Discrepancies and Evaluation of Needle-Leaf and Tracheid Traits of Qinhai Spruce in Qilian Mountains, Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Laboratory Analysis
2.3. Data Processing
3. Results
3.1. Multiple Comparisons of Tracheid and Needle-Leaf Traits
3.2. Analysis of Variation in Tracheid and Needle-Leaf Traits
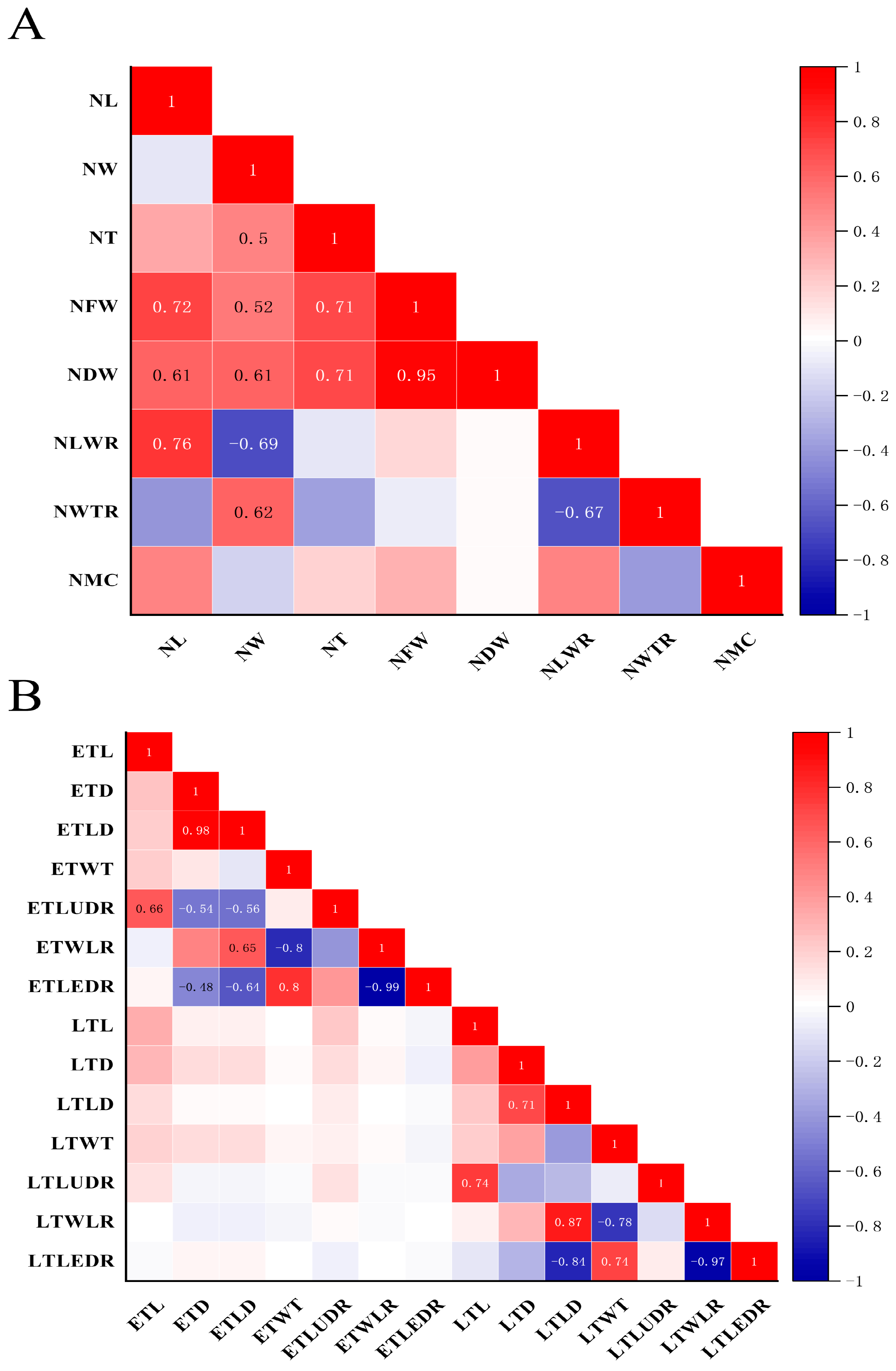
3.3. Correlation Analysis of Tracheid and Needle-Leaf Traits
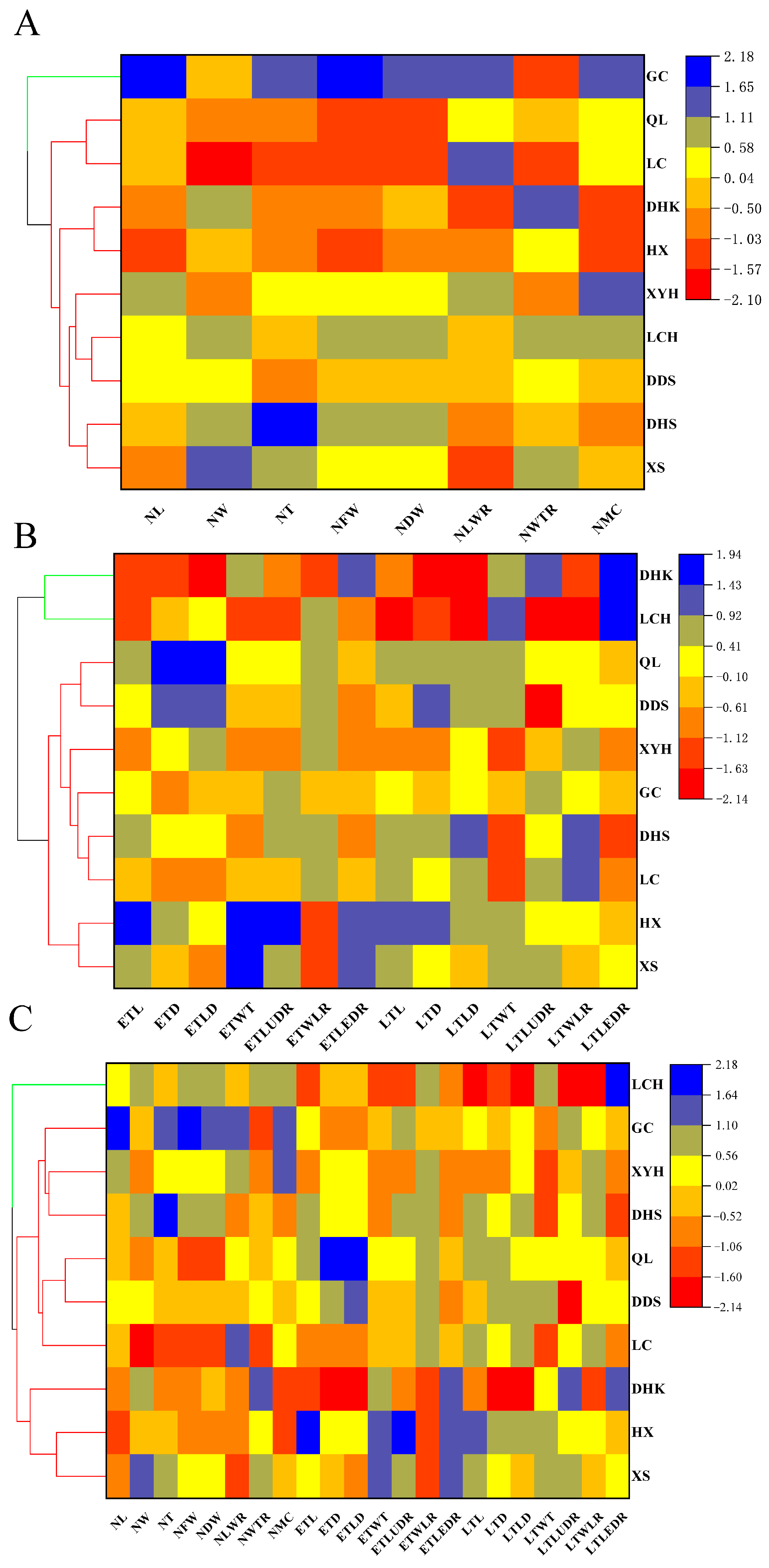
3.4. Cluster Analysis of Tracheid and Needle-Leaf Traits
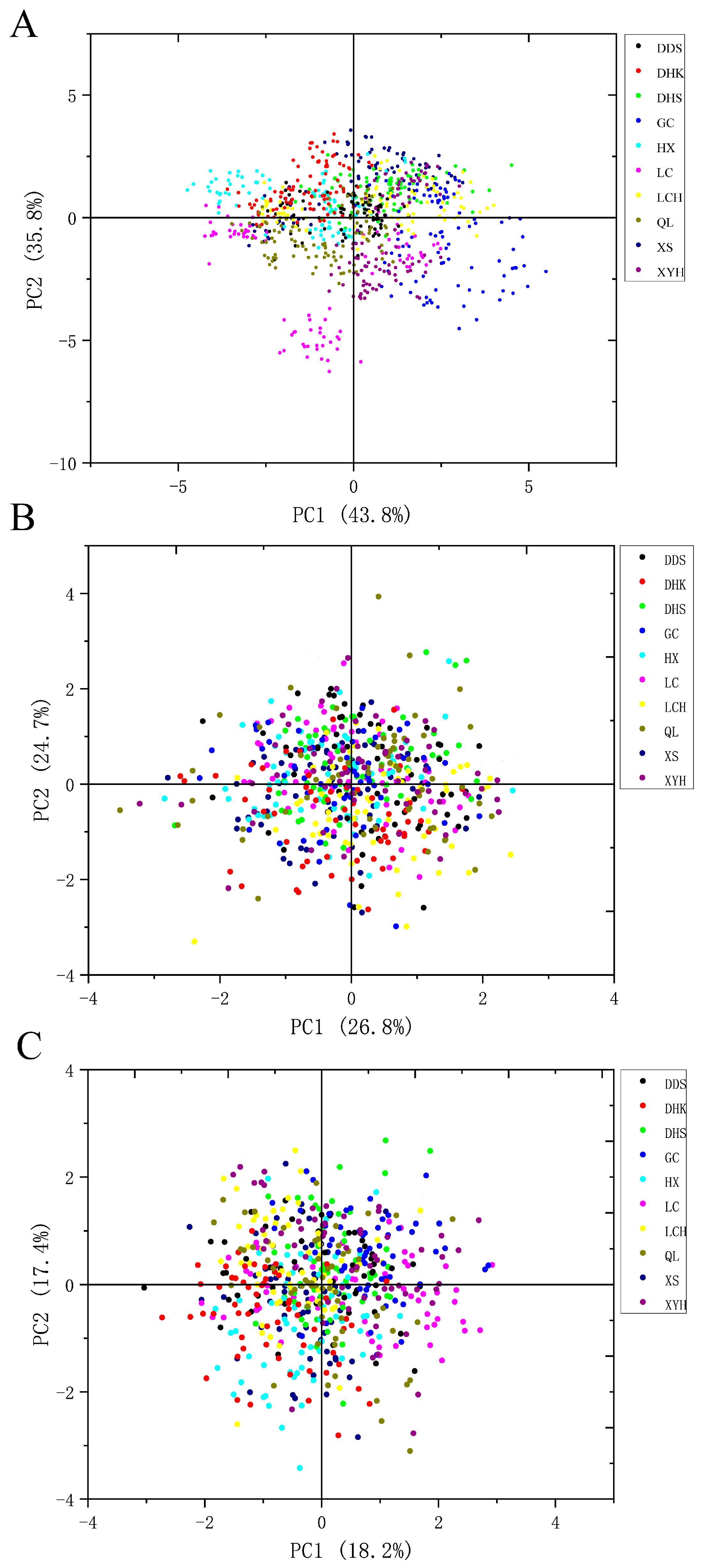
3.5. Principal Component Analysis and Comprehensive Evaluation of Tracheid and Needle-Leaf Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, F.; Wen, S.; Wei, S.; Ou, H.B.; Wang, Z.H. Variation Analysis of Seed and Leaf Phenotypic Traits of Albizia Odoratissima from Different Provenances. J. Cent. South Univ. For. Technol. 2023, 2, 1–10. [Google Scholar] [CrossRef]
- Varghese, M.; Harwood, C.E.; Bush, D.J.; Baltunis, B.; Kamalakannan, R.; Suraj, P.G.; Hegde, D.; Meder, R. Growth and wood properties of natural provenances, local seed sources and clones of Eucalyptus camaldulensis in southern India: Implications for breeding and deployment. New For. 2017, 48, 67–82. [Google Scholar] [CrossRef]
- Perek, M.; Hodge, G.; Tambarussi, E.V.; Biernaski, F.A.; Acosta, J. Predicted genetic gains for growth traits and wood resistance in Pinus maximinoi and Pinus tecunumanii. Crop Breed. Appl. Biotechnol. 2022, 22, e391022213. [Google Scholar] [CrossRef]
- Gao, J.; Yang, B.; Peng, X.; Rossi, S. Tracheid development under a drought event producing intra-annual density fluctuations in the semi-arid China. Agric. For. Meteorol. 2021, 308, 108572. [Google Scholar] [CrossRef]
- Chin, A.R.; Sillett, S.C. Phenotypic plasticity of leaves enhances water-stress tolerance and promotes hydraulic conductivity in a tall conifer. Am. J. Bot. 2016, 103, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhao, G.H.; Zhang, L.S.; Sun, X.Y.; Yin, S.P.; De, Y.L.; Li, Y.; Mi, Z.; Zhao, X. Genetic and variation analyses of growth traits of half-sib Larix olgensis families in northeastern China. Euphytica 2016, 212, 387–397. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, H.; Tan, J.; Feng, Y.; Huang, Y. Selection of superior families of Pinus massoniana in southern China for large-diameter construction timber. J. For. Res. 2020, 31, 475–484. [Google Scholar] [CrossRef]
- Benavides, R.; Carvalho, B.; Bastias, C.C.; López-Quiroga, D.; Mas, A.; Cavers, S.; Gray, A.; Albet, A.; Alía, R.; Ambrosio, O.; et al. The GenTree Leaf Collection: Inter-and intraspecific leaf variation in seven forest tree species in Europe. Glob. Ecol. Biogeogr. 2021, 30, 590–597. [Google Scholar] [CrossRef]
