Determination and Analysis of Endogenous Hormones and Cell Wall Composition between the Straight and Twisted Trunk Types of Pinus yunnanensis Franch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Morphology, Histology and Imaging
2.3. Determination of Lignin, Hemicellulose and Cellulose Content
2.4. UPLC/MS Analysis of Plant Hormone Content
2.5. RNA Extraction and RT-qPCR Expression Validation
2.6. Statistics and Data Analysis
3. Results
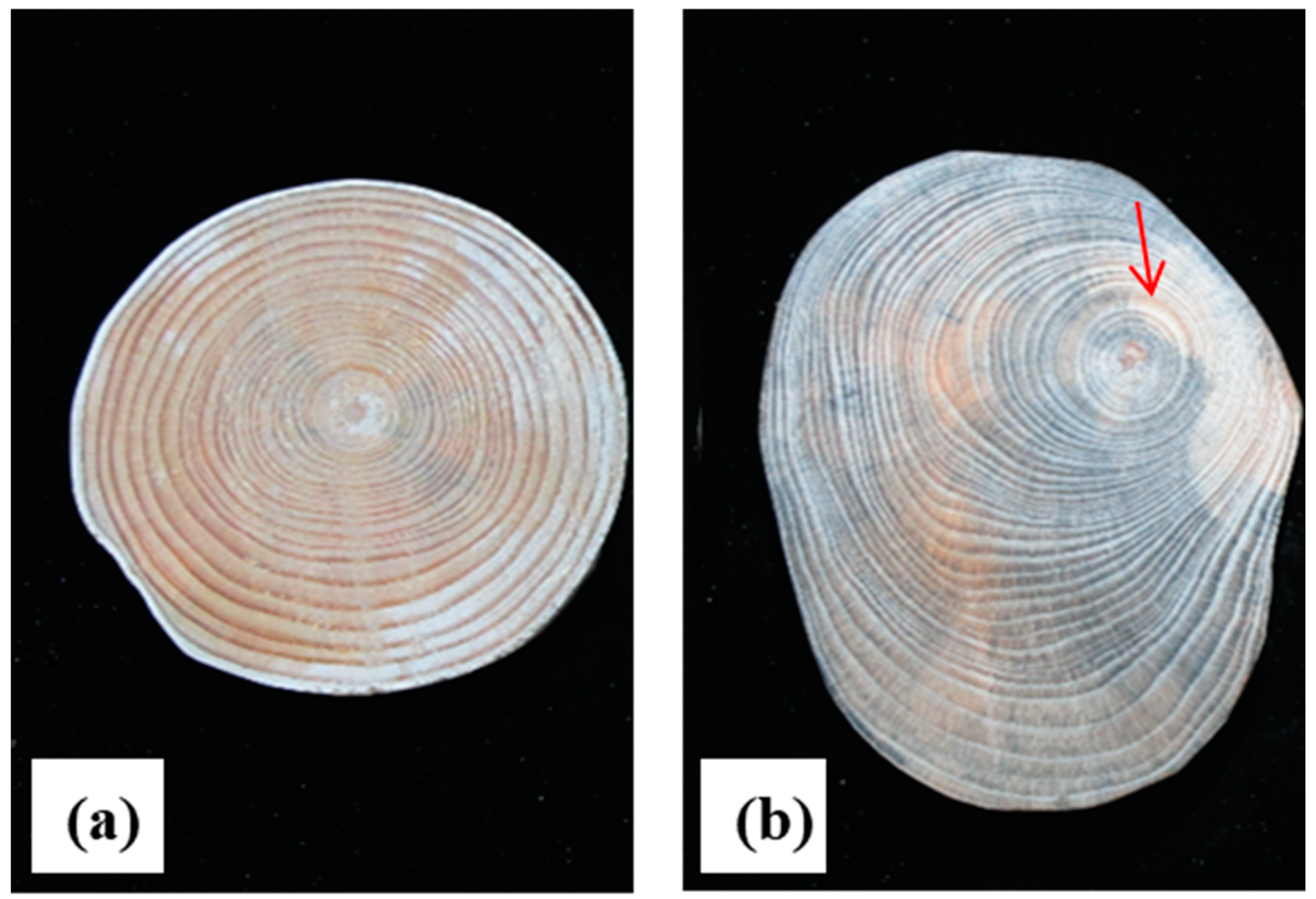
3.1. Macrostructural Analysis
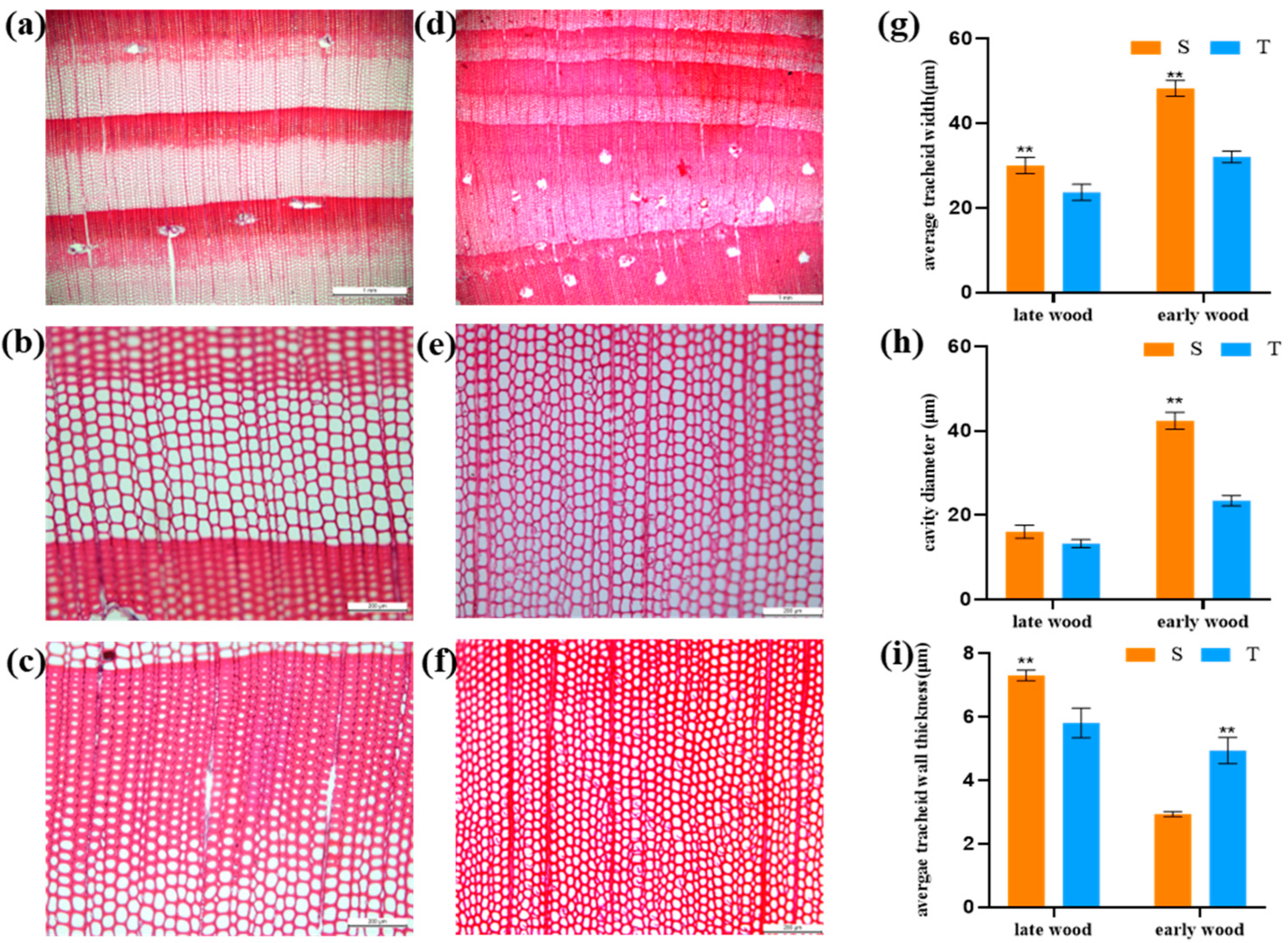
3.2. Microanatomical and Structural Characteristics
3.3. Determination of Lignin, Hemicellulose and Cellulose Content in Different Parts of the Plant
3.4. Differences in Endogenous Hormone Content
3.5. Differential Gene Expression (DGE) in Straight and Twisted Trunk Types of P. yunnanensis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, N.; Vanhaeren, H.; Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hannrup, B.; Grabner, M.; Karlsson, B.; Müller, U.; Wimmer, R. Erratum: Genetic parameters for spiral-grain angle in two 19-year-old clonal Norway spruce trials. Ann. For. Sci. 2005, 59, 551–556. [Google Scholar] [CrossRef]
- Steeves, T.A.; Sussex, I.M. Secondary growth: The vascular cambium. In Patterns in Plant Development; Cambridge University Press: Cambridge, UK, 1989; pp. 311–332. [Google Scholar]
- Harris, J.M. Spiral Grain and Wave Phenomena in Wood Formation; Springer Science & Business Media: Berlin, Germany, 1989; Volume 64. [Google Scholar]
- Jones, B.; Jones, B. Cell adjustments accompanying the development of spiral grain in a specimen of Pseudotsuga taxifolia Brit. Commonw. For. Rev. 1963, 43, 151–158. [Google Scholar]
- Zheng, T.; Li, L.; Zhang, Q. Advances in research on tortuous traits of plants. Euphytica 2018, 214, 224. [Google Scholar] [CrossRef]
- Schulgasser, K.; Witztum, A. The mechanism of spiral grain formation in trees. Wood Sci. Technol. 2007, 41, 133–156. [Google Scholar] [CrossRef]
- MacMillan, C.P.; Birke, H.; Chuah, A.; Brill, E.; Tsuji, Y.; Ralph, J.; Dennis, E.S.; Llewellyn, D.; Pettolino, F.A. Tissue and cell-specific transcriptomes in cotton reveal the subtleties of gene regulation underlying the diversity of plant secondary cell walls. BMC Genom. 2017, 18, 539. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB Transcription factors and its regulation in secondary cell wall formation and lignin biosynthesis during xylem development. Int. J. Mol. Sci. 2021, 22, 3560. