Abstract
Pinus yunnanensis Franch., one of the pioneer species of wild mountain afforestation in southwest China, plays an essential role in the economy, society and environment of Yunnan Province. Nonetheless, P. yunnanensis’ trunk twisting and bending phenomenon has become more common, which significantly restricts its use and economic benefits. In order to clarify the compositional differences between the straight and twisted trunk types of P. yunnanensis and to investigate the reasons for the formation of twisted stems, the present study was carried out to dissect the macroscopic and microscopic structure of the straight and twisted trunk types of P. yunnanensis, to determine the content of cell wall components (lignin, cellulose, hemicellulose), determine the content of endogenous hormones, and the expression validation of phytohormone-related differential genes (GA2OX, COI1, COI2) and cell wall-related genes (XTH16, TCH4). The results showed that the annual rings of twisted trunk types were unevenly distributed, eccentric growth, insignificant decomposition of early and late wood, rounding and widening of the tracheid cells, thickening of the cell wall, and reduction of the cavity diameter; the lignin and hemicellulose contents of twisted trunk types were higher; in twisted trunk types, the contents of gibberellin (GA) and jasmonic acid (JA) increased, and the content of auxin (IAA) was reduced; the GA2OX were significantly down-regulated in twisted trunk types, and the expressions of the genes associated with the cell wall, COI1, COI2, TCH4 and XTH16, were significantly up-regulated. In conclusion, the present study found that the uneven distribution of endogenous hormones may be an important factor leading to the formation of twisted trunk type of P. yunnanensis, which adds new discoveries to reveal the mechanism of the genesis of different trunk types in plants, and provides a theoretical basis for the genetic improvement of forest trees.
1. Introduction
The process of plant growth and development relies on flexible control of cell division and cell proliferation, which in some cases can be twisted, spiraled or coiled [1]. Plants that undergo spiraling, especially wood, tend to twist or torsion, rendering them unsuitable for applications such as lumber, furniture, etc. [2]. Typically, plants grow and develop as a through-and-through development [3], but in some cases twisting, spiraling, or coiling occurs, and these three categories are often attributed to spirals. As a common form of variation in the development of plants, spiral grain is often considered one of the important wood characteristics that affect the value of wood products [4]. Spiral grain, as a slanting grain of trunk distortion, is due to the fact that the wood fibers are not parallel to the axis of the tree and present an angular inclination, as well as a high degree of complexity. Harris [4] and Jones [5] found that the helical angle was close to 90° in Pinus sylvestris L. and Pseudotsuga menziesii (Mirv.) Franco. Corylus avellana L. and Salix matsudana Koidz have twisted woods with a spiral grain, while Pinus sylvestris shows a distinct spiral character after development and maturity [6]. Foresters and botanists have been researching the reasons for spiral grain in trees for centuries. They look at the tree as a whole in its growing environment and try to figure out how much genetic or environmental factors contribute to this occurrence [7].
The structure of secondary cell wall determines the characteristics of plant cells and organ development [8]. Plants produce the spiral phenotypes due to the changes in xylem, and lignification is the process of secondary cell wall thickening in which lignin, cellulose, and hemicellulose, as the main components of the secondary cell wall, are essential for the development of secondary xylem in plants [9]. Lignin is mechanically weaker than cellulose but has an important reinforcing effect on cell walls [10] and plays an important role in plant growth and development, biotic stress resistance, and defense responses against various pathogens [11,12]. Lignin enhances plant resistance by depositing large amounts of itself or stimulating related hormone signaling pathways [13]. Transgenic alfalfa and Arabidopsis thaliana C4H mutants were shown to have lower lignin contents, which led to stunted plants, decreased biomass, delayed flowering, and loss of apical dominance [14]. It has been shown that cellulose microfibrils (CMF) bear most of the stress during cell wall extension that the orientation of the microfibrils is usually transverse to the direction of growth, and that widening of the spacing between the microfibrils or sliding of the microfibrils against each other leads to cell wall deformation [9,15,16]. When cellulose is arranged in a helical form, it causes the cell to assume a rotational orientation opposite to the helical direction of the cellulose microfibrils [17]. Hemicellulose is a heterogeneous polymer composed of several different types of monosaccharides, including xylose, arabinose, galactose, etc., which are found in the cell walls of almost all plants [18]. Reis found that hemicellulose plays a significant role in driving helicoidal architectures of cellulose in quince mucilage [19]. Chang et al. proposed that when CMF is deposited into the matrix of most hemicellulose cell walls, a helical structure is formed [20].
Endogenous plant hormones also play an important role in plant growth and development [21]. It was found that auxin (IAA)-inducible genes are mainly expressed in the formation layer and its neighboring cells to promote xylem differentiation [22]. Mirza et al. found that in aux1 mutants, roots exhibited left-handed spiral growth [23]. In the deep-seeded wheat variety ‘Red Mangrove’, the first internode of this variety is capable of extreme elongation and also shows a right-handed twist, which in turn favors plant elongation against soil resistance [24]. This complex inter-regulatory mechanism often exists between endogenous plant hormones, and jasmonic acid (JA) is commonly associated with responses to a range of biotic and abiotic stresses [25]. It has been found that JA controls the polar transport of IAA to regulate root differentiation [22,26]. Gibberellins (GAs) control IAA distribution in the formation layer, which can promote the creation of xylem [27]. Chen et al. found that the first internode of wheat plants is extremely sensitive to GA, which in turn produces variability in GA content, ultimately leading to stem spiraling [28].