- Zhao, R.; Chu, X.; He, Q.; Liu, W.; Zhu, Z. Leaf phenotypic variation in natural populations of Carpinus tschonoskii in China. J. For. Res. 2023, 34, 1591–1602. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, X.Y. Phenotypic diversity of natural populations in Picea crassifolia in Qilian mountains. Sci. Silvae Sin. 2008, 44, 70–77. [Google Scholar]
- Zhou, C.; Guo, Y.; Chen, Y.; Zhang, H.; El-Kassaby, Y.A.; Li, W. Genome Wide Association Study Identifies Candidate Genes Related to the Earlywood Tracheid Properties in Picea crassifolia Kom. Forests 2022, 13, 332. [Google Scholar] [CrossRef]
- Qin, H.; Jiao, L.; Zhou, Y.; Wu, J.; Che, X. Elevation affects the ecological stoichiometry of Qinghai spruce in the Qilian Mountains of northwest China. Front. Plant Sci. 2022, 13, 917755. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Yang, B.; Li, G. Assessing the influence of local environment, regional climate and tree species on radial growth in the Hexi area of arid northwest China. Front. Plant Sci. 2022, 13, 1046462. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Han, Y.; Yuan, S.; Gao, Y.; Rong, Z.; Zhao, C. Simulating the canopy photosynthesis of Qinghai spruce (Picea crassifolia Kom.) in the Qilian Mountains, Northwestern China. New For. 2022, 53, 511–531. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, B.P.; Zhao, F.; Yao, Y.H.; Zhang, S.; Qi, W.W. Omni-directional distribution patterns of montane coniferous forest in the Helan Mountains of China. J. Mt. Sci. 2013, 10, 724–733. [Google Scholar] [CrossRef]
- Bakeer, S.M.; Makpoul, K.R.; Abou-Ellail, M. Evaluation of ten jojoba clones (Simmondsia chinensis) grown under Middle Sinai conditions. Ind. Crops Prod. 2017, 107, 30–37. [Google Scholar] [CrossRef]
- Ji, M.; Deng, J.; Yao, B.; Chen, R.; Fan, Z.; Guan, J.; Li, X.; Wu, F.; Niklas, K.J. Ecogeographical variation of 12 morphological traits within Pinus tabulaeformis: The effects of environmental factors and demographic histories. J. Plant Ecol. 2017, 10, 386–396. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Cortesao, M.; Garcia, C. Ecological marginalization facilitated diversification in conifers. Evol. Biol. 2015, 42, 146–155. [Google Scholar] [CrossRef]
- Weng, Y.H.; Liu, K.J.; Chen, Y.B.; Li, Y.; Wang, J.; Meng, Q.F. Variation in cone and seed traits in a clonal seed orchard of red pine (Pinus koraiensis Sieb. et Zucc.). Scand. J. For. Res. 2020, 35, 1–9. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, H.; Zhang, L.; Guan, C. Genetic variation of 17-year-old hybrid larch and its superior family selection. J. Northeast For. Univ. 2010, 38, 8–11. [Google Scholar] [CrossRef]
- Dong, X.S.; Chen, X.X.; Gai, Z.S.; Zhai, J.T.; Guo, X.F.; Han, X.L.; Zhang, S.H.; Wu, Z.H.; Li, Z.J. Phenotypic Diversity and Variation in Natural Populus euphratica Populations Shaped by Environmental Factors. Contemp. Probl. Ecol. 2023, 16, 230–252. [Google Scholar] [CrossRef]