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Shadle, G.; Chen, F.; Srinivasa Reddy, M.S.; Jackson, L.; Nakashima, J.; Dixon, R.A. Down-regulation of hydroxycinnamoyl CoA: Shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry 2007, 68, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Green, P.B. Expression of pattern in plants: Combining molecular and calculus-based biophysical paradigms. Am. J. Bot. 1999, 86, 1059–1076. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, D.B.; Cosgrove, D.J. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 2009, 19, R800–R811. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Luo, J.; Dyson, R.J. Lockhart with a twist: Modelling cellulose microfibril deposition and reorientation reveals twisting plant cell growth mechanisms. J. Theor. Biol. 2021, 525, 110736. [Google Scholar] [CrossRef]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Reis, D.; Vian, B. Helicoidal pattern in secondary cell walls and possible role of xylans in their construction. C.R. Biol. 2004, 327, 785–790. [Google Scholar] [CrossRef]
- Chang, Y.; Middleton, R.; Ogawa, Y.; Gregory, T.; Steiner, L.M.; Kovalev, A.; Karanja, R.H.N.; Rudall, P.J.; Glover, B.J.; Gorb, S.N. Cell wall composition determines handedness reversal in helicoidal cellulose architectures of Pollia condensata fruits. Proc. Natl. Acad. Sci. USA 2021, 118, e2111723118. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence and function. In Plant Hormones Physiology Biochemistry & Molecular Biology; Springer: Dordrecht, The Netherlands, 1987; pp. 1–15. [Google Scholar]
- Brackmann, K.; Qi, J.; Gebert, M.; Jouannet, V.; Schlamp, T.; Grünwald, K.; Wallner, E.S.; Novikova, D.D.; Levitsky, V.G.; Agustí, J.; et al. Spatial specificity of auxin responses coordinates wood formation. Nat. Commun. 2018, 9, 875. [Google Scholar] [CrossRef]
- Mirza, J.I. Spiral growth of hypocotyls in mutant aux-1 of Arabidopsis thaliana. Arab. Inf. Serv. 1987, 23, 62–63. [Google Scholar]
- Okada, K.; Shimura, Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 1990, 250, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Yoon, Y.; Choi, Y.D. Jasmonic acid modulates xylem development by controlling expression of PIN-FORMED 7. Plant Signal. Behav. 2019, 14, 1637664. [Google Scholar] [CrossRef] [PubMed]
- Mäkilä, R.; Wybouw, B.; Smetana, O.; Vainio, L.; Solé-Gil, A.; Lyu, M.; Ye, L.; Wang, X.; Siligato, R.; Jenness, M.K.; et al. Gibberellins promote polar auxin transport to regulate stem cell fate decisions in cambium. Nat. Plants 2023, 9, 631–644. [Google Scholar] [CrossRef]
