A New Species of Biscogniauxia Associated with Pine Needle Blight on Pinus thunbergii in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
2.2. Colony Observations
2.3. Genomic DNA Extraction, PCR, and Sequencing
2.4. Morphological Identification
2.5. Phylogenetic Analyses
2.6. Genealogical Concordance Phylogenetic Species Recognition Analysis
3. Results
3.1. Sampling and Isolation
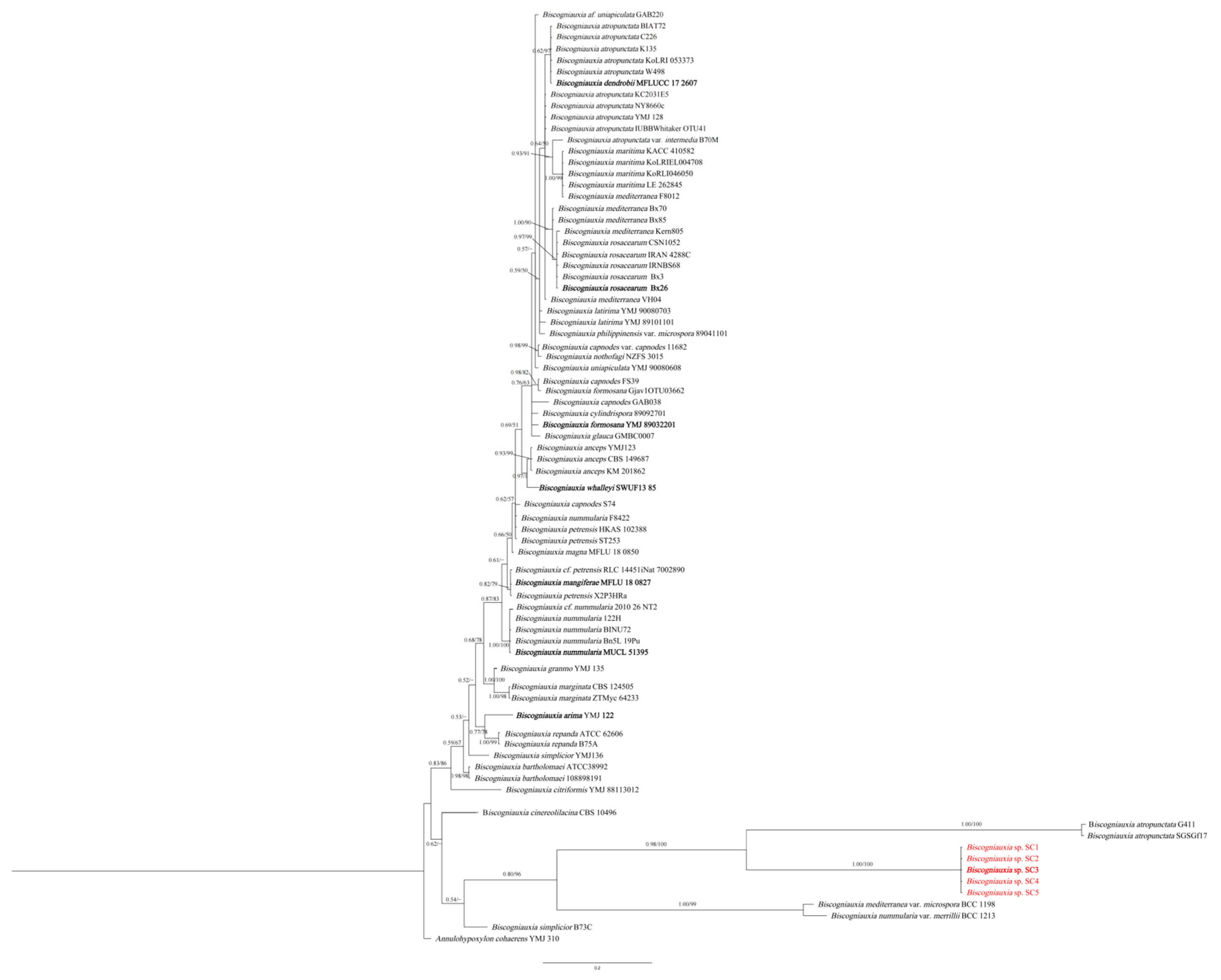
3.2. Phylogenetic Analyses
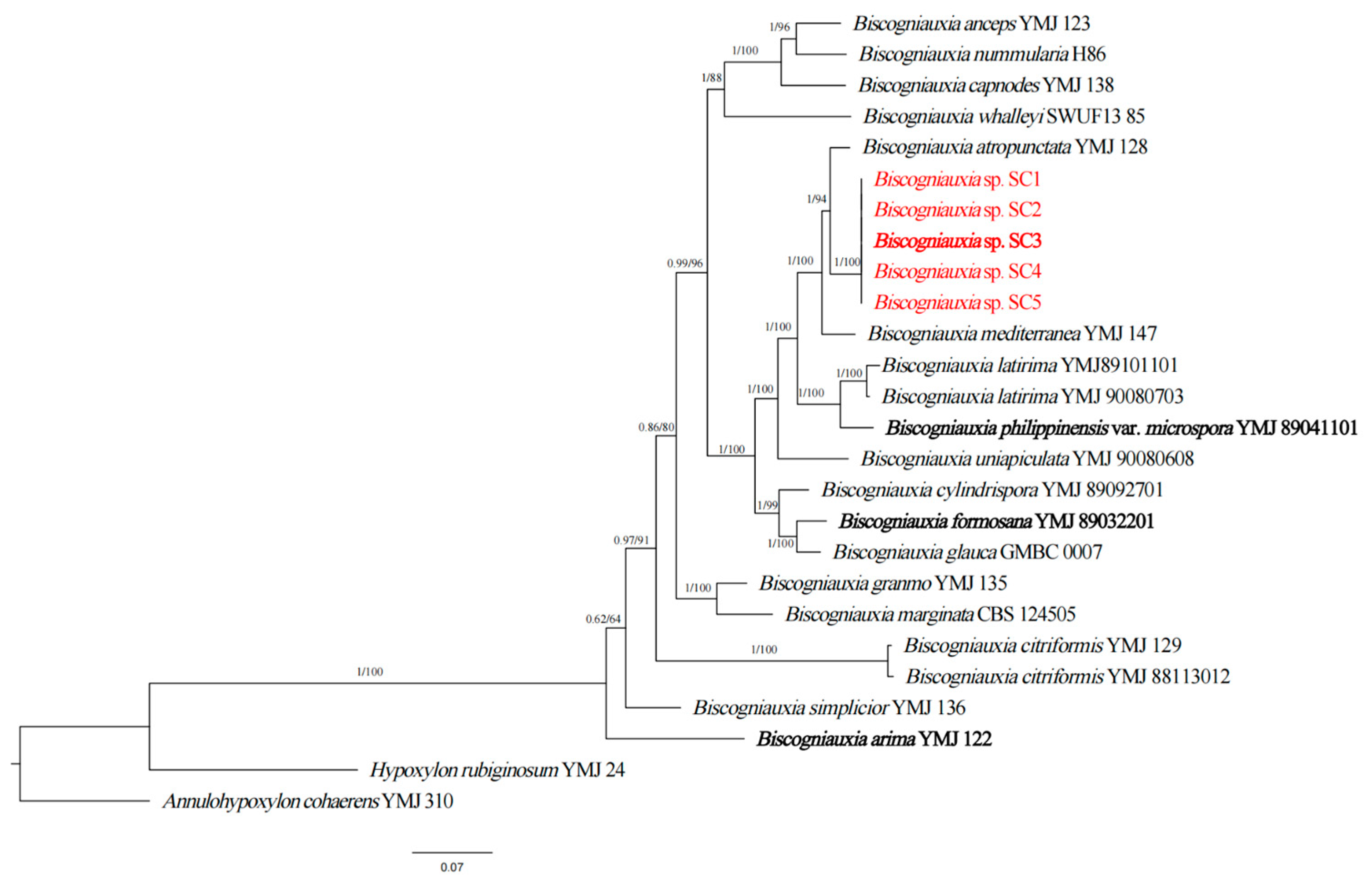
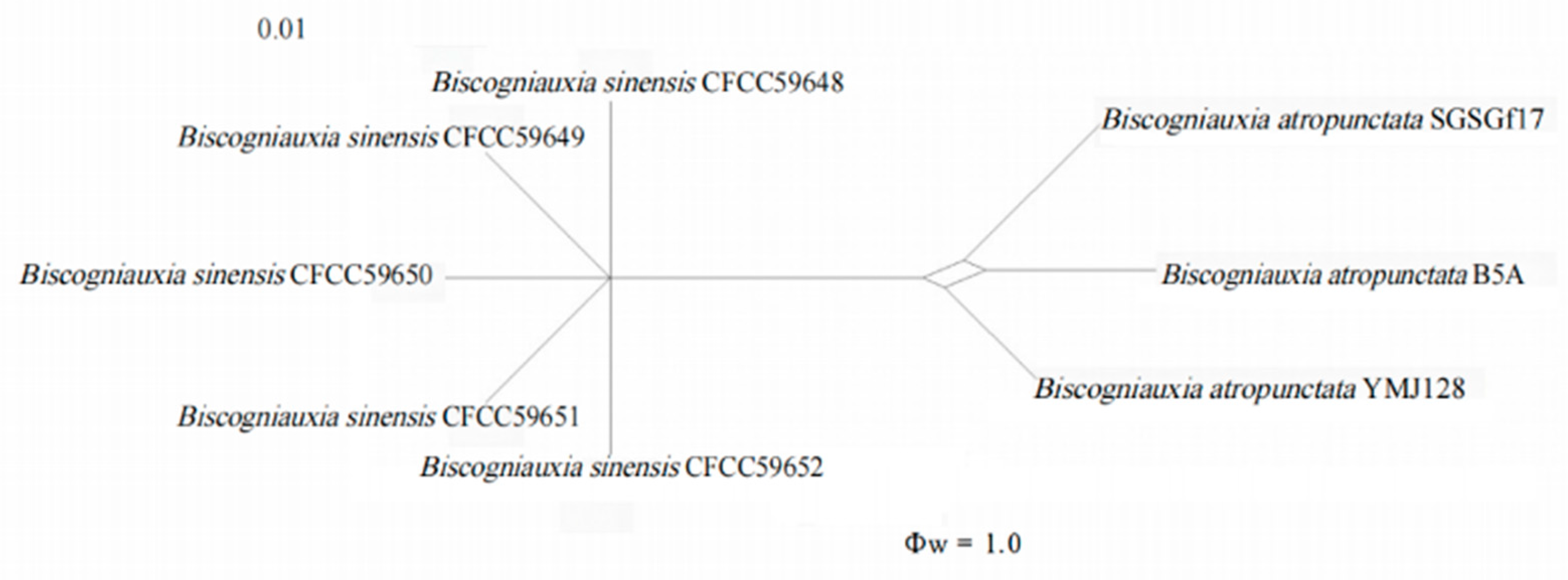
3.3. Genealogical Concordance Phylogenetic Species Recognition
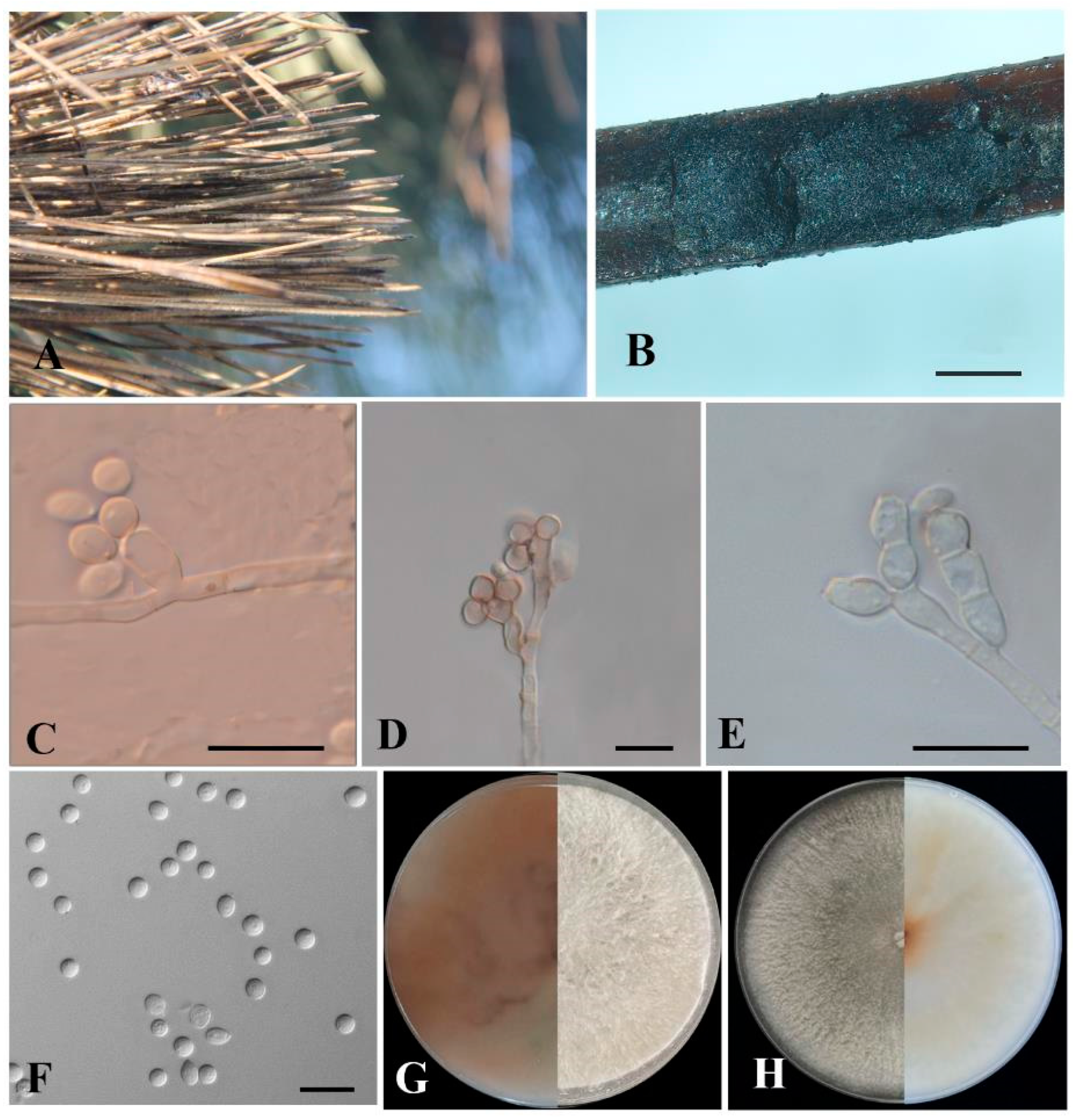
3.4. Taxonomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Wang, Z.; Song, W.; Zhao, Z.; Zhao, Y. Isolation of proanthocyanidins from Pinus thunbergii needles and tyrosinase inhibition activity. Process Biochem. 2021, 100, 245–251. [Google Scholar] [CrossRef]
- Tanaka, S.; Tomita, R.; Saijo, H.; Takahashi, K.; Ashitani, T. Growth-inhibitory activity of components in Cryptomeria japonica leaves against Robinia pseudoacacia. J. For. Res. 2020, 25, 192–197. [Google Scholar] [CrossRef]
- Masaka, K.; Torita, H.; Sato, H.; Kon, H.; Fukuchi, S.M. Decline of Pinus thunbergii Parlat. stands due to excess soil moisture caused by a buried andosol layer at a coastal sand site in Hokkaido, northern Japan. J. For. Res. 2010, 15, 341–346. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Lee, J. Studies on the characteristics of growth of Pinus thunbergii planted in a Costal Sand Zone. J. Korean For. Soc. 2012, 101, 656–662. [Google Scholar]