- Garcia, R.; Siepielski, A.M.; Benkman, C.W. Cone and seed trait variation in whitebark pine (Pinus albicaulis; Pinaceae) and the potential for phenotypic selection. Am. J. Bot. 2009, 96, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Lv, D.; Zhao, M.; Zhao, X.P.; Zhao, H.; Li, W. Genetic structure analysis of Picea crassifolia based on genome-wide SNP molecular markers. Bull. Bot. Res. 2022, 42, 373–382. [Google Scholar]
- Chen, Y.L.; Lv, D.; Zhao, M.; Zhao, H.; Zhao, X.P.; Li, W. Half-sib progeny test and evaluation of parents of Picea crassifolia. J. Beijing For. Univ. 2021, 43, 17–24. [Google Scholar] [CrossRef]
- Yücedağ, C.; Gezer, A.; Orhan, H. The genetic variation in Crimean juniper populations from the Lakes District of Turkey. Rom. Biotechnol. Lett. 2010, 15, 5487–5492. [Google Scholar]
- Li, Q.; Wang, J.; Li, D.; Hu, C.; Qi, S. Genetic variation of wood traits in asexual lines of Qinghai spruce. J. Northeast For. Univ. 2015, 43, 12–16+35. [Google Scholar] [CrossRef]
- Bodare, S. Conservation Genetics and Speciation in Asian Forest Trees. Doctoral Dissertation, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2013. [Google Scholar]
- Zhang, S.; Zhang, H.; Ding, L.; Xia, Y.; Dai, W.; Han, X.; Siqin, T.; You, X. Evaluation and Selection of Excellent Provenances of Eleutherococcus senticosus. Forests 2023, 14, 1359. [Google Scholar] [CrossRef]
- Donaldson, L.A. Within-and between-tree variation in microfibril angle in Pinus radiata. N. Z. J. For. Sci. 1992, 22, 77–86. [Google Scholar]
- Pan, Y.; Li, S.; Wang, C.; Ma, W.; Xu, G.; Shao, L.; Li, K.; Zhao, X.; Jiang, T. Early evaluation of growth traits of Larix kaempferi clones. J. For. Res. 2018, 29, 1031–1039. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Liu, R.J.; Yao, L.H.; Guo, Y. Compative ative study on the tracheid moprphology and basic density of clonal chinese fir. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2020, 41, 54–60. [Google Scholar] [CrossRef]
- Gao, X.X.; Chen, J.; Xu, R.; Yu, J.; Yang, C.M.; Peng, F.; Zhang, Y.Q.; Peng, Y.L. Variation and correlation analysis on phenotypic traits of Scutellaria baicalensis from different strains. China J. Chin. Mater. Medica 2016, 41, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Y.F.; Tang, L.; Yang, C.Y.; Li, R.Y.; Ma, X.J. Phenotypic Trait Variation, Principal Component, Correlation and Path Analysis of Paris polyphylla var. Yunnanensis. J. Chin. Med. Mater. 2015, 38, 1339–1342. [Google Scholar] [CrossRef]
- Du, Q.; Xu, B.; Gong, C.; Yang, X.; Pan, W.; Tian, J.; Li, B.; Zhang, D. Variation in growth, leaf, and wood property traits of Chinese white poplar (Populus tomentosa), a major industrial tree species in Northern China. Can. J. For. Res. 2014, 44, 326–339. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ma, J.; Zhang, X.; Xu, L.A. Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area. J. For. Res. 2018, 29, 35–44. [Google Scholar] [CrossRef]
