Exploring the Efficient Irrigation Period for Larix kaempferi Seedlings in Nursery Pots in Greenhouse Conditions Using Optical Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Drought Stress and Rehydration Treatments
2.3. Greenhouse and Soil Environment
2.4. Growth Characteristic Measurements
2.5. Optical Measurements
| Type | Parameter | Principle and Equation | Reference |
|---|---|---|---|
| VI | NDVI | (RNIR − RRed)/(RNIR + RRed) | [36] |
| OSAVI | (1 + 0.16) × (R790 − R670)/(R790 − R670 + 0.16) | [25] | |
| MCARI | [(R700 − R670) − 0.2 × (R700 − R550)] × (R700/R670) | [37] | |
| PRI | (R415 − R435)/(R415 + R435) | [38] | |
| FL imaging | Fo | Minimum fluorescence in a dark-adapted state | [39] |
| Fm | Maximum fluorescence in a dark-adapted state | [40] | |
| QY_max | (Fm − Fo)/Fm | [41] | |
| QY_Lss | (Fm_Lss − Ft_Lss)/Fm_Lss | ||
| NPQ_Lss | (Fm − Fm_Lss)/Fm_Lss | [42] | |
| Rfd_Lss | (Fp − Ft_Lss)/Ft_Lss | [43] | |
| TH imaging | CWSI | (Tl − Tw)/(Td − Tw) | [44] |
| LTD | Tc − Tl |
2.6. Statistical Analysis
3. Results
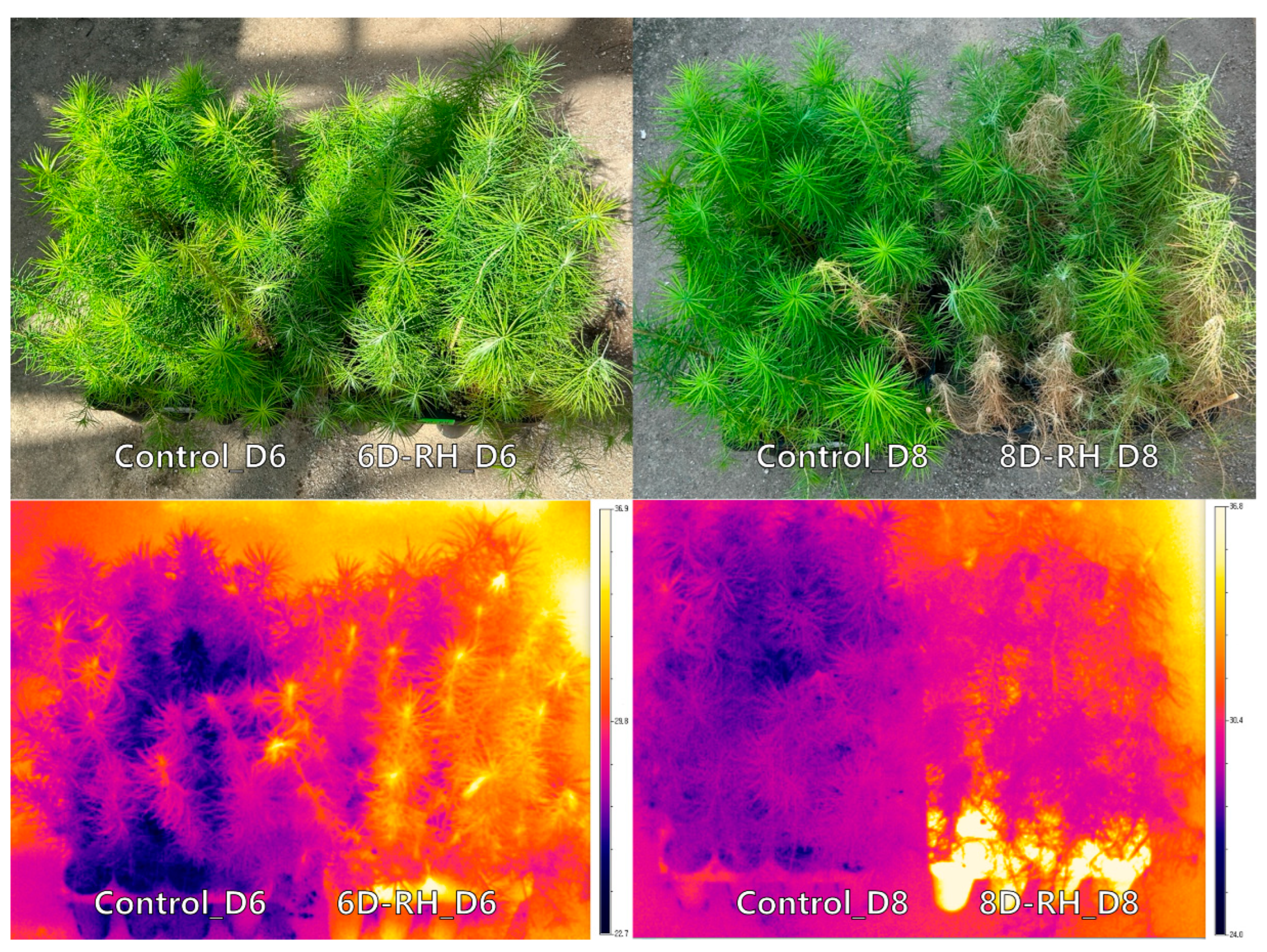
3.1. Results of Growth Characteristics
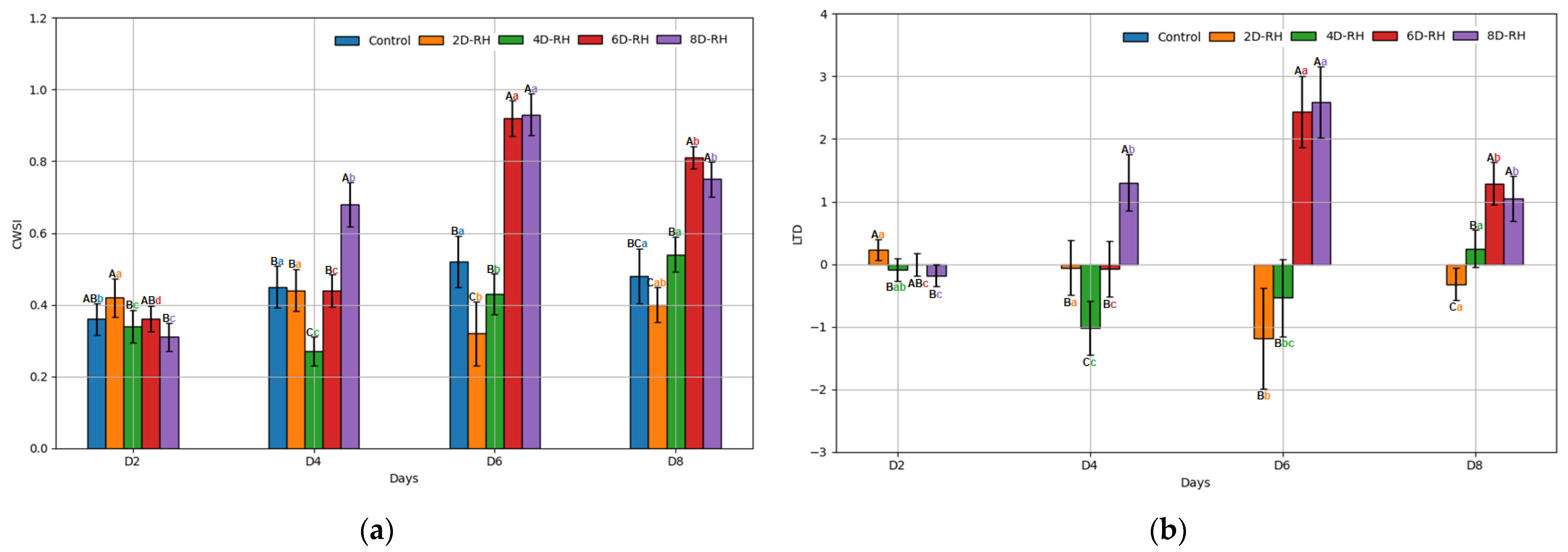
3.2. The Results of the ANOVA
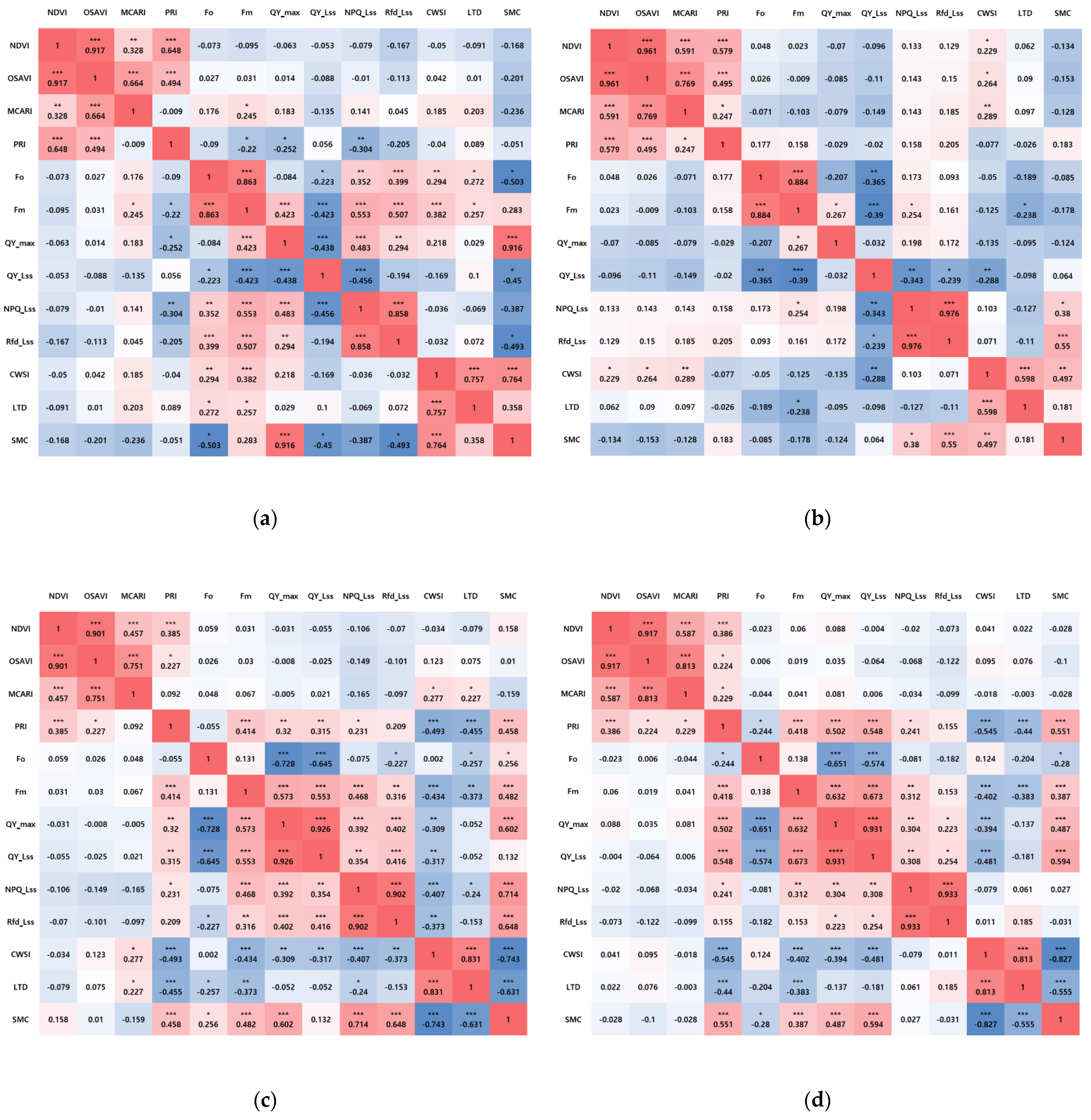
3.3. The Results of the Correlation Analysis
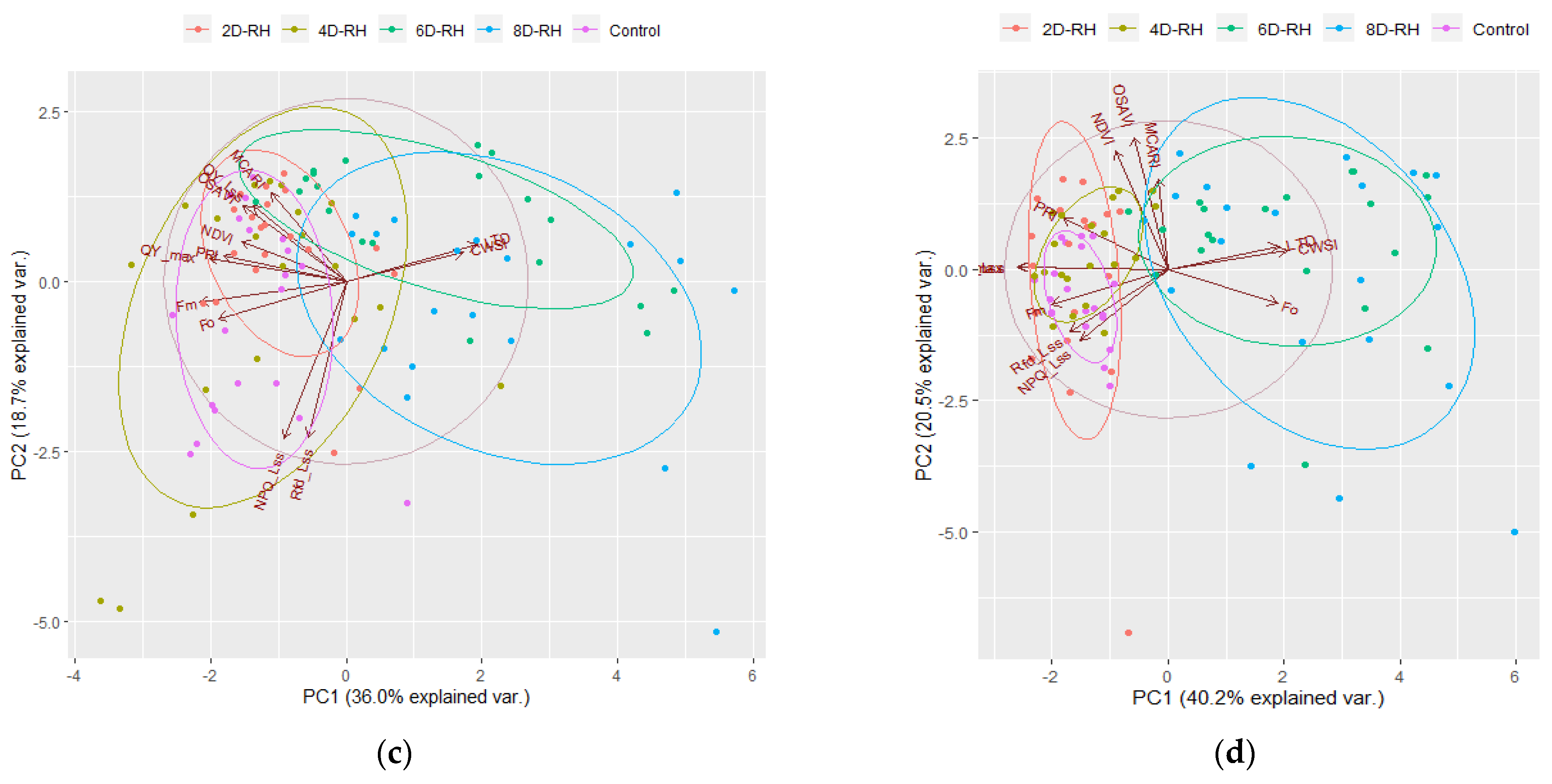
3.4. The Results of the PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plesa, I.M.; González-Orenga, S.; Al Hassan, M.; Sestras, A.F.; Vicente, O.; Prohens, J.; Sestras, R.E.; Boscaiu, M. Effects of drought and salinity on European Larch (Larix decidua Mill.) seedlings. Forests 2018, 9, 320. [Google Scholar] [CrossRef]
- Sparks, A.M.; Talhelm, A.F.; Feltrin, R.P.; Smith, A.M.; Johnson, D.M.; Kolden, C.A.; Boschetti, L. An experimental assessment of the impact of drought and fire on western larch injury, mortality and recovery. Int. J. Wildland Fire 2018, 27, 490–497. [Google Scholar] [CrossRef]
- Kitao, M.; Agathokleous, E.; Harayama, H.; Kitaoka, S.; Uemura, A.; Yazaki, K.; Tobita, H. Tolerance of Japanese larch to drought is modified by nitrogen and water regimes during cultivation of container seedlings. Eur. J. For. Res. 2022, 141, 699–712. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Noh, N.J.; Kim, G.J.; Son, Y.; Cho, M.S. Early growth responses of Larix kaempferi (Lamb.) carr. seedling to short-term extreme climate events in summer. Forests 2021, 12, 1595. [Google Scholar] [CrossRef]