- Beccaccioli, M.; Grottoli, A.; Scarnati, L.; Faino, L.; Reverberi, M. Nanopore hybrid assembly of Biscogniauxia mediterranea isolated from Quercus cerris affected by charcoal disease in an endangered coastal wood. Microbiol. Resour. Announc. 2021, 10, e0045021. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G. Control Measures against Main Pinus koraiensis Diseases in Liaoning Province. Plant Dis. Pests 2022, 13, 5–6. [Google Scholar] [CrossRef]
- Hirose, D.; Osono, T. Development and seasonal variations of Lophodermium populations on Pinus thunbergii needle litter. Mycoscience 2006, 47, 242–247. [Google Scholar] [CrossRef]
- Du, Z. Common diseases of pine needles and comprehensive prevention and control measures. Mod. Agric. Technol. 2022, 31–32. [Google Scholar]
- Li, C.D.; Zhu, X.Q.; Han, Z.M. Investigation on brown-spot needle blight of pines in China. J. Nanjing For. Univ. 1986, 29, 11–18. [Google Scholar]
- Ding, X.L.; Zhao, R.W.; Lin, S.X.; Ye, J.R. Morphological and phylogenetic resolution of Diplodia neojuniperi emerging Diplodia top dieback of Pinus thunbergii Parl. in China. Phyton 2022, 91, 2813–2825. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.C.; Zhang, Y.; Li, D.W.; Ye, J.R. Colletotrichum gloeosporioides sensu stricto is a pathogen of leaf anthracnose on evergreen spindle tree (Euonymus japonicus). Plant Dis. 2015, 100, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Mostert, L.; Crous, P.W.; Fourie, P.H. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 2008, 20, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Mirabolfathy, M.; Ju, Y.M.; Hsieh, H.M.; Rogers, J.D. Obolarina persica sp. nov., associated with dying Quercus in Iran. Mycoscience 2013, 54, 315–320. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Lousie, G.N.