- Chen, L.; Higashitani, A.; Suge, H.; Takeda, K.; Takahashi, H. Spiral growth and cell wall properties of the gibberellin-treated first internodes in the seedlings of a wheat cultivar tolerant to deep-sowing conditions. Physiol. Plant. 2003, 118, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, R.; Tian, B.; Bai, Q.; Wang, D.; Cai, N.; He, C.; Kang, X.; Duan, A. Development of novel microsatellite markers for P. yunnanensis and their cross amplification in congeneric species. Conserv. Genet. Resour. 2013, 5, 1113–1114. [Google Scholar] [CrossRef]
- Eklund, L.; Säll, H. The influence of wind on spiral grain formation in conifer trees. Trees 2000, 14, 324–328. [Google Scholar] [CrossRef]
- Koehler, A. More about twisted grain in trees. Science 1931, 73, 477. [Google Scholar] [CrossRef]
- Kubler, H. Function of spiral grain in trees. Trees 1991, 5, 125–135. [Google Scholar] [CrossRef]
- Chen, S.; Lyu, Y. The causes and prevention of stem torsion of P. yunnanensis. Sci. Silvae Sin. 1962, 2, 131–140. [Google Scholar]
- Champion, H.G. More about spiral grain in conifers. Indian For. 1929, 55, 57–58. [Google Scholar]
- Cai, N.; Xu, Y.; Li, G.; Deng, L.; Li, W.; Wang, D. Planning, research status and prospect of the crooked and twisted characteristics of P. yunnanensis stem. For. Invent. Plan. 2016, 41, 19–23. [Google Scholar]
- Chen, Q.C.E.; Dong, F.; Fan, G.C.; Yin, J.Q.; Zhan, J.G.; Wang, J.L. The half-sib progeny test on natural elite stand of Pinus yunanensis in west of Yunnan. J. West China For. Sci 1997, 1, 9–21. [Google Scholar] [CrossRef]
- Anpei, Z.; Dan, Z.; Jiashan, L.; Dezhou, S.; Runxi, H.; Bin, T.; Yulan, X.; Chengzhong, H. AFLP analysis on genetic variation of stem forms in P. yunnanensis. Mol. Plant Breed 2016, 14, 186–194. [Google Scholar]
- He, F. Genetic experiment on texture phenotypic characters of P. yunnanensisc wood. Yunnan For. Sci. Technol. 1994, 2, 1–7. [Google Scholar]
- Gan, P.; Li, P.; Zhang, X.; Li, H.; Ma, S.; Zong, D.; He, C. Comparative transcriptomic and metabolomic analyses of differences in trunk spiral grain in P. yunnanensis. Int. J. Mol. Sci. 2023, 24, 14658. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Y.; Ran, M.; Qiu, J. Study on the anatomical structural characteristics of Manglietiastrum sinicum. J. Southwest For. Univ. 2020, 40, 161. [Google Scholar]