- Popović, V.; Nikolić, B.; Lučić, A.; Rakonjac, L.; Šešlija Jovanović, D.; Miljković, D. Morpho-anatomical trait variability of the Norway spruce (Picea abies (L.) Karst.) needles in natural populations along elevational diversity gradient. Trees 2022, 36, 1131–1147. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Xin, Y.; Wang, F.; Xin, P.; Gao, J. Needle phenotype variation among natural populations of Pinus yunnanensis, P. kesiya var. langbianensis and P. kesiya. Chin. J. Plant Ecol. 2023, 47, 833–846. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.Q.; Sun, P.; Huang, X.J.; Li, S.Q.; Huang, C.L. Phenotypic variation in natural population of Picea asperata. J. Northeast For. Univ. 2003, 31, 9–11. [Google Scholar]
- Luo, J.; Gu, W.C. Cone and seed variation of natural population in Picea asperata. J. Northwest Sci-Tech Univ. Agri. For. 2004, 32, 60–66. [Google Scholar]
- Luo, J.; Gu, W.C. Study on phenotypic diversity of natural population in Picea asperata. Sci. Silvae Sin. 2005, 41, 66–73. [Google Scholar]
- Meng, J.; Chen, X.; Huang, Y.; Wang, L.; Xing, F.; Li, Y. Environmental contribution to needle variation among natural populations of Pinus tabuliformis. J. For. Res. 2019, 30, 1311–1322. [Google Scholar] [CrossRef]
- Lewandowska, A.; Boratynska, K.; Marcysiak, K.; Gómez, D.; Romo, A.; Malicki, M.; Iszkulo, G.; Boratynski, A. Phenotypic differentiation of Rhododendron ferrugineum populations in European mountains. Dendrobiology 2022, 87, 1–12. [Google Scholar] [CrossRef]
- Gülcü, S.; Üçler, A.Ö. Genetic variation of Anatolian black pine (Pinus nigra Arnold. subsp. pallasiana (Lamb.) Holmboe) in the lakes district of Turkey. Silvae Genet. 2008, 57, 1–5. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, Y.; Zhang, J.; Li, Y. Variation of phenotypic and physiological traits of Robinia pseudoacacia L. from 20 provenances. PLoS ONE 2022, 17, e0262278. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, I.R.; Wang, T.; Hamann, A.; Smets, P.; Aitken, S.N. Selective breeding of lodgepole pine increases growth and maintains climatic adaptation. For. Ecol. Manag. 2017, 391, 404–416. [Google Scholar] [CrossRef]
- Liu, K.; Han, Y.; Wang, X.; Li, L.; Shen, Q.; Zheng, Y.; Rong, J.; Chen, L. Difference and evaluation of grow traits of Fokienia hodginsii caused by different provenances. J. Cent. South Univ. For. Technol. 2023, 43, 62–72. [Google Scholar] [CrossRef]
- He, Q.; Yang, S.; Li, Y.; Shen, X.; Liu, X. Phenotypic variations in seed and fruit traits of Liquidambar formosana populations. Chin. J. Plant Ecol. 2018, 42, 752–763. [Google Scholar] [CrossRef]
- Metougui, M.L.; Mokhtari, M.; Maughan, P.J.; Jellen, E.N.; Benlhabib, O. Morphological variability, heritability and correlation studies within an argan tree population (Argania spinosa (L.) Skeels) preserved in situ. Int. J. Agric. For. 2017, 7, 42–51. [Google Scholar]