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.M.; Ju, Y.M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Rogers, J.D. A Revision of the Genus Hypoxylon; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Sohrabi, M.; Mohammadi, H.; Armengol, J.; León, M. New report of Biscogniauxia rosacearum as a pathogen on almond trees in Iran. J. Plant Dis. Prot. 2022, 129, 411–417. [Google Scholar] [CrossRef]
- Rajpoot, A.; Kumar, V.; Kumari, R.; Singh, T. Molecular Characteristic of Indian Pangolin with Existing Manidae Species Based on Mitochondrial Genes: A Wildlife Forensic Approach. In Proceedings of the 4th International Arab Forensic Sciences & Forensic Medicine Conference, Riyadh, Saudi Arabia, 27–29 November 2018. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Tang, X.Y.; Hu, S.; Zhu, W.Y.; Wu, X.P.; Sang, W.J.; Ding, H.X.; Peng, L.J. First report of gray spot on tobacco caused by Alternaria alstroemeriae in China. Plant Dis. 2023, 107, 2546. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Kalyaanamoorthy; Subha; Minh, B.Q.; Wong; Thomas, K.F.; Haeseler, V.; Arndt; Jermiin; Lars, S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Dong, H.-P.; Hou, L.-J.; Liu, Y.; Ou, Y.-F.; Zheng, Y.-L.; Han, P.; Liang, X.; Yin, G.-Y.; Wu, D.-M.; et al. Newly discovered Asgard archaea Hermodarchaeota potentially degrade alkanes and aromatics via alkyl/benzyl-succinate synthase and benzoyl-CoA pathway. ISME J. 2021, 15, 1826–1843. [Google Scholar] [CrossRef]
- Wan, Y.; Li, D.W.; Si, Y.Z.; Li, M.; Huang, L.; Zhu, L.H. Three new species of Diaporthe causing leaf blight on Acer palmatum in China. Plant Dis. 2023, 107, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 4.0. A Graphical Viewer of Phylogenetic Trees; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2012. [Google Scholar]