- Sasani, N.; Pâques, L.E.; Boulanger, G.; Singh, A.P.; Gierlinger, N.; Rosner, S.; Brendel, O. Physiological and anatomical responses to drought stress differ between two larch species and their hybrid. Trees 2021, 35, 1467–1484. [Google Scholar] [CrossRef] [PubMed]
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2023. (In Korean) [Google Scholar]
- Dulamsuren, C.; Hauck, M.; Leuschner, C. Recent drought stress leads to growth reductions in Larix sibirica in the western Khentey, Mongolia. Glob. Chang. Biol. 2010, 16, 3024–3035. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Manzanedo, R.D.; D’Orangeville, L.; Lv, P.; Wang, C.; Xu, C.; Hou, M.; Huang, X.; Rademacher, T. High risk of growth cessation of planted larch under extreme drought. Environ. Res. Lett. 2021, 16, 014040. [Google Scholar] [CrossRef]
- Naik, M.S.; Desai, S.; Sairam, K.; Chaitra, S.N. IoT-Based Nursery Management System. In Advances in Artificial Intelligence and Data Engineering: Select Proceedings of AIDE 2019, 1st ed.; Chiplunkar, N.N., Fukao, T., Eds.; Springer Nature: Singapore, 2020; Volume 1133, pp. 1335–1344. [Google Scholar]
- Dünisch, O. Relationship between the anatomical structure and the swelling of conditioned wood surfaces. IAWA J. 2013, 34, 197–208. [Google Scholar] [CrossRef]
- Sicher, R.C.; Timlin, D.; Bailey, B. Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J. Plant Physiol. 2012, 169, 686–695. [Google Scholar] [CrossRef]
- Florea, A.; Popa, D.I.; Morariu, D.; Maniu, I.; Berntzen, L.; Fiore, U. Digital farming based on a smart and user-friendly IoT irrigation system: A conifer nursery case study. IET Cyber-Phys. Syst. Theory Appl. 2023, 9, 150–168. [Google Scholar] [CrossRef]
- South, D.B.; Starkey, T.E.; Enebak, S.A. Forest nursery practices in the southern United States. Reforesta 2016, 1, 106–146. [Google Scholar] [CrossRef]
- Korea Forest Service. The Guidelines for Seed and Nursery Practices; Korea Forest Service: Daejeon, Republic of Korea, 2020. (In Korean) [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Ubierna, N.; Jenkins, M.W.; Garrity, S.R.; Rahn, T.; Powers, H.H.; Hanson, D.T.; Sevanto, S.; Wong, S.C.; McDowell, N.G.; et al. Unsaturation of vapour pressure inside leaves of two conifer species. Sci. Rep. 2018, 8, 7667. [Google Scholar] [CrossRef] [PubMed]
