance in high-resource habitats, and males would increase their dominance in relatively low-resource habitats [66]. Accordingly, males of P. cathayana exhibit higher Anet, biomass accumulation, and carbon balance mechanisms under elevated CO2 than females [67]. No experiment about the physiology of yerba mate was performed under elevated CO2, but some simulations projected that current favorable areas for yerba mate cultivation would reduce significantly in Brazil [3,16]. How gender dominance in yerba mate carbon acquisition and WUE would change under elevated CO2 is a topic for further research.
5. Conclusions
As a novelty, we found a modification of SSD expression in leaf gas exchange traits over the rhythmic growth period, which was unknown in yerba mate. Then, our initial hypothesis that females would have higher photosynthetic performance than males was strongly modified by growth rhythmicity. In fact, Anet, Anet/Ci, and WUE were higher in females than males during the summer rest (R1) and spring growth unit (GU2) periods. Also, gs and E were higher in females than males during summer R1. Oppositely, higher WUE in males than in females was observed during the fall growth unit (GU1) and winter rest (R2), together with the Anet/Ci ratio during winter R2 and E during the spring GU2 periods. This means that the gender dominance changed over the growth periods and phenology of yerba mate. All those leaf gas exchange traits were modified by the light environment, with higher values found generally under MO as compared with AFS. Despite the strong effect of the cultivation system on LAI and leaf gas exchange traits over the diurnal course, SSD expression was rarely modified by the cultivation system, being expressed for E and WUE only in MO during spring GU2 and winter R2, respectively. Higher WUE of females during early vegetative (R1) and early reproductive (GU2) growth periods would confer an ecological advantage during warmer periods. On the other hand, higher WUE in males during fall GU1 and winter R2 would benefit plants during colder and drier annual periods. The superior Anet in females during early vegetative (R1) and early reproductive (GU2) stages can be underlined as a generalized SSD expression in yerba mate and could be considered as a fitness strategy of female plants in their additional reproduction efforts. While long-term experiments would reveal if the ontogeny changes the gender dominance in leaf gas exchanges of yerba mate, our study highlights the importance of the period of rhythmic growth when the ecophysiological traits were evaluated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16010161/s1, Figure S1: Flowchart showing measurements of light (photosynthetically active photon flux density, PPFD) and Anet/PPFD curves for further modeling the instantaneous leaf gas exchanges along the diurnal period in monoculture (MO) and agroforestry (AFS); Figure S2: Yerba mate stomatal conductance (gs) estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS), for two genders (female—♀ and male—♂), during four seasons: (A) summer—rest 1 (R1), (B) fall—growth unit 1 (GU1), (C) winter—rest 2 (R2), and (D) spring—growth unit 2 (GU2)] for each hour. Estimated mean ± SE, and P-values (bold when significant) are shown (n = 6). Lowercase colored letters compare gender (Gen) responses under each cultivation system (Sys), for each hour, and each season; uppercase colored letters compare cultivation systems for each gender, for each hour, and for each season. The uppercase bold green letters in parenthesis on the right side compare general gs seasonal responses (P < 0.0001). Different y-axis scales for seasons are used to observe the gender segregations; Figure S3: Intercellular CO2 concentration (Ci) estimated in two cultivation systems (monoculture—MO and agroforestry system—AFS), for two genders (female—♀ and male—♂), during four seasons: (A) summer—rest 1 (R1), (B) fall—growth unit 1 (GU1), (C) winter—rest 2 (R2), and (D) spring—growth unit 2 (GU2)] for each hour. Estimated mean ± SE, and P-values (bold when significant) are shown (n = 6). Lowercase colored letters compare gender (Gen) responses under each cultivation system (Sys), for each hour, and each season; uppercase colored letters compare cultivation systems for each gender, for each hour, and for each season. The uppercase bold green letters in parenthesis on the right side compare general Ci seasonal responses (P < 0.0001).
Author Contributions
Conceptualization, M.R.; data curation, M.R. and E.R.B.; formal analysis, M.R.; funding acquisition, M.R. and I.W.; investigation, M.R., E.R.B., R.L.d.A. and R.V.R.; methodology, M.R. and E.R.B.; project administration, M.R. and I.W.; resources, M.R.; E.R.B., R.V.R. and I.W.; validation, all authors; writing—original draft, M.R.; writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq, Brazil) with the fellowship for Invited Researcher awarded to Miroslava Rakočević (350509/2020-4). R.V.R. is a CNPq fellow (3042950/2022-1).