- Hsieh, H.M.; Lin, C.R.; Fang, M.J.; Rogers, J.D.; Ju, Y.M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenetics Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Crous, P.W.; Akulov, A.; Balashov, S.; Boers, J.; Braun, U.; Castillo, J.; Delgado, M.A.; Denman, S.; Erhard, A.; Gusella, G.; et al. New and Interesting Fungi. Fungal Syst. Evol. 2023, 11, 109–156. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ballesteros, J.; González, V.; Salazar, O.; Acero, J.; Portal, M.A.; Julián, M.; Rubio, V.; Bills, G.F.; Polishook, J.D.; Platas, G.; et al. Phylogenetic study of Hypoxylon and related genera based on ribosomal ITS sequences. Mycologia 2000, 92, 964–977. [Google Scholar] [CrossRef]
- Pinto, T. The Phyloecology of Hypoxylon sensu lato. Master’s Thesis, University of California, Berkeley, CA, USA, 2001. [Google Scholar]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Ju, Y.M.; Rogers, J.D. New and interesting Biscogniauxia taxa, with a key to the world species. Mycol. Res. 2001, 105, 1123–1133. [Google Scholar] [CrossRef]
- Ma, X.Y.; Nontachaiyapoom, S.; Hyde, K.D.; Jeewon, R.; Doilom, M.; Chomnunti, P.; Kang, J.C. Biscogniauxia dendrobii sp. nov. and B. petrensis from Dendrobium orchids and the first report of cytotoxicity (towards A549 and K562) of B. petrensis (MFLUCC 14-0151) in vitro. S. Afr. J. Bot. 2020, 134, 382–393. [Google Scholar] [CrossRef]