P. yunnanensis is one of the pioneering species for the reforestation of barren mountains in southwest China and plays an important role in soil and water conservation, which is crucial for the economy, society and environment of Yunnan Province [29]. However, P. yunnanensis forests in natural habitats are experiencing serious problems with decline, and the proportion of twisted trunk types in this species (spiral) has increased, which greatly affects the economic benefits of its forests. Experts have different suggestions for the causes of twisted trunk types in the species, and some of them believe that environmental factors are the main reasons for inducing twisted trunk type, with different levels of wind [30], soil [31], sun [32], slope orientation [33], elevation [34], and stress [4] all contributing to the change in tree trunk types. Another group of experts believes that trunk type distortion is mainly controlled by genes [35]. Combining investigation methods with traditional breeding, it was found that the twisted trunk phenotypes of parents could be passed on to offspring [36]. Meanwhile modern molecular biology methods also revealed that trunk twisting is mainly regulated by genetic factors [37,38]. Gan et al. analyzed the transcriptome of the vascular cambium of straight-twisted P. yunnanensis and found that genes related to the plant cell wall and endogenous hormones had a certain influence on the formation of trunk types [39]. In the present investigation, we conducted wood dissection, measurements of lignin, cellulose, and hemicellulose content, and RT-qPCR expression validation of endogenous plant hormone-related genes (GA2OX, COI1, COI2) and cell wall-related genes (XTH16, TCH4) screened out based on the pre-transcriptomic data [39] of the straight and twisted trunk types. The aim was to analyze the compositional differences between straight and twisted trunk types in the species, to explore the reasons for the formation of twisted trunk types, and to provide a theoretical basis for the genetic improvement of P. yunnanensis trunk types.
2. Materials and Methods
2.1. Experimental Materials
The experimental materials came from Chuxiong Prefecture in Yunnan Province, China, within the longitudinal and latitudinal range from 101°14′ to 101°49′ E and 25°4′ to 26°7′ N, with a subtropical monsoon climate, where P. yunnanensis grows at elevations ranging from 2200 to 2377 m. We collected five straight-stemmed and five twisted-stemmed P. yunnanensis of about 25 years of age, with similar appearance in height and diameter at breast height. The vascular cambium was collected at 1.2 m above ground for the purpose of verifying differential gene expression and determining the contents of endogenous hormones. The trunk discs were collected for morphological, histological, and imaging observations, as well as for the determination of lignin, cellulose, and hemicellulose contents.
2.2. Morphology, Histology and Imaging
The trunks were cut into 1–2 cm thick discs at a height of 1.2 m, then cut into 2 × 2 × 2 cm3 squares and put in an autoclave for four hours to soften. The Leica 2000R slide walker slicer was used to cut the 10 μm thick slices, which were then placed in an embedding cassette, debris removed from the slices (rinsed in distilled water for 3 min), the slices stained (immersed in 1% aqueous fennel solution for 4 h), and the stains subsequently removed (washed out in distilled water for 5 min). Next, the slices were dehydrated in an ethanol gradient (concentration gradients of 35%, 50%, 70%, and anhydrous ethanol). The final transparency treatment was three minutes of ethanol-n-butanol with a 1:1 volume ratio, three minutes of n-butanol, three minutes of n-butanol-xylene with a 1:1 volume ratio, and three minutes of xylene. After being taken out of the xylene and placed on slides, sections were sealed with coverslips after the proper quantity of neutral gum was added. The anatomical features were observed under a NikonECLIPSE80i bio-digital microscope (Nikon, Tokyo, Japan).
From the straight trunk types and twisted trunk types of P. yunnanensis discs, 5 wooden strips about 1.5 cm long and 0.2 cm wide were taken and placed in atest tube containing a 30% hydrogen peroxide–ice acetic acid mixture (volume ratio of 1:1). The mixture did not exceed the small wooden strips. The test tube was placed in a 90 °C water bath heated until the wooden strips whitened, were then washed with water, and 1% red stain was added (not over the wood). The wooden strips were stained for eight hours, temporary slices for tracheid cell dissociation were prepared, and distilled water was used to remove any remaining color after sealing [40]. The tracheid cells were photographed using a NikonECLIPSE80i bio-digital microscope. The width and cavity diameter of the tracheid cells were measured using Image, and the double-wall thickness was computed.
2.3. Determination of Lignin, Hemicellulose and Cellulose Content
Five cm thick discs were collected at 1/5 (about 1.5 m above ground level), 1/3 (about 2.5 m above ground level), and 2/3 (about 5 m above ground level) of total tree height in both straight and twisted trunk types of P. yunnanensis (Figure S1); these samples were named as the base (1/5), the middle part (1/3), and the tip (2/3) of the tree. The discs were then cut into 2 × 2 × 2 cm small pieces of wood, baked in an oven to a constant weight, and ground into a powder using a pulverizer, sieved gradually (40–60 mesh), and kept in an oven at 40 °C. Using the colorimetric approach, the absolute concentrations of cellulose, hemicellulose, and lignin were determined in both straight and twisted dried P. yunnanensis wood.