- Chen, Y. Selection of Superior Half-Sib Families and Genome-Wide Association Study of Important Traits in Picea crassifolia. Master’s Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar] [CrossRef]
- Kaviriri, D.K.; Li, Y.; Zhang, D.; Li, H.; Fan, Z.; Wang, J.; Wang, L.; Wang, Q.; Wang, D.; Chiang, V.L.; et al. Clonal variations in cone, seed and nut traits in a Pinus koraiensis seed orchard in Northeast China. J. For. Res. 2021, 32, 171–179. [Google Scholar] [CrossRef]
- Barzdajn, W. An assessment of diagnostic value of morphological traits of Norway spruce (Picea abies (L.) Karst.) cones for discrimination of spruce provenances. Sylwan 1996, 140, 61–75. [Google Scholar]
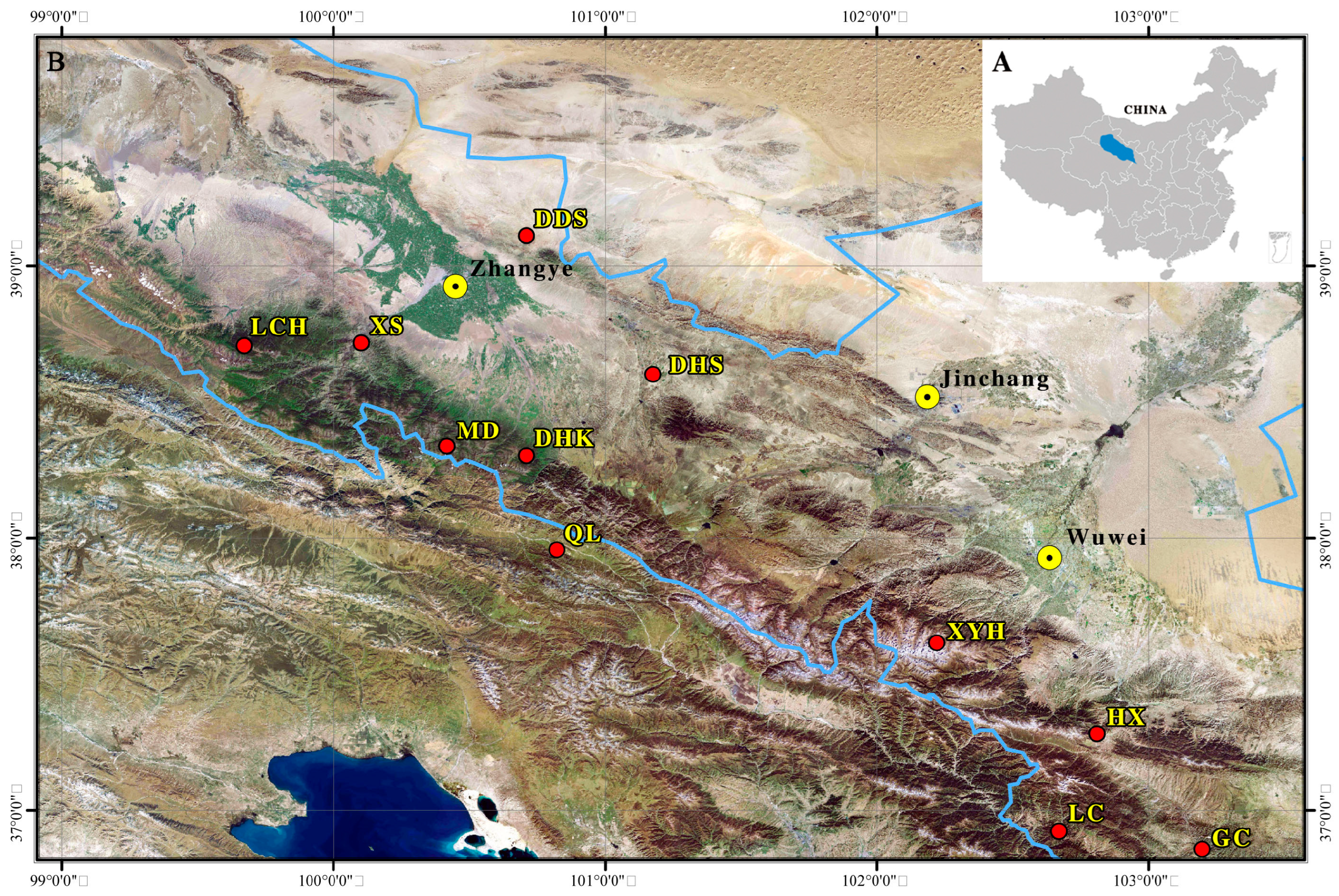

| Material Sources | Longitude | Latitude | Elevation (m) | Quantity |
|---|---|---|---|---|
| XS | 100°03′~100°23′ | 38°23′~38°48′ | 2735 | 5 |
| LC | 102°26′~102°55′ | 36°33′~36°48′ | 2790 | 5 |
| DHS | 101°00′~101°30′ | 38°20′~38°30′ | 2752 | 5 |
| HX | 102°01′~102°51′ | 37°16′~37°45′ | 2613 | 5 |
| DDS | 100°45′~100°51′ | 39°00′~39°04′ | 2712 | 5 |
| LCH | 99°32′~100°01′ | 38°39′~38°56′ | 2589 | 5 |
| GC | 102°26′~102°51′ | 36°45′~37°07′ | 2595 | 5 |
| DHK | 100°31′~100°56′ | 37°54′~38°31′ | 2468 | 5 |
| QL | 102°06′~102°25′ | 37°31′~37°51′ | 2485 | 5 |
| XYH | 102°24′~102°66′ | 36°57′~37°25′ | 2605 | 5 |
| Needle-Leaf | Abridge | Earlywood | Abridge | Latewood | Abridge |
|---|---|---|---|---|---|
| Fresh weight | NFW | Length | ETL | Length | LTL |
| Length | NL | Diameter | ETD | Diameter | LTD |
| Width | NW | Lumen diameter | ETLD | Lumen diameter | LTLD |
| Thickness | NT | Wall thickness | ETWT | Wall thickness | LTWT |
| Dry weight | NDW | Length/diameter ratio | ETLEDR | Length/diameter ratio | LTLEDR |
| Length/width ratio | NLWR | Lumen/diameter ratio | ETLUDR | Lumen/diameter ratio | LTLUDR |
| Width/thickness ratio | NWTR | Wall/lumen ratio | ETWLR | Wall/lumen ratio | LTWLR |
| Moisture content | NMC |
| Provenance | Earlywood Tracheid (μm) | ||||||
|---|---|---|---|---|---|---|---|
| ETL | ETD | ETLD | ETWT | ETLEDR | ETLUDR | ETWLR | |
| XS | 3370.68 ± 63.38 b | 52.18 ± 0.86 bcd | 44.33 ± 0.85 cd | 7.85 ± 0.22 ab | 65.48 ± 1.54 ab | 0.85 ± 0.04 c | 0.18 ± 0.01 a |
| LC | 3101.49 ± 70.41 c | 51.13 ± 0.90 cd | 44.31 ± 0.86 cd | 6.83 ± 0.19 cde | 61.64 ± 1.62 bcd | 0.87 ± 0.01 a | 0.16 ± 0.01 c |
| DHS | 3402.97 ± 65.54 b | 52.82 ± 1.01 bc | 46.16 ± 1.01 abc | 6.66 ± 0.18 de | 65.87 ± 1.81 ab | 0.87 ± 0.01 a | 0.15 ± 0.01 c |
| HX | 3674.02 ± 60.06 a | 53.51 ± 0.88 abc | 45.59 ± 0.92 bc | 7.91 ± 0.22 a | 69.51 ± 1.44 a | 0.85 ± 0.01 bc | 0.18 ± 0.01 ab |
| DDS | 3303.31 ± 73.26 b | 54.54 ± 1.14 ab | 47.64 ± 1.11 ab | 6.90 ± 0.19 cde | 61.39 ± 1.50 bcd | 0.87 ± 0.01 a | 0.15 ± 0.01 c |
| LCH | 2906.80 ± 85.47 c | 51.81 ± 1.14 bcd | 45.40 ± 1.34 bc | 6.40 ± 0.19 e | 56.76 ± 1.66 d | 0.87 ± 0.01 a | 0.15 ± 0.01 c |