- Batke, S.P.; Yiotis, C.; Elliott-Kingston, C.; Holohan, A.; McElwain, J. Plant responses to decadal scale increments in atmospheric CO2 concentration: Comparing two stomatal conductance sampling methods. Planta 2020, 251, 52. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Kyratzis, A.; Skarlatos, D.; Fotopoulos, V.; Vamvakousis, V.; Katsiotis, A. Investigating correlation among NDVI index derived by unmanned aerial vehicle photography and grain yield under late drought stress conditions. Procedia Environ. Sci. 2015, 29, 225–226. [Google Scholar] [CrossRef][Green Version]
- Thapa, S.; Rudd, J.C.; Xue, Q.; Bhandari, M.; Reddy, S.K.; Jessup, K.E.; Liu, S.; Devkota, R.N.; Baker, J.; Baker, S. Use of NDVI for characterizing winter wheat response to water stress in a semi-arid environment. J. Crop Improv. 2019, 33, 633–648. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.L. Thermal imaging for plant stress detection and phenotyping. Remote Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Guilioni, L.; Jones, H.G.; Leinonen, I.; Lhomme, J.P. On the relationships between stomatal resistance and leaf temperatures in thermography. Agric. For. Meteorol. 2008, 148, 1908–1912. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Köksal, E.S.; Candoğan, B.N.; Yıldırım, Y.E.; Yazgan, S. Determination of water use and water stress of cherry trees based on canopy temperature, leaf water potential and resistance. ZEMDIRBYSTE 2010, 97, 57–64. [Google Scholar]
- Yun, S.K.; Kim, S.J.; Nam, E.Y.; Kwon, J.H.; Do, Y.S.; Song, S.Y.; Kim, M.; Choi, Y.; Kim, G.; Shin, H. Evaluation of Water Stress Using Canopy Temperature and Crop Water Stress Index (CWSI) in Peach Trees. J. Bio-Enviorn. Con. 2020, 29, 20–27. (In Korean) [Google Scholar] [CrossRef]
- Zhou, Z.; Majeed, Y.; Naranjo, G.D.; Gambacorta, E.M. Assessment for crop water stress with infrared thermal imagery in precision agriculture: A review and future prospects for deep learning applications. Comput. Electron. Agric. 2021, 182, 106019. [Google Scholar] [CrossRef]
- National Institute of Agricultural Science and Technology. Methods of Soil and Plant Analysis; Rural Development Administration (NIAST): Suwon, Republic of Korea, 2000. (In Korean) [Google Scholar]
- Jones, H.G.; Stoll, M.; Santos, T.; Sousa, C.D.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.W., Jr.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA: Greenbelt, MD, USA, 1974. [Google Scholar]
- Daughtry, C.S.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey Iii, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Serôdio, J.; Marques da Silva, J.; Catarino, F. Nondestructive Tracing of Migratory Rhythms of Intertidal Benthic Microalgae Using In Vivo Chlorophyll a Fluorescence. J. Phycol. 1997, 33, 542–553. [Google Scholar] [CrossRef]