2.4. UPLC/MS Analysis of Plant Hormone Content
The vascular cambium was gathered 1.2 m above the ground, and 100 mg was extracted after being crushed using liquid nitrogen. Next, 200 μL of pre-cooled ultrapure water was added, and 800 μL of methanol/acetonitrile (2:2 v/v) was added after sufficient homogenization, vortexing and mixing. Sonication was performed in an ice bath for 60 min, incubated at −20 °C for 1 h to precipitate the protein, centrifuged at 15,000× g for 20 min at 4 °C, and the supernatant extracted and dried under vacuum. The sample was redissolved in 80 μL of 50% acetonitrile solution, centrifuged at 20,000× g for 10 min, and the supernatant was prepared for mass spectrometry analysis. The separation was performed on a Nexera X2 LC-30AD ultra-high pressure liquid chromatograph (Shimadzu, Shanghai, China). A 5500QTRAP mass spectrometer (AB SCIEX, Shanghai, China) was used for mass spectrometry analysis in positive/negative ion mode. The peak areas and retention times were extracted using MultiQuant V3.0.3 software. Retention time was corrected using phytohormone standards for metabolite identification.
2.5. RNA Extraction and RT-qPCR Expression Validation
Based on the previous transcriptome data, five differential genes were selected and primers were designed using Primer Premier 5.0. MolPure® Plant Plus RNA Kit (YEASEN Biotech, Shanghai, China) was used to extract RNA from the vascular cambium of P. yunnanensis, and the purity and concentration of RNA were determined by UV spectrophotometer (NanoDrop 2000, Thermo Scientific, Wilmington, DE, USA). The cDNA was synthesized using Hifair ® Ⅲ 1st Strand cDNA Synthesis SuperMix (YEASEN Biotech, Shanghai, China) for qPCR kit.
Differential gene expression was detected on a real-time PCR instrument (Rotor-Gene Q; Qiagen, Dusseldorf, Germany) using chemofluorescence (ChamQ SYBY qPCR Master Mix; Nanjing, China). The internal reference primer was the reference gene AT2G24020 from Pinus pinaster Aiton [41]. The amplification program was 95 °C for 2 min and 45 cycles of 95 °C for 10 s, 56 °C for 30 s, and 72 °C for 60 s. The 2−ΔΔCT method [42] was used to calculate the relative expression levels.
2.6. Statistics and Data Analysis
Microsoft Excel was used to conduct data analysis on the average tracheid cell width, cavity diameter size, and average double-wall thickness; on lignin, cellulose, and hemicellulose content; and on endogenous hormone content and RT-qPCR data of straight and twisted trunk types of P. yunnanensis. Single-factor analysis of variance (ANOVA) in SPSS 18.0 software was used for statistical analysis, and the Duncan test was used to compare significant differences between factors under different treatments. Asterisk (*) means a significant difference at p < 0.05, double asterisk (**) means an extremely significant difference at p < 0.01, and three asterisks (***) means an extremely significant difference at p < 0.001. Graphing was carried out on Graphpad Prism 10.
3. Results
3.1. Macrostructural Analysis
Macrostructural observation of the xylem of straight and twisted trunk types of Pinus yunnanensis revealed that the width of the annual rings of the straight trunk type was uniformly distributed in all directions, with concentric circles and no eccentricity (Figure 1a). The width of the annual rings of the twisted stem shape was uniformly distributed in 4~5 years, but after 5 years of growth, the overall disc appeared to be eccentric (Figure 1b).
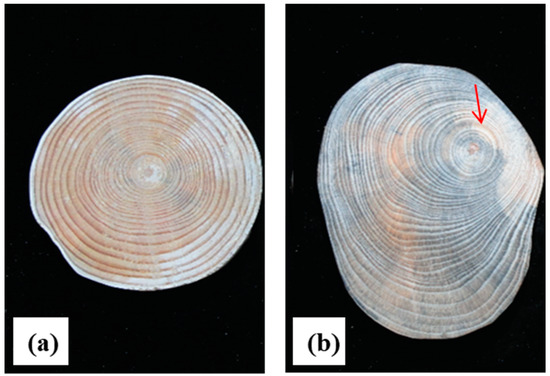
Figure 1.
Macrostructural observation of straight and twisted P. yunnanensis. (a): Straight type (S), (b): twisted type (T); the arrows point to the eccentricity of the distribution of annual rings from the fifth year onwards.
3.2. Microanatomical and Structural Characteristics
In the analysis of the xylem cross-sectional structure of the straight and twisted trunk types, the boundary between the early and late wood of the straight trunk was obvious, the tracheid cells were neatly arranged and of uniform size, with no damage, extrusion and other undesirable conditions, and the single resin ducts were sporadically distributed in the latewood. In contrast, the early and late wood of the twisted trunk type had no clear demarcation, the tracheid cells were arranged in an irregular way, there was a serious extrusion phenomenon, and the resin ducts were distributed in groups in the latewood. The widths of earlywood of the straight and twisted trunk types were larger than that of latewood (Figure 2a,d), in which the cross-section of the earlywood tracheids of the straight trunk type showed polygonal (quadrilateral, hexagonal) shapes, and the tracheids of the latewood showed mostly quadrilateral shapes. In the twisted trunk types of P. yunnanensis, there were cases in which polygonal shapes and ellipsoidal shapes alternated between the earlywood and the latewood (Figure 2b,c,e,f). Further measurements of tracheid width, cavity diameter and tracheid wall thickness (Figure 2g–i) revealed that the tracheid width and cavity diameter of straight trunk P. yunnanensis were significantly larger than those of twisted trunk type in both early and latewood. The tracheid wall thickness was significantly higher in the latewood of straight trunk type than in the twisted trunk type, while it was significantly lower in the earlywood than in the twisted trunk type.