Data Availability Statement
The authors can provide the experimental data for all interested researchers.
Acknowledgments
To Mário Dobner, Kelen Haygert Lencina, and Ana Clara Dondoerfer Teixeira from UFSC, Brazil, for assisting us in the data collection and maintenance of the experimental field.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rakočević, M.; Janssens, M.; Scherer, R. Light responses and gender issues in the domestication process of yerba-mate, a sub-tropical evergreen. In Evergreens: Types, Ecology and Conservation; Bezerra, A.D., Ferreira, T.S., Eds.; Nova Science Publisher: New York, NY, USA, 2012; pp. 63–95. [Google Scholar]
- Croge, C.P.; Cuquel, F.L.; Pintro, P.T.M. Yerba mate: Cultivation systems, processing, and chemical composition. A review. Sci. Agric. 2021, 78, e20190259. [Google Scholar] [CrossRef]
- Torsoni, G.B.; de Oliveira Aparecido, L.E.; Lorençone, P.A.; Lorençone, J.A.; de Lima, R.F.; de Souza Rolim, G. Climatic zoning of yerba mate and climate change projections: A CMIP6 approach. Int. J. Biometeorol. 2024, 68, 979–990. [Google Scholar] [CrossRef]
- Zaions, I.; Picolo, A.P.; Gonçalves, I.L.; Borges, A.C.P.; Valduga, A.T. Physico-chemical characterization of Ilex paraguariensis St. Hil. during the maturation. Braz. Arch. Biol. Technol. 2014, 57, 663–667. [Google Scholar] [CrossRef]
- Valduga, A.T.; Gonçalves, I.L.; Dartora, N.; Mielniczki-Pereira, A.A.; Souza de, L.M. Phytochemical profile of morphologically selected yerba-mate progenies. Ciência Agrotecnol. 2016, 40, 114–120. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Albrecht, C.; Miranda, A.R.; Mazzuduli, G.M.; Soria, E.A.; Repossi, G. Neuroprotective effect of Ilex paraguariensis intake on brain myelin of lung adenocarcinoma-bearing male BALB/c mice. Nutr. Cancer 2019, 71, 629–633. [Google Scholar] [CrossRef]
- Rakočević, M.; Maia, A.H.d.N.; de Liz, M.V.; Imoski, R.; Helm, C.V.; Cardozo Junior, E.L.; Wendling, I. Stability of leaf yerba mate (Ilex paraguariensis) metabolite concentrations over time from the prism of secondary sexual dimorphism. Plants 2023, 12, 2199. [Google Scholar] [CrossRef]
- Westphalen, D.J.; Angelo, A.C.; Rossa, U.B.; Helm, C.V.; Radetski, C.M.; Gomes, E.N. Phytochemical composition of yerba mate leaves (Ilex paraguariensis) and its relation with cultivation conditions. Braz. J. Med. Plants 2024, 22, 99–107. [Google Scholar]
- Matsunaga, F.T.; Rakočević, M.; Brancher, J.D. Modelling the 3D structure and rhythmic growth responses to environment in dioecious yerba-mate. Ecol. Model. 2014, 290, 34–44. [Google Scholar] [CrossRef]
- Rakočević, M.; Costes, E.; Assad, E.D. Structural and physiological sexual dimorphism estimated from three-dimensional virtual trees of yerba-mate (Ilex paraguariensis) is modified by cultivation environment. Ann. Appl. Biol. 2011, 159, 178–191. [Google Scholar] [CrossRef]
- Rakočević, M.; Martim, S.F. Time series in analysis of yerba-mate biennial growth modified by environment. Int. J. Biometeorol. 2011, 55, 161–171. [Google Scholar] [CrossRef]
- Marques, A.d.C.; dos Reis, M.S.; Denardin, V.F. Yerba mate landscapes: Forest use and socio-environmental conservation. Ambiente Soc. 2019, 22, e02822. [Google Scholar] [CrossRef]
- De David, P.R.; de David, F.A.; Toso, J.O.; Pasinato, C.; Müller, C.; Galon, L.; Perin, G.F. Growth and development of yerba mate seedlings associated with different winter cover species. Braz. J. Sci. 2024, 3, 34–42. [Google Scholar] [CrossRef]
- Dos Santos, E.R.; Vestena, L.R.; Serrato, F.B. Interception loss by yerba mate (Ilex paraguariensis) in production systems in Southern Brazil. Pesq. Agropecu. Trop. 2024, 54, e77226. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to human-induced environmental stresses: Issues, problems, and strategies for alleviating stress. Crit. Rev. Plant Sci. 2000, 19, 91–170. [Google Scholar] [CrossRef]