- Rysgaard, S.; Kühl, M.; Glud, R.N.; Hansen, J.W. Biomass, production and horizontal patchiness of sea ice algae in a high-Arctic fjord (Young Sound, NE Greenland). Mar. Ecol. Prog. Ser. 2001, 223, 15–26. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Flexas, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Medrano, H. The response of photosynthesis to soil water stress. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Heidelberg, Germany, 2012; pp. 129–144. [Google Scholar]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [PubMed]
- Blake, T.J.; Li, J. Hydraulic adjustment in jack pine and black spruce seedlings under controlled cycles of dehydration and rehydration. Physiol. Plant. 2003, 117, 532–539. [Google Scholar] [CrossRef]
- Rolando, C.A.; Little, K.M. Measuring water stress in Eucalyptus grandis Hill ex Maiden seedlings planted into pots. S. Afr. J. Bot. 2008, 74, 133–138. [Google Scholar] [CrossRef][Green Version]
- Turner, N.C. Imposing and maintaining soil water deficits in drought studies in pots. Plant Soil 2019, 439, 45–55. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.C.Y.; Lew, T.T.S. Non-destructive technologies for plant health diagnosis. Front. Plant Sci. 2022, 13, 884454. [Google Scholar] [CrossRef] [PubMed]
- Asaari, M.S.M.; Mertens, S.; Verbraeken, L.; Dhondt, S.; Inzé, D.; Bikram, K.; Scheunders, P. Non-destructive analysis of plant physiological traits using hyperspectral imaging: A case study on drought stress. Comput. Electron. Agric. 2022, 195, 106806. [Google Scholar] [CrossRef]
- Ye, D.; Wu, L.; Li, X.; Atoba, T.O.; Wu, W.; Weng, H. A synthetic review of various dimensions of non-destructive plant stress phenotyping. Plants 2023, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Thénot, F.; Méthy, M.; Winkel, T. The Photochemical Reflectance Index (PRI) as a water-stress index. Int. J. Remote Sens. 2002, 23, 5135–5139. [Google Scholar] [CrossRef]
- Kovar, M.; Brestic, M.; Sytar, O.; Barek, V.; Hauptvogel, P.; Zivcak, M. Evaluation of hyperspectral reflectance parameters to _gassess the leaf water content in soybean. Water 2019, 11, 443. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Williams, L.E.; Suarez, L.; Berni, J.A.; Goldhamer, D.; Fereres, E. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band airborne imagery and the CWSI thermal index. Remote Sens. Environ. 2013, 138, 38–50. [Google Scholar] [CrossRef]