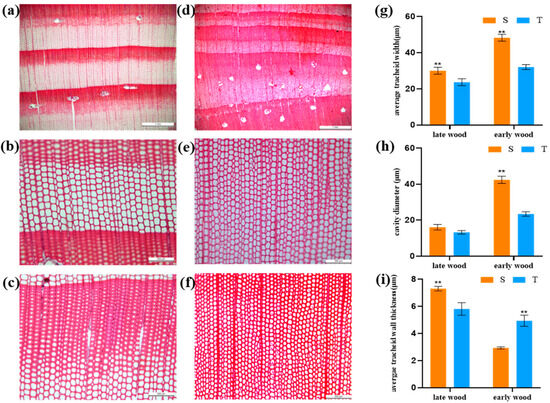
Figure 2.
Microanatomy of straight and twisted trunk types of P. yunnanensis. Straight trunked type (a–c), twisted trunk type (d–f). Comparison of early and latewood demarcation (a,d); comparison of earlywood (b,e); comparison of latewood (c,f); comparison of average tracheid cell width (g), comparison of cavity diameter size (h), comparison of average double-wall thickness (i). S, straight trunk type; T, twisted trunk type. Double asterisk (**) means extremely significant difference (p < 0.01).
3.3. Determination of Lignin, Hemicellulose and Cellulose Content in Different Parts of the Plant
Both in the straight and twisted trunk types, lignin and cellulose contents decreased with the increase of the collection height; the lignin and hemicellulose contents of the twisted trunk were higher than that of straight trunk in the base, middle and tip, but the cellulose contents of the straight trunk types were higher than in the twisted trunk types (Figure 3).
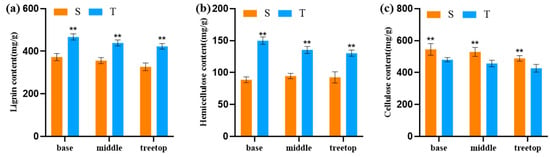
Figure 3.
Differences in lignin (a), hemicellulose (b) and cellulose (c) contents of straight and twisted trunk types of P. yunnanensis. S, straight trunk type; T, twisted trunk type. Double asterisk (**) means extremely significant difference (p < 0.01).
3.4. Differences in Endogenous Hormone Content
The twisted trunk types had significantly higher GA3 and GA4 contents than the straight trunk types in terms of gibberellin (GA) content (p < 0.01) (Figure 4). They also had a lower GA7 content than the straight trunk types, and there was no significant difference between the two types of trunks’ GA1 content in the vascular cambium. Three hormone types were identified in the auxin content: indole-3-carboxaldehyde (ICA), indolebutyric acid (IBA), and indoleacetic acid (IAA). The findings indicate that the IAA and ICA content in the twisted trunk types were lower than that in the straight trunk types. Moreover, there was an extremely significant difference in the contents of IAA (p < 0.01) and a significant difference in ICA among them (p < 0.05), while IBA content in the twisted trunk types was significantly higher than that in the straight trunk types (p < 0.05). Three different hormone types were found in the cytokinin content: trans-zeatin (TZ), N6-isopentenyladenine (IP), and isopentenyladenine (IPR). The twisted trunk had an extremely significantly greater TZ content compared with the straight trunk varieties (p < 0.01). There was no discernible difference in the IPR, and the IP was substantially higher than that of straight trunk kinds (p < 0.05). When it came to the salicylic acid content, the twisted trunk’s SA level was noticeably lower than in the straight trunk’s (p < 0.01). The jasmonic acid concentration of twisted trunk varieties was higher than that of straight trunk, with respect to jasmonic acid isoleucine (JA-ILE, p < 0.05), jasmonic acid (JA, p < 0.01) and dihydrojasmonic acid (H2JA, p < 0.01). Furthermore, there was no discernible difference in the BR contents between twisted and straight types.
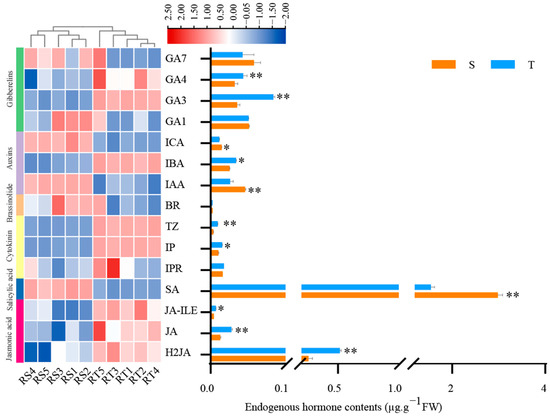
Figure 4.