- Wrege, M.S.; Soares, M.T.S.; Fritzsons, E.; de Sousa, V.A.; de Aguiar, A.V.; Bognola, I.A.; de Sousa, L.P. Natural distribution of yerba mate in brazil in the current and future climatic scenarios. Agrometeoros 2020, 28, e026795. [Google Scholar] [CrossRef]
- Caron, B.O.; Schmidt, D.; Balbinot, R.; Behling, A.; Eloy, E.; Elli, E.F. Efficiency of the use of yerba mate solar radiation in intercropping or monocropping for the accumulation of carbon. Rev. Árvore 2016, 40, 983–990. [Google Scholar] [CrossRef]
- Hallé, F.; Oldemann, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests; Springer: Berlin, Germany, 1978. [Google Scholar]
- Hallé, F.; Martin, R. Étude de la croissance rythmique de l’hévéa (Hevea brasiliensis Müll Arg. Euphorbiacées Crotonoïdées). Adansonia 1968, 2–8, 475–503. [Google Scholar] [CrossRef]
- Guédon, Y.; Costes, E.; Rakočević, M. Modulation of the yerba-mate metamer production phenology by the cultivation system and the climatic factors. Ecol. Model. 2018, 384, 188–197. [Google Scholar] [CrossRef]
- Galfrascoli, G.M.; Calviño, A. Secondary sexual dimorphism in a dioecious tree: A matter of inter-plant variability? Flora 2020, 266, 151595. [Google Scholar] [CrossRef]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Rocheleau, A.-F.; Houle, G. Different cost of reproduction for the males and females of the rare dioecious shrub Corema conradii (Empetraceae). Am. J. Bot. 2001, 88, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wright, V.; Dorken, M.E. Sexual dimorphism in leaf nitrogen content but not photosynthetic rates in Sagittaria latifolia (Alismataceae). Botany 2017, 92, 109–112. [Google Scholar] [CrossRef]
- Delph, L.F.; Gehring, J.L.; Arntz, A.M.; Levri, M.; Frey, F.M. Genetic correlations with floral display lead to sexual dimorphism in the cost of reproduction. Am Nat. 2005, 166 (Suppl. S4), S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Wei, X.; Wei, Q. Responses of early distribution and developmental traits of male and female trees to stand density in Fraxinus mandshurica Rupr. plantation. Forests 2022, 13, 472. [Google Scholar] [CrossRef]
- Cepeda-Cornejo, V.; Dirzo, R. Sex-related differences in reproductive allocation, growth, defense, and herbivory in three dioecious neotropical palms. PLoS ONE 2010, 5, e9824. [Google Scholar] [CrossRef]
- Geber, M.A.; Dawson, T.E.; Delph, L.F. Gender and Sexual Dimorphism in Flowering Plants; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Dawson, T.E.; Ward, J.K.; Ehleringer, J.R. Temporal scaling of physiological responses from gas exchange to tree rings: A gender-specific study of Acer negundo (Boxelder) growing under different conditions. Funct. Ecol. 2004, 18, 212–222. [Google Scholar] [CrossRef]
- Hadad, M.A.; Roig, F.A.; Molina, J.G.A.; Hacket-Pain, A. Growth of male and female Araucaria araucana trees respond differently to regional mast events, creating sex-specific patterns in their tree-ring chronologies. Ecol. Indic. 2021, 122, 107245. [Google Scholar] [CrossRef]
- Rakočević, M.; Maia, A.H.N.; Duarte, M.M.; Wendling, I. Secondary sexual dimorphism in biomass production of ilex paraguariensis progenies associated to their provenances and morphotypes. Exp. Agric. 2023, 59, e3. [Google Scholar] [CrossRef]
- Marcheafave, G.G.; Pauli, E.D.; Wendling, I.; Rakočević, M.; Scarminio, I.S.; Bruns, R.E. A Comparative Study Using UV-Vis, NIR, and FTIR spectral fingerprinting in yerba mate leaves through mixture design extractions and ASCA models. J. Braz. Chem. Soc. 2025, 36, e-20240073. [Google Scholar] [CrossRef]