| GC | 3304.39 ± 56.70 b | 51.39 ± 0.89 bcd | 44.39 ± 0.89 cd | 7.01 ± 0.18 cde | 65.32 ± 1.53 ab | 0.86 ± 0.01 ab | 0.16 ± 0.01 c |
| DHK | 2924.40 ± 55.41 c | 49.58 ± 0.85 d | 42.21 ± 0.82 d | 7.37 ± 0.24 abc | 59.66 ± 1.28 cd | 0.85 ± 0.01 bc | 0.18 ± 0.01 ab |
| QL | 3396.29 ± 50.89 b | 56.20 ± 1.32 a | 48.94 ± 1.33 a | 7.26 ± 0.17 bcd | 62.82 ± 2.07 bc | 0.87 ± 0.01 a | 0.16 ± 0.01 c |
| XYH | 3056.54 ± 63.75 c | 52.99 ± 1.20 bc | 46.31 ± 1.21 abc | 6.68 ± 0.23 de | 58.75 ± 1.44 cd | 0.87 ± 0.01 a | 0.15 ± 0.01 c |
| F-value | 13.805 ** | 3.292 ** | 3.418 ** | 6.196 ** | 5.809 ** | 4.805 ** | 4.595 ** |
| Provenance | Latewood Tracheid (μm) | ||||||
|---|---|---|---|---|---|---|---|
| LTL | LTD | LTLD | LTWT | LTLEDR | LTLUDR | LTWLR | |
| XS | 3637.78 ± 64.19 ab | 40.67 ± 0.61 abc | 20.53 ± 0.51 b | 20.14 ± 0.41 a | 90.47 ± 1.94 a | 0.50 ± 0.01 b | 1.03 ± 0.04 b |
| LC | 3635.91 ± 64.17 ab | 40.84 ± 0.61 abc | 22.20 ± 0.61 ab | 18.64 ± 0.42 bc | 89.59 ± 1.59 a | 0.54 ± 0.01 a | 0.89 ± 0.04 bc |
| DHS | 3645.44 ± 68.45 ab | 40.99 ± 0.51 ab | 22.36 ± 0.50 a | 18.63 ± 0.40 bc | 89.34 ± 1.70 a | 0.54 ± 0.01 a | 0.87 ± 0.03 c |
| HX | 3728.44 ± 77.22 a | 41.96 ± 0.49 a | 21.98 ± 0.49 ab | 19.98 ± 0.42 ab | 89.03 ± 1.70 a | 0.52 ± 0.01 ab | 0.95 ± 0.03 bc |
| DDS | 3456.91 ± 79.43 bc | 41.91 ± 0.48 a | 21.74 ± 0.66 ab | 20.17 ± 0.49 a | 82.87 ± 1.92 b | 0.52 ± 0.01 ab | 1.01 ± 0.05 b |
| LCH | 3161.01 ± 93.30 d | 38.05 ± 0.56 de | 17.74 ± 0.53 c | 20.31 ± 0.35 a | 82.82 ± 1.94 b | 0.46 ± 0.01 c | 1.22 ± 0.05 a |
| GC | 3598.50 ± 58.38 abc | 40.00 ± 0.65 bc | 20.93 ± 0.61 ab | 19.07 ± 0.34 abc | 90.88 ± 1.70 a | 0.52 ± 0.01 ab | 0.98 ± 0.04 bc |
| DHK | 3404.44 ± 50.38 c | 37.47 ± 0.54 e | 17.58 ± 0.47 c | 19.89 ± 0.50 ab | 91.84 ± 1.79 a | 0.47 ± 0.01 c | 1.19 ± 0.05 a |
| QL | 3665.32 ± 76.60 ab | 41.57 ± 0.63 ab | 21.65 ± 0.66 ab | 19.92 ± 0.61 ab | 88.70 ± 1.86 a | 0.52 ± 0.01 ab | 0.99 ± 0.05 bc |
| XYH | 3389.00 ± 66.38 c | 39.19 ± 0.53 cd | 20.86 ± 0.54 ab | 18.34 ± 0.43 d | 86.90 ± 1.68 ab | 0.53 ± 0.01 ab | 0.93 ± 0.04 bc |
| F-value | 6.110 ** | 7.779 ** | 9.620 ** | 2.888 * | 3.091 * | 7.077 ** | 7.320 ** |
| Provenance | NL (mm) | NW (mm) | NT (mm) | NFW (mg) | NDW (mg) | NLWR | NWTR | NMC% |
|---|---|---|---|---|---|---|---|---|
| XS | 15.10 ± 0.22 e | 1.76 ± 0.02 a | 1.26 ± 0.01 b | 21.22 ± 0.58 c | 9.75 ± 0.25 c | 8.61 ± 0.10 f | 1.39 ± 0.01 bc | 53.78 ± 0.30 bc |
| LC | 15.92 ± 0.27 d | 1.26 ± 0.02 g | 1.09 ± 0.02 f | 15.49 ± 0.55 f | 7.01 ± 0.26 g | 13.06 ± 0.36 b | 1.16 ± 0.02 f | 54.69 ± 0.55 b |
| DHS | 15.56 ± 0.19 de | 1.69 ± 0.01 b | 1.35 ± 0.01 a | 22.58 ± 0.38 b | 10.63 ± 0.19 b | 9.26 ± 0.14 e | 1.25 ± 0.01 d | 52.90 ± 0.17 c |
| HX | 13.7 ± 0.19 g | 1.56 ± 0.01 cd | 1.16 ± 0.01 d | 16.40 ± 0.38 f | 7.96 ± 0.17 f | 8.81 ± 0.13 ef | 1.36 ± 0.02 c | 51.16 ± 0.32 d |