Differences in endogenous hormone content of straight and twisted trunk types of P. yunnanensis. S, straight trunk type; T, twisted trunk type. Asterisk (*) means significant difference (p < 0.05), and double asterisk (**) means extremely significant difference (p < 0.01). GA7: Gibberellin A7; GA4: Gibberellin A4; GA3: Gibberellin A3;GA1: Gibberellin A1; ICA: Indole-3-carboxaldehyde; IBA: 3-Indolebutyric acid; IAA: Indole-3-acetic acid; BR: Brassinolide; TZ: trans-Zeatin; IP: N6-Isopentenyladenine; IPR: isopentenyladenine riboside; SA: Salicylic acid; JA-ILE: Jasmonoyl-L-Isoleucine; JA: Jasmonic acid; H2JA: Dihydrojasmonic acid.
3.5. Differential Gene Expression (DGE) in Straight and Twisted Trunk Types of P. yunnanensis
The expression of the GA2OX gene was significantly down-regulated in twisted trunks, while the expression of COI1, COI2, and XTH16 was significantly up-regulated in them, which was higher than that of straight trunk types. However, the expression of TCH4 was similar in both trunk types (Figure 5).
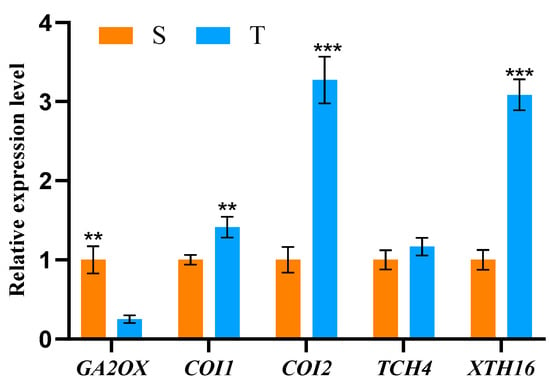
Figure 5.
Differential gene expression of straight and twisted trunk types of P. yunnanensis. S, straight trunk type; T, twisted trunk type. Double asterisk (**) means extremely significant difference (p < 0.01), and three asterisks (***) means extremely significant difference (p < 0.001).
4. Discussion
Since the properties of wood are directly related to the anatomical and structural characteristics of wood, this study found that the annual rings of the straight trunk type in P. yunnanensis were uniformly distributed through macroscopic structural observation, while the twisted trunk type was characterized by eccentricity and the annual rings were different in width. Further microanatomy of the wood found that the early- and latewood of the straight trunk was clearly demarcated and were due to acute transition (earlywood to latewood transformation and transition processes are rapid and pronounced), while the early- and latewood of the twisted trunk was obviously excessive and the demarcation not obvious, which is due to slow transition. The earlywood in the twisted trunk type appeared to have the characteristics of rounded tracheid cells, a thickened tracheid wall and an enlarged cavity diameter, which was similar to that of the earlywood of Pinus massoniana Lamb. [43], Pinus taeda L. [44], Pinus koraiensis Siebold & Zucc. [45], and Pinus bungeana Zucc. ex Endl. [46]; other anatomical results are also consistent. Therefore, we hypothesize that the uneven development of xylem may lead to the distortion of P. yunnanensis trunk type.
As the plant grows, the cell wall will continue to remodel to the extent that the xylem structure changes, and the accumulation of cell wall components may vary in order to accommodate the cell wall, lignin, cellulose, and hemicellulose, the three main components that play important roles. Cellulose acts as a cytoskeleton through the orderly deposition of microfilaments, and hemicellulose connects lignin to cellulose with hydrogen bonds [47]. Lignin is primarily responsible for strengthening the cell walls and intermediate layers of all woody plants, which allows trees to resist gravity and grow [48]. The present study selected the 1/5 (base), 1/3 (middle), and 2/3 (tip) of the tree height for the determination of cell wall components and found that the content of lignin and hemicellulose was higher in twisted types. It is speculated that P. yunnanensis needs to resist stronger growth stress when it undergoes twisting, so its cell wall deposits more lignin to increase the cell wall mechanical stress. This result is similar to the results of Schuetz et al. who found in the tracheid development of Arabidopsis thaliana L. xylem that there was more lignin deposition in the secondary cell walls of spiral or circular tracheids [49]. In addition, we found that cellulose and lignin content decreased with height of sample collection within the tree; the base had the highest content while the tip had a lower content, which we hypothesized was due to the fact that the wood formation time in the tip was not as long as that of the base, and thus cellulose and lignin were not as deposited as in the base.
Plant endogenous hormones play an important role in regulating the rate of formation layer division and the content of substances related to cell wall composition [30]. Earlier studies found that exogenous IAA could induce cell division and xylem differentiation in the formation layer of sunflower instead of acropetal buds [50]. Simon and Petrášek, in their experiments on externally applied IAA in decapitated poplars, found that IAA, which is supplied to the forming tissues via polar transport, is a key regulator of secondary xylem formation and induces the expression of lignin biosynthesis genes [51]. In this study, we found that the auxin content of twisted trunk types of P. yunnanensis was significantly lower than that of straight trunk type, and the twisted trunk appeared to be characterized by rounded tracheid cells and thickened cell walls, which is in line with the results of Chakraborty et al., who treated the stems of poplar with auxin and found that the thickness of its secondary cell walls was reduced. In addition, some researchers have shown that GAs play a role in influencing cell elongation by affecting the cell wall extensibility and the osmotic potential of the cells and thus the cell elongation [17]. GAs promote cellulose synthesis by inducing the release of secondary cell wall protein regulators through the DELLA repression cascade, which may potentially enhance and correlate with lignin deposition and increased lignin content [52]. In this study, high levels of lignin and GA were detected in the twisted trunk types, which is more consistent with the results of Zhou et al., who found an increase in the lignin content of horsetail pine after treating it with GA [53].