- Diggle, P.K.; Di Stilio, V.S.; Gschwend, A.R.; Golenberg, E.M.; Moore, R.C.; Russel, J.R.; Sinclair, J.P. Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet. 2011, 27, 368–376. [Google Scholar] [CrossRef]
- Leite, M.A.P.; Kersten, B.; Fladung, M.; Müller, N.A. Diversity and dynamics of sex determination in dioecious plants. Front. Plant Sci. 2021, 11, 580488. [Google Scholar]
- Rakočević, M.; Batista, E.R.; Matsunaga, F.T.; Wendling, I.; Marcheafave, G.G.; Bruns, R.E.; Scarminio, I.S.; Ribeiro, R.V. Canopy architecture and diurnal CO2 uptake in male and female clones of yerba-mate cultivated in monoculture and agroforestry. Ann. Appl. Biol. 2024, 184, 210–225. [Google Scholar] [CrossRef]
- Rakočević, M.; Medrado, M.J.S.; Lavoranti, O.J.; Valduga, A.T. Quality of yerba-mate leaves originating from male and female plants. Pesq. Florest. Bras. 2007, 54, 71–83. [Google Scholar]
- Letts, M.G.; Phelan, C.A.; Johnson, D.R.E.; Rood, S.B. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiol. 2008, 28, 1037–1048. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Serrano-Romero, E.A.; Cousins, A.B. Cold acclimation of mesophyll conductance, bundle-sheath conductance and leakiness in Miscanthus × giganteus. New Phytol. 2020, 226, 1594–1606. [Google Scholar] [CrossRef]
- Correia, O.; Barradas, M.C.D. Ecophysiological differences between male and female plants of Pistacia lentiscus L. Plant Ecol. 2000, 149, 131–142. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef]
- Penteado Junior, J.F.; Goulart, I.C.G.R. Erva 20: Sistema de Produção de Erva-Mate; Embrapa: Brasília, Brazil, 2019. [Google Scholar]
- Kok, B. A Critical consideration of the quantum yield of chlorella-photosynthesis. Separatum Enzymol. 1948, 13, 1–56. [Google Scholar]
- Da Silva, J.R.; Patterson, A.E.; Rodrigues, W.P.; Campostrini, E.; Griffin, K.L. Photosynthetic acclimation to elevated CO2 combined with partial root zone drying results in improved water use efficiency, drought tolerance, and leaf carbon balance of grapevines (Vitis labrusca). Environ. Exp. Bot. 2017, 134, 82–95. [Google Scholar] [CrossRef]
- R Core Team. Available online: https://www.r-project.org// (accessed on 24 September 2024).
- Lobo, F.A.; de Barros, M.P.; Dalmagro, H.J.; Dalmolin, A.C.; Pereira, W.E.; de Souza, E.C.; Vourlitis, G.L.; Rodríguez Ortíz, C.E. Fitting net photosynthetic light-response curves with Microsoft Excel—A critical look at the models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Retuerto, R.; Lema, B.F.; Roiloa, S.R.; Obeso, J.R. Gender, light and water effects in carbon isotope discrimination, and growth rates in the dioecious tree Ilex aquifolium. Funct. Ecol. 2000, 14, 529–537. [Google Scholar] [CrossRef]
- Hultine, K.R.; Grady, K.C.; Wood, T.E.; Shuster, S.M.; Stella, J.C.; Whitham, T.G. Gender specific patterns of carbon uptake and water use in a dominant riparian tree species exposed to a warming climate. Nat. Plants 2016, 2, 16109. [Google Scholar] [CrossRef]
- Van Blerk, J.J.; West, A.G.; Skelton, R.P.; Midgley, J.J. Phenological asynchrony between sexes of Restionaceae can explain culm δ13C differences. Austral Ecol. 2022, 47, 1200–1207. [Google Scholar] [CrossRef]
- Petriţan, A.M.; von Lüpke, B.; Petriţan, I.C. Influence of light availability on growth, leaf morphology and plant architecture of beech (Fagus sylvatica L.), maple (Acer pseudoplatanus L.) and ssh (Fraxinus excelsior L.) saplings. Eur. J. For. Res. 2009, 128, 61–74. [Google Scholar] [CrossRef]
- Gehring, J.L.; Monson, R.K. Sexual differences in gas exchange and response to environmental stress in dioecious Silene latifolia (Caryophyllaceae). Am. J. Bot. 1994, 81, 166–174. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; FitzSimons, T.; Roberts, T.L.; Oosterhuis, D.M.; Govindjee, G. Evaluation of secondary sexual dimorphism of the dioecious Amaranthus palmeri under abiotic stress. Sci. Rep. 2023, 13, 13156. [Google Scholar] [CrossRef]