- Tan, C.W.; Wang, D.L.; Zhou, J.; Du, Y.; Luo, M.; Zhang, Y.J.; Guo, W.S. Assessment of Fv/Fm absorbed by wheat canopies employing in-situ hyperspectral vegetation indexes. Sci. Rep. 2018, 8, 9525. [Google Scholar] [CrossRef]
- Nakaji, T.; Takeda, T.; Mukai, Y.; Koike, T.; Oguma, H.; Fujinuma, Y. Relationships between Photosynthesis and Spectral Reflectance Indices in Japanese Larch Needles. J. Jpn. For. Soc. 2003, 85, 205–213. (In Japanese) [Google Scholar]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Miller, J.R.; Jiménez-Muñoz, J.C.; Sobrino, J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Suárez, L.; Zarco-Tejada, P.J.; Berni, J.A.J.; González-Dugo, V.; Fereres, E. Modelling PRI for water stress detection using radiative transfer models. Remote Sens. Environ. 2009, 113, 730–744. [Google Scholar] [CrossRef]
- Panigada, C.; Rossini, M.; Meroni, M.; Cilia, C.; Busetto, L.; Amaducci, S.; Boschetti, M.; Cogliati, S.; Picchi, V.; Pinto, F.; et al. Fluorescence, PRI and canopy temperature for water stress detection in cereal crops. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 167–178. [Google Scholar] [CrossRef]
- Banks, J.M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. Exp. Bot. 2018, 155, 118–127. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, M.; Grosch, R.; Graefe, J. The use of features from fluorescence, thermography, and NDVI imaging to detect biotic stress in lettuce. Plant Dis. 2018, 102, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Tang, H.; Fu, L.; Tan, J.; Govindjee, G.; Guo, Y. Determination of Fv/Fm from chlorophyll a fluorescence without dark adaptation by an LSSVM model. Plant Phenomics 2023, 5, 0034. [Google Scholar] [CrossRef] [PubMed]
- Belin, É.; Rousseau, D.; Boureau, T.; Caffier, V. Thermography versus chlorophyll fluorescence imaging for detection and quantification of apple scab. Comput. Electron. Agric. 2013, 90, 159–163. [Google Scholar] [CrossRef]
- Granum, E.; Pérez-Bueno, M.L.; Calderón, C.E.; Ramos, C.; de Vicente, A.; Cazorla, F.M.; Barón, M. Metabolic responses of avocado plants to stress induced by Rosellinia necatrix analysed by fluorescence and thermal imaging. Eur. J. Plant Pathol. 2015, 142, 625–632. [Google Scholar]
- Saglam, A.; Chaerle, L.; Van Der Straeten, D.; Valcke, R. Promising monitoring techniques for plant science: Thermal and chlorophyll fluorescence imaging. In Photosynthesis, Productivity and Environmental Stress; Wiley: Hoboken, NJ, USA, 2019; pp. 241–266. [Google Scholar]