Low expression of GA2OX, the rate-limiting enzyme for active gibberellin synthesis, promotes gibberellin synthesis [54]. In this study, we found that the GA2OX gene was significantly down-regulated in the twisted trunk types, while the content of endogenous plant hormone GA3 was significantly higher than that of the straight trunk type, suggesting that low expression of GA2OX gene in the twisted trunk types promotes the synthesis of GA3, a result consistent with the conclusion that treatment of exogenous GAs in oat [55], bamboo [56], rice [57] and other plants, increases the ductility of their cell walls. Hamann et al. found that exogenous application of JA to Arabidopsis induced additional lignin synthesis [58]. In the present study, JA receptors (COI1, COI2) were significantly up-regulated and expressed in the twisted trunk type, which had higher lignin content, consistent with these results. Plant growth and development often occur through cell wall relaxation and remodeling, and changes in the cellulose–half-cellulose network are key to cell wall remodeling, which is regulated by xyloglucan endoglucan transglucosylase/hydrolysase (XTH) [59]. XTH typically has two catalytic functions: a xyloglucan endoglucosidase (XEH) activity and a xyloglucan endohydrolase (XET) activity [60]. TCH4 has been identified as a class of xyloglucan endoglycosyltransferases (XETs) that are involved in cell expansion and cell wall synthesis and modification [61]. Enzymes of the XTH family and members of the glycoside hydrolase family 16 (XTH16) are thought to contribute to cell wall mechanics and expansion through xyloglucan cleavage and reattachment [62]. In this study, TCH4 and XTH16 were found to be up-regulated in twisted trunk types, which at the same time had low cellulose content. Therefore, we hypothesized that high expression of TCH4 and XTH16 promotes xyloglucan hydrolysis to the extent of altering cellulose microfibril arrangement, which leads to increased cell wall extensibility, plasticity, and more susceptibility to deformation.
5. Conclusions
In this study, we observed the macroscopic and microscopic structures of two kinds of trunk types of P. yunnanensis and measured the content of their cell wall components, as well as the content of endogenous plant hormones, for comparative study and also verified the expression of genes related to the cell wall. The lignin and hemicellulose contents were high, the cellulose content was low, and the xyloglucan endotransglucosylase/hydrolyzing enzymes (XTH16 and TCH4) were highly expressed in the twisted trunk type. At the same time, the content of GA3 and JA was high, while the content of auxin was low. GA2OX was lowly expressed in the twisted trunk types, while COI1 and COI2 were significantly high in them. Based on this, we conclude that the differences in endogenous hormone content may be an important factor affecting the formation of twisted trunk types in P. yunnanensis. We surmise that in the twisted stem form, differential expression of genes such as GA2OX, COI1, and COI2 led to an abnormally high endogenous hormone content. Meanwhile, endogenous hormones acted on the cell wall to elevate the expression of xyloglucan endotransglycosylases (XTH16, TCH4), which changed the mechanical properties of the cell wall and made it more susceptible by resisting growing gravity through the large deposition of lignin in the cell wall. The plant continues to grow despite changes in the mechanical properties of the cell wall that make it more susceptible to deformation. The results of this study may add new discoveries to reveal the mechanism of plant genesis in different trunk types and provide a theoretical basis for genetic improvement of forest trees.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15091626/s1, Figure S1: Sampling location of twisted Pinus yunnanensis.
Author Contributions
D.Z. and Z.W. conceived and designed the experiment. R.X., C.W. and D.Z. analyzed the data. H.L., X.Z. and P.L. performed the experiments. H.L. wrote the manuscript. D.Z. and Z.W. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Postgraduate Fund Project of the Yunnan Education Department (2023Y0787) and the Youth Talents Special Project of Yunnan Province “Xingdian Talents Support Program” (XDYC-QNRC-2022-0232).