- Rocha, S.P.; Borrero, P.A.P.; Niella, F.; Morales, A.V.; Danner, M.A. Sexual differentiation in yerba mate plants: The role of stomatal density. Braz. Arch. Biol. Technol. 2024, 67, e24230896. [Google Scholar] [CrossRef]
- Tomazelli, D.; Costa, M.D.; Primieri, S.; Rech, T.D.; Santos, J.C.P.; Klauberg-Filho, O. Inoculation of arbuscular mycorrhizal fungi improves growth and photosynthesis of Ilex paraguariensis (St. Hil) seedlings. Braz. Arch. Biol. Technol. 2022, 65, e22210333. [Google Scholar] [CrossRef]
- Zanotto, G.S.; Montes, A.L.; Touguinha, L.B.A.; Silvestre, W.P.; dos Santos Branco, C.; Schwambach, J. Cultivation conditions and their impact on yerba mate ecophysiology and antioxidant potential. Pesqui. Agropec. Gaúch. 2024, 30, 68–79. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; FitzSimons, T.; Roberts, T.L.; Oosterhuis, D.M. Differential response of Palmer amaranth (Amaranthus palmeri) gender to abiotic stress. Weed Sci. 2017, 65, 213–227. [Google Scholar] [CrossRef]
- Li, J.-Y.; Zhang, X.-C.; Li, D.; Sun, M.-Y.; Shi, L. Energy response patterns to light spectrum at sex differentiation stages of Drynaria roosii gametophytes. Environ. Exp. Bot. 2020, 172, 103996. [Google Scholar] [CrossRef]
- Robakowski, P.; Pers-Kamczyc, E.; Ratajczak, E.; Thomas, P.A.; Ye, Z.-P.; Rabska, M.; Iszkuło, G. Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Front. Plant Sci. 2018, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, L.; Zhang, X.; Korpelainen, H.; Li, C. sexual differences in photosynthetic activity, ultrastructure and phytoremediation potential of Populus cathayana exposed to lead and drought. Tree Physiol. 2013, 33, 1043–1060. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Meng, Q.; Fang, S.; Kang, J.; Guo, Q. Differences in carbon and nitrogen metabolism between male and female Populus cathayana in response to deficient nitrogen. Tree Physiol. 2021, 41, 119–133. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Wang, H.; Zhang, X. Foliar water uptake and its relationship with photosynthetic capacity and anatomical structure between female and male Populus euphratica at different growth stages. Forests 2023, 14, 1444. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Xu, X.; Fowler, J.C.; Miller, T.E.X.; Dong, T. Effect of summer warming on growth, photosynthesis, and water status in female and male Populus cathayana: Implications for sex-specific drought and heat tolerances. Tree Physiol. 2020, 40, 1178–1191. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Allelochemicals and soil microorganisms jointly mediate sex-specific belowground interactions in dioecious Populus cathayana. New Phytol. 2023, 240, 1519–1533. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Borkhert, E.V.; Snezhkina, A.V.; Kudryavtseva, A.V.; Dmitriev, A.A. Sex-specific response to stress in Populus. Front. Plant Sci. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Vilas, J.S.; Hernández-Alonso, H.; Rozas, V.; Retuerto, R. Differential growth rate, water-use efficiency and climate sensitivity between males and females of Ilex aquifolium in North-Western Spain. Ann. Bot. 2024, 20, mcae126. [Google Scholar] [CrossRef]
- Dudley, L.S. Ecological correlates of secondary sexual dimorphism in Salix glauca (Salicaceae). Am. J. Bot. 2006, 93, 1775–1783. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Yu, L.; Korpelainen, H.; Niinemets, Ü.; Li, C. Elevated temperature and CO2 interactively modulate sexual competition and ecophysiological responses of dioecious Populus cathayana. For. Ecol. Manag. 2021, 481, 118747. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).