- Li, Q.R.; Gong, X.F.; Zhang, X.; Pi, Y.H.; Long, S.H.; Wu, Y.P.; Shen, X.C.; Kang, Y.Q.; Kang, J.C. Phylogeny of Graphostromatacea with two new species (Biscogniauxia glaucae sp. nov. and Graphostroma guizhouensis sp. nov.) and new record of Camillea broomeana isolated in China. Arch. Microbiol. 2021, 203, 6119–6129. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Stadler, M.; Gareth Jones, E.B.; Promputtha, I.; Suwannarach, N.; Camporesi, E.; Bulgakov, T.S.; Liu, J.K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.; Hyde, K.; Jones, E.; McKenzie, E.; Bhat, D.J.; Dayarathne, M.; Huang, S.-K.; Norphanphoun, C.; Senanayake, I.; Perera, R.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Raimondo, M.L.; Lops, F.; Carlucci, A. Charcoal canker of pear, plum, and quince trees caused by Biscogniauxia rosacearum sp. nov. in Southern Italy. Plant Dis. 2016, 100, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Travadon, R.; Lawrence, D.P.; Moyer, M.M.; Fujiyoshi, P.T.; Baumgartner, K. Fungal species associated with grapevine trunk diseases in Washington wine grapes and California table grapes, with novelties in the genera Cadophora, Cytospora and Sporocadus. Frontiers 2022, 3, 1018140. [Google Scholar] [CrossRef]
- Okane, I.; Srikitikulchai, P.; Toyama, K.; Læssøe, T.; Sivichai, S.; Hywel-Jones, N.; Nakagiri, A.; Potacharoen, W.; Suzuki, K.-i. Study of endophytic Xylariaceae in Thailand: Diversity and taxonomy inferred from rDNA sequence analyses with saprobes forming fruit bodies in the field. Mycoscience 2008, 49, 359–372. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P.; Zając, J.; Jobczyk, A.; Taerum, S.J. The culturable leaf mycobiome of Viscum album subsp. austriacum. For. Pathol. 2023, 53, e12821. [Google Scholar] [CrossRef]