Data Availability Statement
The transcriptome data in this study are available from the NCBI SRA database under accession number PRJNA507489.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gonzalez, N.; Vanhaeren, H.; Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hannrup, B.; Grabner, M.; Karlsson, B.; Müller, U.; Wimmer, R. Erratum: Genetic parameters for spiral-grain angle in two 19-year-old clonal Norway spruce trials. Ann. For. Sci. 2005, 59, 551–556. [Google Scholar] [CrossRef][Green Version]
- Steeves, T.A.; Sussex, I.M. Secondary growth: The vascular cambium. In Patterns in Plant Development; Cambridge University Press: Cambridge, UK, 1989; pp. 311–332. [Google Scholar]
- Harris, J.M. Spiral Grain and Wave Phenomena in Wood Formation; Springer Science & Business Media: Berlin, Germany, 1989; Volume 64. [Google Scholar]
- Jones, B.; Jones, B. Cell adjustments accompanying the development of spiral grain in a specimen of Pseudotsuga taxifolia Brit. Commonw. For. Rev. 1963, 43, 151–158. [Google Scholar]
- Zheng, T.; Li, L.; Zhang, Q. Advances in research on tortuous traits of plants. Euphytica 2018, 214, 224. [Google Scholar] [CrossRef]
- Schulgasser, K.; Witztum, A. The mechanism of spiral grain formation in trees. Wood Sci. Technol. 2007, 41, 133–156. [Google Scholar] [CrossRef]
- MacMillan, C.P.; Birke, H.; Chuah, A.; Brill, E.; Tsuji, Y.; Ralph, J.; Dennis, E.S.; Llewellyn, D.; Pettolino, F.A. Tissue and cell-specific transcriptomes in cotton reveal the subtleties of gene regulation underlying the diversity of plant secondary cell walls. BMC Genom. 2017, 18, 539. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB Transcription factors and its regulation in secondary cell wall formation and lignin biosynthesis during xylem development. Int. J. Mol. Sci. 2021, 22, 3560. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Shadle, G.; Chen, F.; Srinivasa Reddy, M.S.; Jackson, L.; Nakashima, J.; Dixon, R.A. Down-regulation of hydroxycinnamoyl CoA: Shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry 2007, 68, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Green, P.B. Expression of pattern in plants: Combining molecular and calculus-based biophysical paradigms. Am. J. Bot. 1999, 86, 1059–1076. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, D.B.; Cosgrove, D.J. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 2009, 19, R800–R811. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Luo, J.; Dyson, R.J. Lockhart with a twist: Modelling cellulose microfibril deposition and reorientation reveals twisting plant cell growth mechanisms. J. Theor. Biol. 2021, 525, 110736. [Google Scholar] [CrossRef]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Reis, D.; Vian, B. Helicoidal pattern in secondary cell walls and possible role of xylans in their construction. C.R. Biol. 2004, 327, 785–790. [Google Scholar] [CrossRef]
- Chang, Y.; Middleton, R.; Ogawa, Y.; Gregory, T.; Steiner, L.M.; Kovalev, A.; Karanja, R.H.N.; Rudall, P.J.; Glover, B.J.; Gorb, S.N. Cell wall composition determines handedness reversal in helicoidal cellulose architectures of Pollia condensata fruits. Proc. Natl. Acad. Sci. USA 2021, 118, e2111723118. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence and function. In Plant Hormones Physiology Biochemistry & Molecular Biology; Springer: Dordrecht, The Netherlands, 1987; pp. 1–15. [Google Scholar]
- Brackmann, K.; Qi, J.; Gebert, M.; Jouannet, V.; Schlamp, T.; Grünwald, K.; Wallner, E.S.; Novikova, D.D.; Levitsky, V.G.; Agustí, J.; et al. Spatial specificity of auxin responses coordinates wood formation. Nat. Commun. 2018, 9, 875. [Google Scholar] [CrossRef]
- Mirza, J.I. Spiral growth of hypocotyls in mutant aux-1 of Arabidopsis thaliana. Arab. Inf. Serv. 1987, 23, 62–63. [Google Scholar]
- Okada, K.; Shimura, Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 1990, 250, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Yoon, Y.; Choi, Y.D. Jasmonic acid modulates xylem development by controlling expression of PIN-FORMED 7. Plant Signal. Behav. 2019, 14, 1637664. [Google Scholar] [CrossRef] [PubMed]
- Mäkilä, R.; Wybouw, B.; Smetana, O.; Vainio, L.; Solé-Gil, A.; Lyu, M.; Ye, L.; Wang, X.; Siligato, R.; Jenness, M.K.; et al. Gibberellins promote polar auxin transport to regulate stem cell fate decisions in cambium. Nat. Plants 2023, 9, 631–644. [Google Scholar] [CrossRef]
- Chen, L.; Higashitani, A.; Suge, H.; Takeda, K.; Takahashi, H. Spiral growth and cell wall properties of the gibberellin-treated first internodes in the seedlings of a wheat cultivar tolerant to deep-sowing conditions. Physiol. Plant. 2003, 118, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, R.; Tian, B.; Bai, Q.; Wang, D.; Cai, N.; He, C.; Kang, X.; Duan, A. Development of novel microsatellite markers for P. yunnanensis and their cross amplification in congeneric species. Conserv. Genet. Resour. 2013, 5, 1113–1114. [Google Scholar] [CrossRef]
- Eklund, L.; Säll, H. The influence of wind on spiral grain formation in conifer trees. Trees 2000, 14, 324–328. [Google Scholar] [CrossRef]
- Koehler, A. More about twisted grain in trees. Science 1931, 73, 477. [Google Scholar] [CrossRef]
- Kubler, H. Function of spiral grain in trees. Trees 1991, 5, 125–135. [Google Scholar] [CrossRef]