| DDS | 16.58 ± 0.10 c | 1.58 ± 0.01 c | 1.16 ± 0.01 d | 19.97 ± 0.29 d | 9.14 ± 0.08 de | 10.59 ± 0.12 d | 1.36 ± 0.01 c | 53.59 ± 0.60 bc |
| LCH | 16.95 ± 0.30 c | 1.65 ± 0.01 b | 1.17 ± 0.01 d | 23.03 ± 0.75 b | 10.03 ± 0.29 c | 10.28 ± 0.17 d | 1.42 ± 0.01 b | 55.99 ± 0.25 a |
| GC | 20.16 ± 0.26 a | 1.52 ± 0.02 de | 1.32 ± 0.01 a | 26.36 ± 0.35 a | 11.40 ± 0.16 a | 13.61 ± 0.32 a | 1.15 ± 0.01 f | 56.72 ± 0.24 a |
| DHK | 14.40 ± 0.07 f | 1.67 ± 0.01 b | 1.13 ± 0.01 e | 17.88 ± 0.25 e | 8.65 ± 0.15 e | 8.66 ± 0.08 f | 1.49 ± 0.01 a | 51.64 ± 0.33 d |
| QL | 15.52 ± 0.20 de | 1.45 ± 0.01 f | 1.16 ± 0.01 de | 16.33 ± 0.33 f | 7.35 ± 0.13 g | 10.76 ± 0.15 d | 1.26 ± 0.13 d | 54.61 ± 0.38 b |
| XYH | 17.80 ± 0.11 b | 1.48 ± 0.02 ef | 1.22 ± 0.01 c | 21.98 ± 0.35 bc | 9.59 ± 0.23 cd | 12.28 ± 0.20 c | 1.21 ± 0.01 e | 56.80 ± 0.37 a |
| F-value | 80.108 ** | 73.481 ** | 52.260 ** | 63.212 ** | 50.398 ** | 87.787 ** | 71.004 ** | 27.501 ** |
| Trait | Mean | Maximum | Minimum | SD | CV (%) |
|---|---|---|---|---|---|
| NL/mm | 16.17 | 26.43 | 10.66 | 2.61 | 16.17 |
| NW/mm | 1.56 | 2.09 | 0.92 | 0.21 | 13.54 |
| NT/mm | 1.20 | 1.52 | 0.83 | 0.14 | 11.33 |
| NFW/mg | 20.12 | 36.3 | 9.30 | 5.38 | 26.74 |
| NDW/mg | 9.15 | 16.6 | 4.00 | 2.32 | 25.32 |
| NLWR | 10.59 | 20.49 | 6.46 | 2.56 | 24.18 |
| NWTR | 1.31 | 1.56 | 0.86 | 0.17 | 12.92 |
| NMC% | 54.19 | 69.58 | 36.57 | 4.00 | 7.39 |
| ETL/μm | 3244.09 | 4753.3 | 1664.4 | 551.34 | 17.00 |
| ETD/μm | 52.61 | 78.8 | 27.6 | 8.10 | 15.39 |
| ETLD/μm | 45.53 | 70.3 | 21.7 | 8.09 | 17.77 |
| ETWT/μm | 7.09 | 13.4 | 2.8 | 1.63 | 23.03 |
| ETLEDR | 62.72 | 110.75 | 34.60 | 12.84 | 20.48 |
| ETLUDR | 0.86 | 0.95 | 0.72 | 0.04 | 4.18 |
| ETWLR | 0.16 | 0.38 | 0.05 | 0.04 | 31.16 |
| LTL/μm | 3532.27 | 4838.1 | 2060.0 | 569.05 | 16.11 |
| LTD/μm | 40.26 | 55.4 | 25.7 | 4.59 | 11.40 |
| LTLD/μm | 20.76 | 43.3 | 8.0 | 4.62 | 22.25 |
| LTWT/μm | 19.51 | 33.4 | 9.7 | 3.48 | 17.84 |
| LTLEDR | 88.24 | 132.54 | 50.92 | 14.05 | 15.93 |
| LTLUDR | 0.51 | 0.78 | 0.28 | 0.08 | 16.24 |
| LTWLR | 1.01 | 2.55 | 0.28 | 0.35 | 35.28 |
| Provenance | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Aggregate Score (Y) | Ranking |
|---|---|---|---|---|---|---|---|---|
| XS | −0.6312 | −0.0932 | 0.4073 | 0.1612 | 0.2087 | 0.0537 | −0.0768 | 7 |
| LC | −0.1007 | −0.1333 | −0.1822 | −0.3000 | −0.0337 | 0.3033 | −0.1112 | 9 |
| DHS | 0.1165 | −0.0592 | 0.2785 | −0.3703 | −0.1027 | −0.2443 | −0.0118 | 4 |
| HX | −0.6723 | −0.1302 | 0.9987 | 0.2305 | 0.1187 | −0.2993 | −0.0240 | 6 |
| DDS | 0.4938 | 0.1158 | 0.2183 | 0.4022 | −0.3590 | −0.0322 | 0.2085 | 2 |
| LCH | 0.7430 | 0.3057 | −0.9878 | 0.1535 | −0.4060 | 0.0403 | 0.0870 | 3 |
| GC | −0.2305 | −0.0603 | 0.0663 | −0.3202 | 0.0173 | −0.0960 | −0.1107 | 8 |
| DHK | −0.4197 | 0.0190 | −0.7743 | −0.1257 | 0.2632 | 0.3205 | −0.2038 | 10 |
| QL | 0.3578 | 0.0623 | 0.6100 | 0.2253 | 0.1815 | −0.0333 | 0.2605 | 1 |