- Granados, J.M.; Ávila, C.; Cánovas, F.M.; Cañas, R.A. Selection and testing of reference genes for accurate RT-qPCR in adult needles and seedlings of maritime pine. Tree Genet. Genomes 2016, 12, 1–15. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin-Xing, L.; Cheng-Lee, Z.L.L. Comparative anatomy of normal wood and compression wood of Masson Pine(Pinus massoniana). J. Integr. Plant Biol. 1993, 35, 201–250. [Google Scholar]
- Liu, Y.; Liu, S. Anatomical properties of compression wood of three-year-old loblolly pine induced by artificial inclination. Sci. Silvae Sin. 2012, 48, 131–137. [Google Scholar]
- Shi, J.; Sun, Q.; Xing, D.; Liu, Y.; Li, J. Effect of stem bending angle on formative tissue during wood formation of Pinus koraiensis. For. Res. 2012, 25, 18. [Google Scholar]
- Zhang, Z.; Ma, J.; Ji, Z.; Xu, F. Comparison of anatomy and composition distribution between normal and compression wood of Pinus bungeana Zucc. revealed by microscopic imaging techniques. Microsc. Microanal 2012, 18, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, W.J.; Pang, B.; Sun, Z.; Lam, S.S.; Sonne, C.; Yuan, T.Q. Ultrastructural change in lignocellulosic biomass during hydrothermal pretreatment. Bioresour. Technol. 2021, 341, 125807. [Google Scholar] [CrossRef]
- Bidlack, J.; Malone, M.; Benson, R. Molecular structure and component integration of secondary cell walls in plants. Proc. Okla. Acad. Sci. 1992, 72, 195–214. [Google Scholar]
- Schuetz, M.; Benske, A.; Smith, R.A.; Watanabe, Y.; Tobimatsu, Y.; Ralph, J.; Demura, T.; Ellis, B.; Samuels, A.L. Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol. 2014, 166, 798–807. [Google Scholar] [CrossRef]
- Snow, R. Activation of cambial growth by pure hormones. Nature 1935, 135, 876. [Google Scholar] [CrossRef]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 454–460. [Google Scholar] [CrossRef]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Fan, F.; Qin, H.; Ding, G. Effects of exogenous GA(3) on stem secondary growth of Pinus massoniana seedlings. Plant Physiol. Biochem. 2024, 206, 108254. [Google Scholar] [CrossRef]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14 (Suppl. S1), S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.A.; Montague, M.J.; Tepfer, M.; Rayle, D.L.; Ikuma, H.; Kaufman, P.B. Effect of gibberellic acid on the plasticity and elasticity of Avena stem segments. Plant Physiol. 