- Vandegrift, R.; Newman, D.S.; Dentinger, B.T.M.; Batallas-Molina, R.; Dueñas, N.; Flores, J.; Goyes, P.; Jenkinson, T.S.; McAlpine, J.; Navas, D.; et al. Richer than Gold: The fungal biodiversity of Reserva Los Cedros, a threatened Andean cloud forest. Bot. Stud. 2023, 64, 17. [Google Scholar] [CrossRef] [PubMed]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Bashiri, S.; Abdollahzadeh, J.; Evidente, A. Diagnosing and pathogenicity of Biscogniauxia species, the causal agents of oak charcoal canker and decline in Zagros forests of Iran. J. Plant Pathol. 2022, 104, 1011–1025. [Google Scholar] [CrossRef]
- Spies, C.F.J.; Mostert, L.; Carlucci, A.; Moyo, P.; van Jaarsveld, W.J.; du Plessis, I.L.; van Dyk, M.; Halleen, F. Dieback and decline pathogens of olive trees in South Africa. Persoonia 2020, 45, 196–220. [Google Scholar] [CrossRef]
- Quaedvlieg, W.; Binder, M.; Groenewald, J.Z.; Summerell, B.A.; Carnegie, A.J.; Burgess, T.I.; Crous, P.W. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 2014, 33, 1–40. [Google Scholar] [CrossRef]
- Royse, D.; Baars, J.J.P.; Tan, Q. Current overview of Mushroom production in the world. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Ju, Y.M.; Rogers, J.D.; Martín, F.S.; Granmo, A. The genus Biscogniauxia. Mycotaxon 1998, 66, 1–98. [Google Scholar]
- Bahmani, Z.; Abdollahzadeh, J.; Amini, J.; Evidente, A. Biscogniauxia rosacearum the charcoal canker agent as a pathogen associated with grapevine trunk diseases in Zagros region of Iran. Sci. Rep. 2021, 11, 14098. [Google Scholar] [CrossRef] [PubMed]
- Pažoutová, S.; Šrůtka, P.; Holuša, J.; Chudíčková, M.; Kolařík, M. The phylogenetic position of Obolarina dryophila (Xylariales). Mycol. Prog. 2010, 9, 501–507. [Google Scholar] [CrossRef]