- Wang, L.; Poque, S.; Valkonen, J.P. Phenotyping viral infection in sweetpotato using a high-throughput chlorophyll fluorescence and thermal imaging platform. Plant Methods 2019, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Vítek, P.; Veselá, B.; Klem, K. Spatial and temporal variability of plant leaf responses cascade after PSII inhibition: Raman, chlorophyll fluorescence and infrared thermal imaging. Sensors 2020, 20, 1015. [Google Scholar] [CrossRef] [PubMed]
- Smigaj, M.; Gaulton, R.; Suarez, J.C.; Barr, S.L. Use of miniature thermal cameras for detection of physiological stress in conifers. Remote Sens. 2017, 9, 957. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Bajons, P.; Klinger, G.; Schlosser, V. Determination of stomatal conductance by means of infrared thermography. Infrared Phys. Technol. 2005, 46, 429–439. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Cho, J.I.; Park, S.H.; Kwon, T.R.; Ahn, B.O.; Lee, G.S.; Jeong, M.J.; Kim, K.W.; Lee, S.K.; Park, S.C. Phenotyping of rice in salt stress environment using high-throughput infrared imaging. Acta Bot. Croat. 2014, 73, 149–158. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Köppl, C.J.; Johnson, M.S.; Gulyas, L.; García, M. Hyperspectral and thermal sensing of stomatal conductance, transpiration, and photosynthesis for soybean and maize under drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Grant, O.M.; Chaves, M.M.; Jones, H.G. Optimizing thermal imaging as a technique for detecting stomatal closure induced by drought stress under greenhouse conditions. Physiol. Plant. 2006, 127, 507–518. [Google Scholar] [CrossRef]
- Grant, O.M.; Tronina, Ł.; Ramalho, J.C.; Kurz Besson, C.; Lobo-do-Vale, R.; Santos Pereira, J.; Jones, H.G.; Chaves, M.M. The impact of drought on leaf physiology of Quercus suber L. trees: Comparison of an extreme drought event with chronic rainfall reduction. J. Exp. Bot. 2010, 61, 4361–4371. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef]
- Jones, H.G.; Leinonen, I. Thermal imaging for the study of plant water relations. J. Agric. Meteorol. 2003, 59, 205–217. [Google Scholar] [CrossRef]
- Dhillon, R.; Rojo, F.; Roach, J.; Upadhyaya, S.; Delwiche, M.A. Continuous leaf monitoring system for precision irrigation management in orchard crops. Tarım Mak. Bilim. Derg. 2014, 10, 267–272. [Google Scholar]
- Prashar, A.; Jones, H.G. Assessing drought responses using thermal infrared imaging. In Environmental Responses in Plants; Humana Press: New York, NY, USA, 2016; pp. 209–219. [Google Scholar]