1975, 56, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Zhu, Q.; Gao, Y.; Wang, H.; Zhao, L.; Wang, Y.; Xi, F.; Wang, W.; Yang, Y.; et al. Transcriptome characterization of moso bamboo (Phyllostachys edulis) seedlings in response to exogenous gibberellin applications. BMC Plant Biol. 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Okuno, A.; Hirano, K.; Asano, K.; Takase, W.; Masuda, R.; Morinaka, Y.; Ueguchi-Tanaka, M.; Kitano, H.; Matsuoka, M. New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS ONE 2014, 9, e86870. [Google Scholar] [CrossRef] [PubMed]
- Hamann, T.; Bennett, M.; Mansfield, J.; Somerville, C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. Cell Mol. Biol. 2009, 57, 1015–1026. [Google Scholar] [CrossRef]
- Vissenberg, K.; Fry, S.C.; Pauly, M.; Höfte, H.; Verbelen, J.-P. XTH acts at the microfibril–matrix interface during cell elongation. J. Exp. Bot. 2005, 56, 673–683. [Google Scholar]
- Du LiPing, D.L.; Shen Xin, S.X.; Chen ShaoLiang, C.S.; Hu ZanMin, H.Z. Research advances on a key cell wall remodeling enzyme xyloglucan endotransglucosylase/hydrolase (XTH). J. Agric. Biotechnol. 2010, 18, 604–609. [Google Scholar]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell Online 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Van Sandt, V.S.; Suslov, D.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann. Bot. 2007, 100, 1467–1473. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xu, R.; Wang, C.; Zhang, X.; Li, P.; Wu, Z.; Zong, D. Determination and Analysis of Endogenous Hormones and Cell Wall Composition between the Straight and Twisted Trunk Types of Pinus yunnanensis Franch. Forests 2024, 15, 1626. https://doi.org/10.3390/f15091626
Li H, Xu R, Wang C, Zhang X, Li P, Wu Z, Zong D. Determination and Analysis of Endogenous Hormones and Cell Wall Composition between the Straight and Twisted Trunk Types of Pinus yunnanensis Franch. Forests. 2024; 15(9):1626. https://doi.org/10.3390/f15091626
Chicago/Turabian StyleLi, Hailin, Rong Xu, Cai Wang, Xiaolin Zhang, Peiling Li, Zhiyang Wu, and Dan Zong. 2024. "Determination and Analysis of Endogenous Hormones and Cell Wall Composition between the Straight and Twisted Trunk Types of Pinus yunnanensis Franch" Forests 15, no. 9: 1626. https://doi.org/10.3390/f15091626
APA StyleLi, H., Xu, R., Wang, C., Zhang, X., Li, P., Wu, Z., & Zong, D. (2024). Determination and Analysis of Endogenous Hormones and Cell Wall Composition between the Straight and Twisted Trunk Types of Pinus yunnanensis Franch. Forests, 15(9), 1626. https://doi.org/10.3390/f15091626