- Chen, S.; Lyu, Y. The causes and prevention of stem torsion of P. yunnanensis. Sci. Silvae Sin. 1962, 2, 131–140. [Google Scholar]
- Champion, H.G. More about spiral grain in conifers. Indian For. 1929, 55, 57–58. [Google Scholar]
- Cai, N.; Xu, Y.; Li, G.; Deng, L.; Li, W.; Wang, D. Planning, research status and prospect of the crooked and twisted characteristics of P. yunnanensis stem. For. Invent. Plan. 2016, 41, 19–23. [Google Scholar]
- Chen, Q.C.E.; Dong, F.; Fan, G.C.; Yin, J.Q.; Zhan, J.G.; Wang, J.L. The half-sib progeny test on natural elite stand of Pinus yunanensis in west of Yunnan. J. West China For. Sci 1997, 1, 9–21. [Google Scholar] [CrossRef]
- Anpei, Z.; Dan, Z.; Jiashan, L.; Dezhou, S.; Runxi, H.; Bin, T.; Yulan, X.; Chengzhong, H. AFLP analysis on genetic variation of stem forms in P. yunnanensis. Mol. Plant Breed 2016, 14, 186–194. [Google Scholar]
- He, F. Genetic experiment on texture phenotypic characters of P. yunnanensisc wood. Yunnan For. Sci. Technol. 1994, 2, 1–7. [Google Scholar]
- Gan, P.; Li, P.; Zhang, X.; Li, H.; Ma, S.; Zong, D.; He, C. Comparative transcriptomic and metabolomic analyses of differences in trunk spiral grain in P. yunnanensis. Int. J. Mol. Sci. 2023, 24, 14658. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Y.; Ran, M.; Qiu, J. Study on the anatomical structural characteristics of Manglietiastrum sinicum. J. Southwest For. Univ. 2020, 40, 161. [Google Scholar]
- Granados, J.M.; Ávila, C.; Cánovas, F.M.; Cañas, R.A. Selection and testing of reference genes for accurate RT-qPCR in adult needles and seedlings of maritime pine. Tree Genet. Genomes 2016, 12, 1–15. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin-Xing, L.; Cheng-Lee, Z.L.L. Comparative anatomy of normal wood and compression wood of Masson Pine(Pinus massoniana). J. Integr. Plant Biol. 1993, 35, 201–250. [Google Scholar]
- Liu, Y.; Liu, S. Anatomical properties of compression wood of three-year-old loblolly pine induced by artificial inclination. Sci. Silvae Sin. 2012, 48, 131–137. [Google Scholar]
- Shi, J.; Sun, Q.; Xing, D.; Liu, Y.; Li, J. Effect of stem bending angle on formative tissue during wood formation of Pinus koraiensis. For. Res. 2012, 25, 18. [Google Scholar]
- Zhang, Z.; Ma, J.; Ji, Z.; Xu, F. Comparison of anatomy and composition distribution between normal and compression wood of Pinus bungeana Zucc. revealed by microscopic imaging techniques. Microsc. Microanal 2012, 18, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, W.J.; Pang, B.; Sun, Z.; Lam, S.S.; Sonne, C.; Yuan, T.Q. Ultrastructural change in lignocellulosic biomass during hydrothermal pretreatment. Bioresour. Technol. 2021, 341, 125807. [Google Scholar] [CrossRef]
- Bidlack, J.; Malone, M.; Benson, R. Molecular structure and component integration of secondary cell walls in plants. Proc. Okla. Acad. Sci. 1992, 72, 195–214. [Google Scholar]
- Schuetz, M.; Benske, A.; Smith, R.A.; Watanabe, Y.; Tobimatsu, Y.; Ralph, J.; Demura, T.; Ellis, B.; Samuels, A.L. Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol. 2014, 166, 798–807. [Google Scholar] [CrossRef]
- Snow, R. Activation of cambial growth by pure hormones. Nature 1935, 135, 876. [Google Scholar] [CrossRef]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 454–460. [Google Scholar] [CrossRef]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Fan, F.; Qin, H.; Ding, G. Effects of exogenous GA(3) on stem secondary growth of Pinus massoniana seedlings. Plant Physiol. Biochem. 2024, 206, 108254. [Google Scholar] [CrossRef]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14 (Suppl. S1), S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.A.; Montague, M.J.; Tepfer, M.; Rayle, D.L.; Ikuma, H.; Kaufman, P.B. Effect of gibberellic acid on the plasticity and elasticity of Avena stem segments. Plant Physiol. 1975, 56, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Zhu, Q.; Gao, Y.; Wang, H.; Zhao, L.; Wang, Y.; Xi, F.; Wang, W.; Yang, Y.; et al. Transcriptome characterization of moso bamboo (Phyllostachys edulis) seedlings in response to exogenous gibberellin applications. BMC Plant Biol. 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Okuno, A.; Hirano, K.; Asano, K.; Takase, W.; Masuda, R.; Morinaka, Y.; Ueguchi-Tanaka, M.; Kitano, H.; Matsuoka, M. New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS ONE 2014, 9, e86870. [Google Scholar] [CrossRef] [PubMed]
- Hamann, T.; Bennett, M.; Mansfield, J.; Somerville, C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. Cell Mol. Biol. 2009, 57, 1015–1026. [Google Scholar] [CrossRef]
- Vissenberg, K.; Fry, S.C.; Pauly, M.; Höfte, H.; Verbelen, J.-P. XTH acts at the microfibril–matrix interface during cell elongation. J. Exp. Bot. 2005, 56, 673–683. [Google Scholar]
- Du LiPing, D.L.; Shen Xin, S.X.; Chen ShaoLiang, C.S.; Hu ZanMin, H.Z. Research advances on a key cell wall remodeling enzyme xyloglucan endotransglucosylase/hydrolase (XTH). J. Agric. Biotechnol. 2010, 18, 604–609. [Google Scholar]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell Online 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Van Sandt, V.S.; Suslov, D.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann. Bot. 2007, 100, 1467–1473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).