- Camoglu, G.; Demirel, K.; Genc, L. Use of infrared thermography and hyperspectral data to detect effects of water stress on pepper. Quant. InfraRed Thermogr. J. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Lee, W.; Choi, H.M.; Kim, D.; Honda, Y.; Guo, Y.L.L.; Kim, H. Synergic effect between high temperature and air pollution on mortality in Northeast Asia. Environ. Res. 2019, 178, 108735. [Google Scholar] [CrossRef]
- Yu, M.H.; Ding, G.D.; Gao, G.L.; Zhao, Y.Y.; Yan, L.; Sai, K. Using plant temperature to evaluate the response of stomatal conductance to soil moisture deficit. Forests 2015, 6, 3748–3762. [Google Scholar] [CrossRef]
- Mao, Z.; Jiang, H.; Wang, Y.; Zu, Y.; Voronin, P.Y. Water balance of birch and larch leaves and their resistance to short and progressive soil drought. Russ. J. Plant Physiol. 2004, 51, 697–701. [Google Scholar] [CrossRef]
- Waseem, M.; Ali, A.; Tahir, M.; Nadeem, M.A.; Ayub, M.; Tanveer, A.; Ahmad, R.; Hussain, M. Mechanism of drought tolerance in plant and its management through different methods. Cont. J. Agric. Sci. 2011, 5, 10–25. [Google Scholar]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Santagata, G.; Schettini, E.; Vox, G.; Immirzi, B.; Scarascia Mugnozza, G.; Malinconico, M. Biodegradable spray mulching and nursery pots: New frontiers for research. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Springer: Berlin, Germany, 2017; pp. 105–137. [Google Scholar]
- Sanchez-Aguilar, H.; Aldrete, A.; Vargas-Hernandez, J.; Ordaz-Chaparro, V. Influence of container type and color on seedling growth of pine in nursery. Agrociencia 2016, 50, 481–492. [Google Scholar]
- South, D.B.; Starkey, T.E.; Lyons, A. Why healthy pine seedlings die after they leave the nursery. Forests 2023, 14, 645. [Google Scholar] [CrossRef]
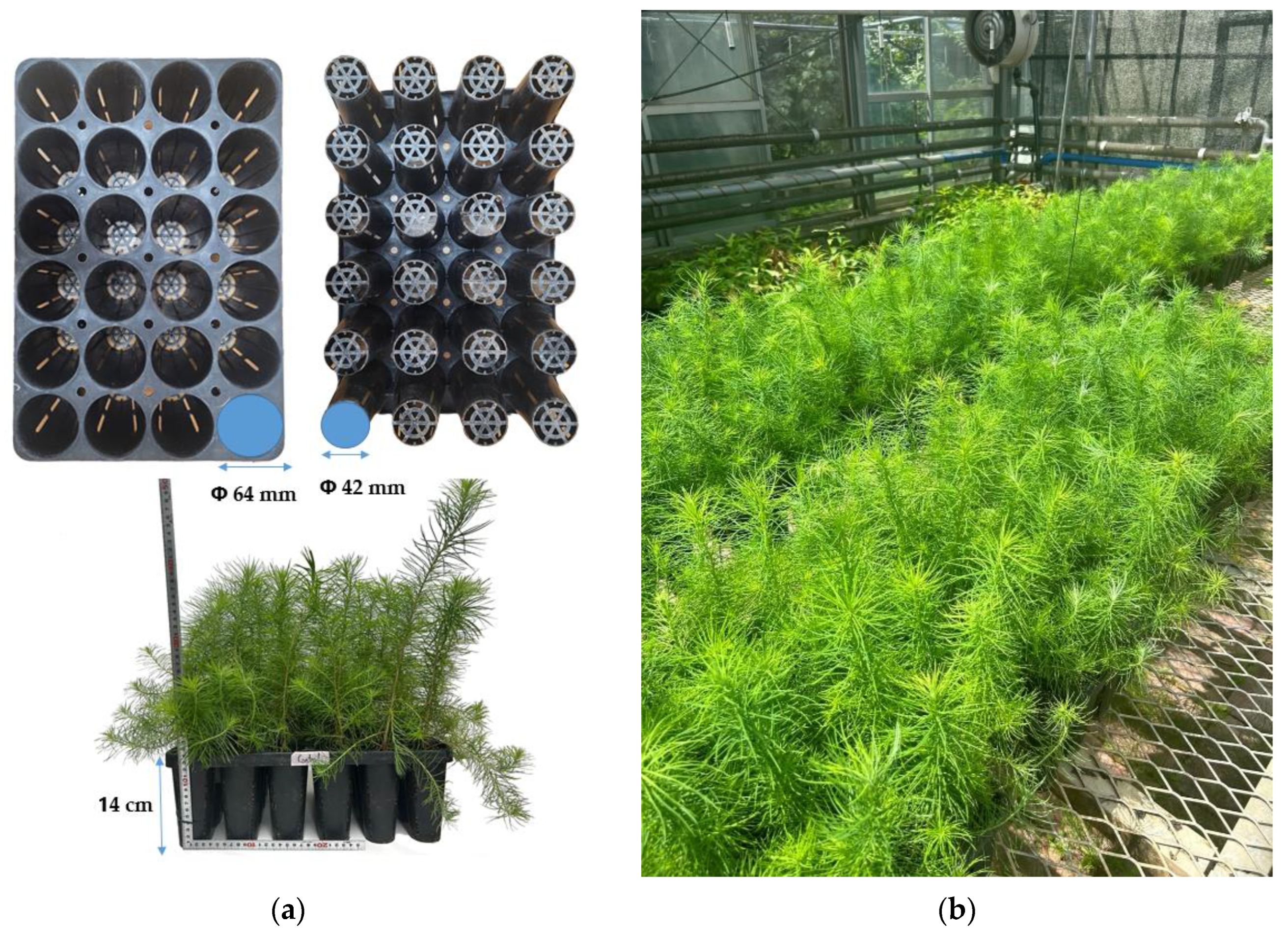
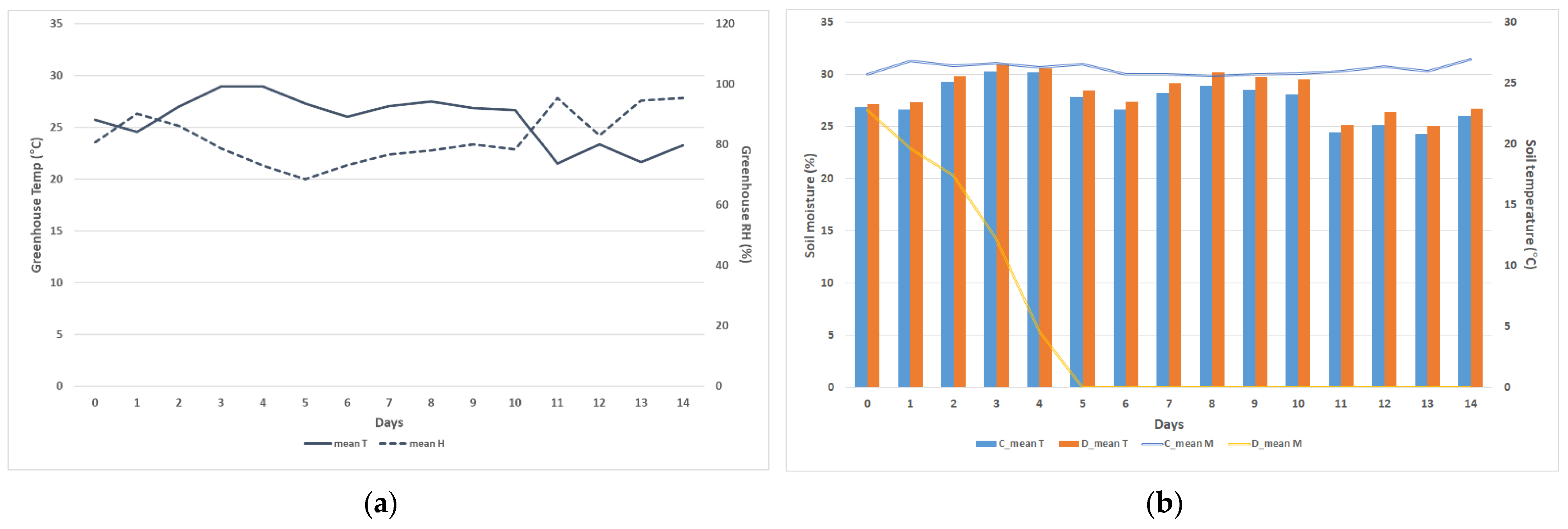
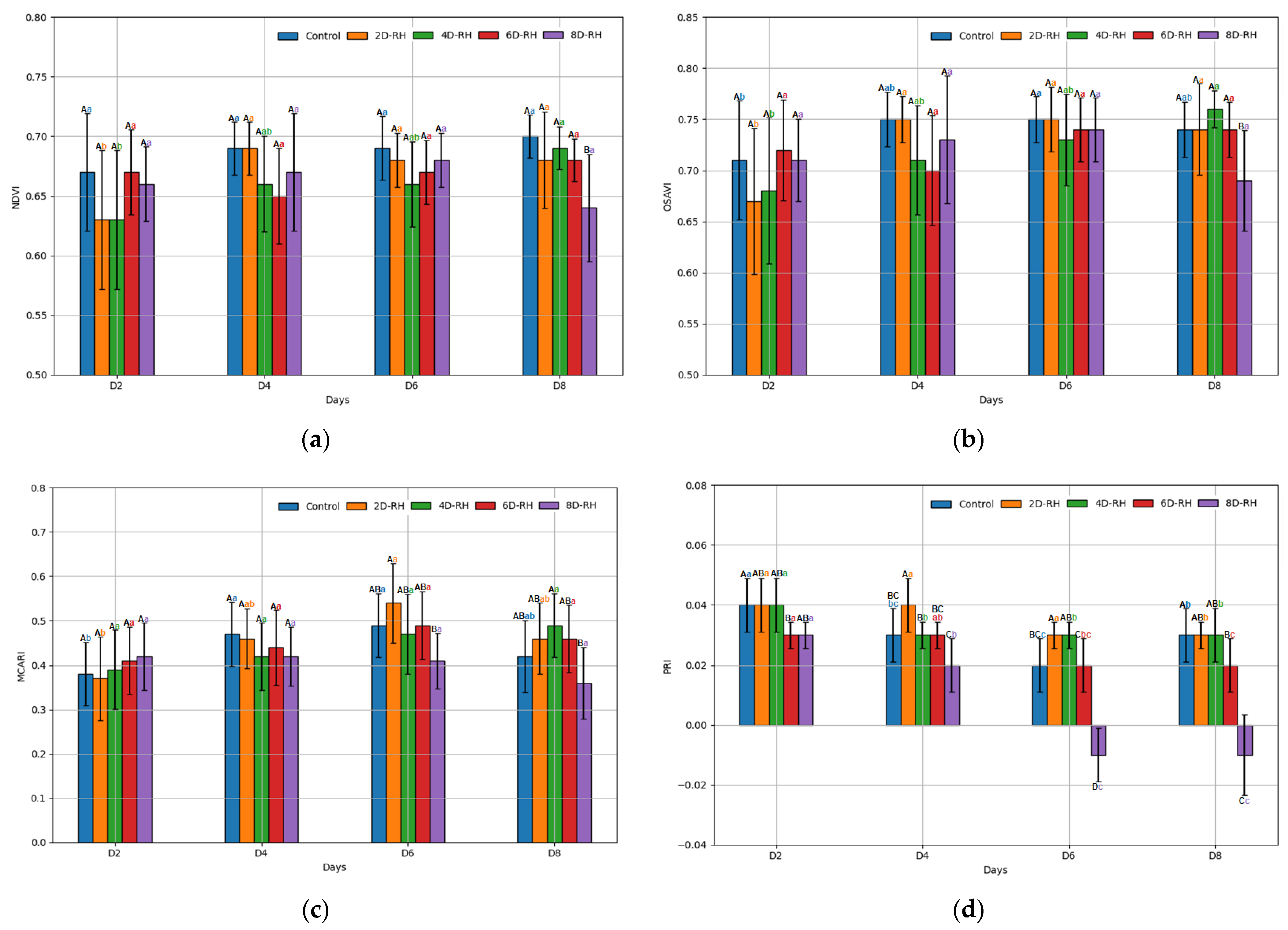

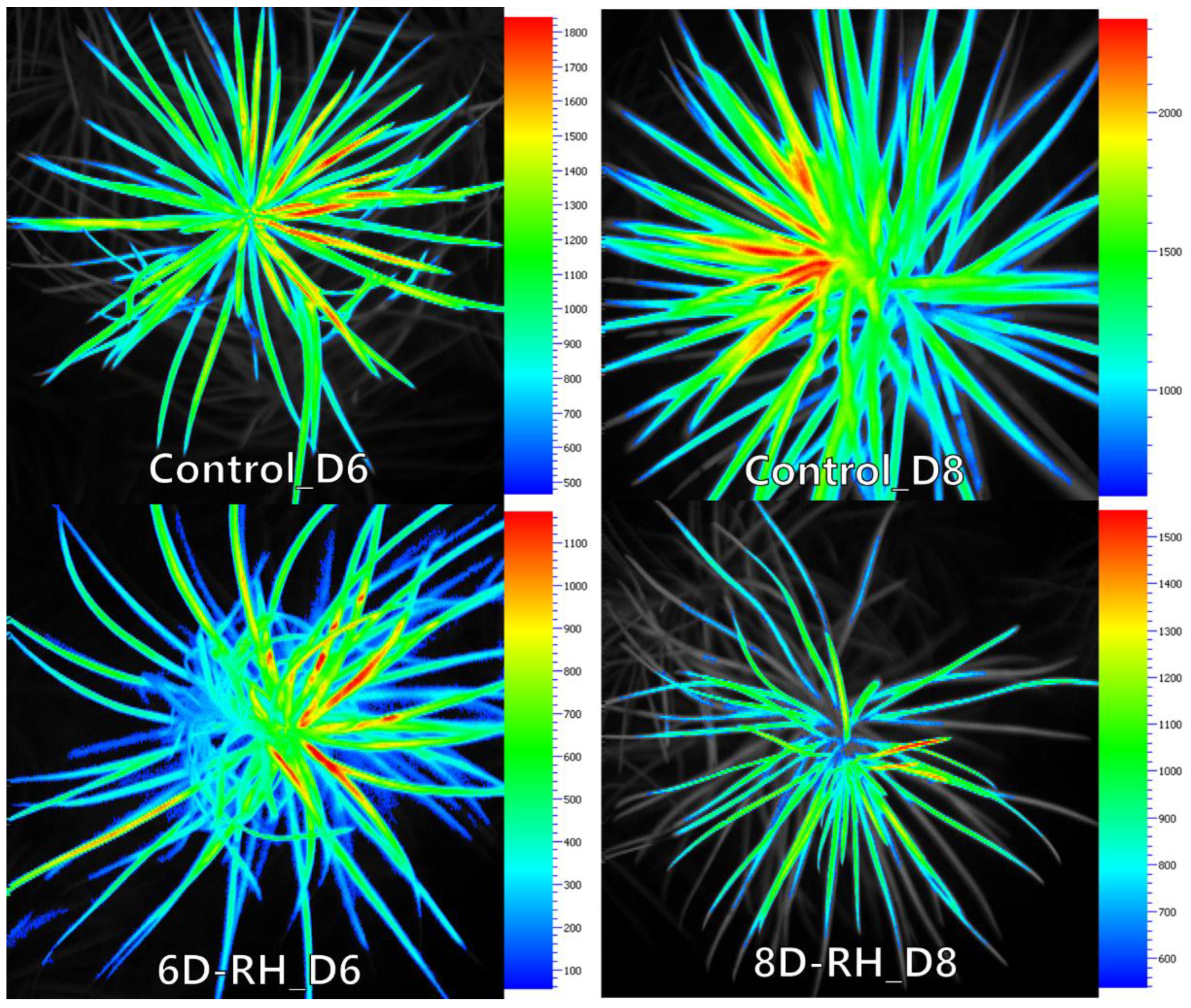

| Bulk Density (g m−3) | pH | EC (ds m−1) | OM (%) | TN (%) | P2O5 (mg kg−1) | Exchangeable Cations (cmolc kg−1) | CEC (cmolc kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | Mg2+ | |||||||
| 0.37 | 6.1 | 0.06 | 4.0 | 0.08 | 3.0 | 0.3 | 3.3 | 2.5 | 24.3 |
| Day | Control | 2D-RH | 4D-RH | 6D-RH | 8D-RH | 10D-RH | 12D-RH | 14D | Average of DTs | |
|---|---|---|---|---|---|---|---|---|---|---|
| D6 | Survival rate (%) | 100 | 100 | 100 | 91.67 | 83.33 | 79.17 | 73.91 | 72.73 | 80.34 |