| XYH | 0.3433 | −0.0237 | −0.6342 | −0.0547 | 0.1083 | −0.0123 | −0.0170 | 5 |
| Provenance | Y1 | Y2 | Aggregate Score (Y) | Ranking |
|---|---|---|---|---|
| XS | 0.0609 | 1.4362 | 0.5414 | 3 |
| LC | −1.0277 | −2.2020 | −1.2386 | 10 |
| DHS | 0.8066 | 1.0488 | 0.7289 | 2 |
| HX | −1.5218 | 0.5850 | −0.4563 | 8 |
| DDS | −0.1550 | 0.1337 | −0.0203 | 6 |
| LCH | 0.5074 | 0.5300 | 0.4120 | 4 |
| GC | 2.6336 | −0.9829 | 0.8001 | 1 |
| DHK | −1.3141 | 1.2407 | −0.1299 | 7 |
| QL | −0.9471 | −0.8134 | −0.7054 | 9 |
| XYH | 0.9574 | −0.9754 | 0.0690 | 5 |
| Provenance | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | Y8 | Aggregate Score (Y) | Ranking |
|---|---|---|---|---|---|---|---|---|---|---|
| XS | −0.7318 | −0.6743 | 0.6520 | 1.1422 | −0.2018 | −0.2838 | 0.1687 | 0.0660 | −0.2673 | 8 |
| LC | 1.2015 | −0.3890 | −1.6312 | −1.3222 | 0.5777 | 0.3677 | −0.0532 | 0.2500 | 0.6518 | 3 |
| DHS | 0.3607 | 0.5868 | 0.5065 | 1.2618 | −0.0417 | −0.6615 | 0.0085 | −0.2113 | −0.2148 | 7 |
| HX | −0.6992 | −1.3117 | −1.1083 | 1.1628 | 0.2830 | 0.0447 | −0.0193 | −0.2967 | −0.2087 | 6 |
| DDS | −0.1943 | 0.4730 | −0.2350 | 0.2970 | −0.1237 | 0.3465 | −0.4102 | −0.0283 | −0.1022 | 5 |
| LCH | −0.9135 | 0.7135 | 0.8720 | −0.8945 | −0.8605 | −0.0573 | −0.4532 | 0.0428 | −1.2065 | 9 |
| GC | 1.6305 | 1.0318 | 1.8897 | −0.4747 | 0.9290 | 0.2473 | 0.3768 | −0.0528 | 2.0397 | 1 |
| DHK | −1.7358 | −1.1343 | 0.0355 | −0.2713 | −1.0073 | −0.6677 | 0.0123 | 0.3643 | −1.8655 | 10 |
| QL | 0.1535 | 0.2693 | −1.5728 | 0.2105 | 0.0940 | 0.2718 | 0.4845 | −0.2550 | 0.3658 | 4 |
| XYH | 0.7334 | 0.3933 | 0.0501 | −0.7806 | 0.2875 | 0.3629 | 0.0345 | 0.0275 | 0.6984 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Xu, E.; Lv, D.; Wang, Y.; Zhao, X.; Wei, N.; Zhang, Z.; Yuan, H.; Ma, X.; Wu, X.; et al. Discrepancies and Evaluation of Needle-Leaf and Tracheid Traits of Qinhai Spruce in Qilian Mountains, Northwest China. Forests 2024, 15, 960. https://doi.org/10.3390/f15060960
Zhao H, Xu E, Lv D, Wang Y, Zhao X, Wei N, Zhang Z, Yuan H, Ma X, Wu X, et al. Discrepancies and Evaluation of Needle-Leaf and Tracheid Traits of Qinhai Spruce in Qilian Mountains, Northwest China. Forests. 2024; 15(6):960. https://doi.org/10.3390/f15060960
Chicago/Turabian StyleZhao, Hu, Erwen Xu, Dong Lv, Yanxia Wang, Xingpeng Zhao, Na Wei, Zhengzhong Zhang, Hao Yuan, Xuee Ma, Xiurong Wu, and et al. 2024. "Discrepancies and Evaluation of Needle-Leaf and Tracheid Traits of Qinhai Spruce in Qilian Mountains, Northwest China" Forests 15, no. 6: 960. https://doi.org/10.3390/f15060960
APA StyleZhao, H., Xu, E., Lv, D., Wang, Y., Zhao, X., Wei, N., Zhang, Z., Yuan, H., Ma, X., Wu, X., & Liu, X. (2024). Discrepancies and Evaluation of Needle-Leaf and Tracheid Traits of Qinhai Spruce in Qilian Mountains, Northwest China. Forests, 15(6), 960. https://doi.org/10.3390/f15060960