| Num of SS/TS | 23/23 | 24/24 | 23/23 | 22/24 | 20/24 | 19/24 | 17/23 | 16/22 | 94/117 | |
| D8 | Survival rate (%) | 95.65 | 100 | 100 | 91.67 | 50.00 | 37.50 | 43.48 | 36.36 | 41.94 |
| Num of SS/TS | 22/23 | 24/24 | 23/23 | 22/24 | 12/24 | 9/24 | 10/23 | 8/22 | 39/93 | |
| D14 | Survival rate (%) | 95.65 | 100 | 100 | 83.33 | 45.83 | 25.00 | 34.78 | 18.18 | 18.18 |
| Num of SS/TS | 22/23 | 24/24 | 23/23 | 20/24 | 11/24 | 6/24 | 8/23 | 4/22 | 4/22 |
| Treatment | SH (cm) | DRC (mm) | RWC (%) | |
|---|---|---|---|---|
| D0 | Control | 25.3 ± 3.2 ab | 3.5 ± 0.6 bcd | - |
| 2D-RH | 25.0 ± 3.1 ab | 3.5 ± 0.5 bcd | - | |
| 4D-RH | 25.0 ± 3.3 ab | 3.4 ± 0.5 cd | - | |
| 6D-RH | 25.0 ± 3.1 ab | 3.5 ± 0.5 bcd | - | |
| 8D-RH | 25.2 ± 3.5 ab | 3.8 ± 0.6 abc | - | |
| 10D-RH | 25.2 ± 3.2 ab | 3.8 ± 0.6 abc | - | |
| 12D-RH | 25.9 ± 3.3 ab | 3.5 ± 0.4 bcd | - | |
| 14D | 25.5 ± 3.0 ab | 3.6 ± 0.4 abcd | - | |
| D14 | Control | 29.4 ± 3.8 a | 4.3 ± 0.7 a | 75.65 ± 1.11 a |
| 2D-RH | 29.0 ± 5.2 ab | 4.1 ± 0.6 ab | 75.33 ± 1.14 a | |
| 4D-RH | 28.8 ± 4.0 ab | 3.9 ± 0.5 abc | 75.06 ± 1.12 a | |
| 6D-RH | 29.1 ± 2.9 ab | 4.1 ± 0.4 abc | 71.37 ± 1.90 b | |
| 8D-RH | 27.8 ± 2.9 ab | 3.6 ± 0.6 abcd | 58.60 ± 3.73 c | |
| 10D-RH | 24.5 ± 4.4 b | 3.4 ± 0.4 cd | 43.27 ± 4.30 d | |
| 12D-RH | 27.3 ± 4.4 ab | 3.8 ± 0.5 abc | 35.99 ± 3.15 e | |
| 14D | 25.6 ± 0.8 ab | 3.0 ± 0.0 d | 13.10 ± 1.50 f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, U.; Han, S.H.; Kim, D.; Kim, S.; Cheong, E.J. Exploring the Efficient Irrigation Period for Larix kaempferi Seedlings in Nursery Pots in Greenhouse Conditions Using Optical Measurements. Forests 2024, 15, 1303. https://doi.org/10.3390/f15081303
Jeong U, Han SH, Kim D, Kim S, Cheong EJ. Exploring the Efficient Irrigation Period for Larix kaempferi Seedlings in Nursery Pots in Greenhouse Conditions Using Optical Measurements. Forests. 2024; 15(8):1303. https://doi.org/10.3390/f15081303
Chicago/Turabian StyleJeong, Ukhan, Seung Hyun Han, Dohee Kim, Sohyun Kim, and Eun Ju Cheong. 2024. "Exploring the Efficient Irrigation Period for Larix kaempferi Seedlings in Nursery Pots in Greenhouse Conditions Using Optical Measurements" Forests 15, no. 8: 1303. https://doi.org/10.3390/f15081303
APA StyleJeong, U., Han, S. H., Kim, D., Kim, S., & Cheong, E. J. (2024). Exploring the Efficient Irrigation Period for Larix kaempferi Seedlings in Nursery Pots in Greenhouse Conditions Using Optical Measurements. Forests, 15(8), 1303. https://doi.org/10.3390/f15081303