- Peláez, F.; Platas, G.; Sánchez-Ballesteros, J. Molecular phylogenetic studies within the Xylariaceae based on ribosomal DNA sequences. Fungal Divers. 2008, 31, 111–134. [Google Scholar]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Stadler, M. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018, 88, 1–165. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Systematic Mycology and Microbiology Laboratory-Fungal Databases; ARS, USDA: Washington, DC, USA, 2023. [Google Scholar]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008, 112, 331–344. [Google Scholar] [CrossRef]
- Yangui, I.; Boutiti, M.Z.; Vettraino, A.M.; Bruni, N.; Vannini, A.; Jamaâ, M.L.B.; Boussaid, M.; Messaoud, C. Biscogniauxia mediterranea associated with cork oak (Quercus suber) in Tunisia: Relationships between phenotypic variation, genetic diversity and ecological factors. Fungal Ecol. 2019, 41, 224–233. [Google Scholar] [CrossRef]
- Bußkamp, J.; Bien, S.; Neumann, L.; Blumenstein, K.; Terhonen, E.; Langer, G.J. Endophytic community in juvenile Acer pseudoplatanus and pathogenicity of Cryptostroma corticale and other associated fungi under controlled conditions. J. Plant Pathol. 2024, 106, 565–577. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. United States National Fungus Collections Fungus-Host Dataset; ARS, USDA: Washington, DC, USA, 2021. [Google Scholar]
- Pusz, W.; Baturo-Ciesniewska, A.; Kaczmarek-Pienczewska, A.; Zwijacz-Kozica, T.; Patejuk, K. The mycobiota of needles and shoots of silver fir (Abies alba Mill.) with symptoms of Herpotrichia needle browning in the Tatra Mts. (Poland). Ann. For. Res. 2020, 63, 45–56. [Google Scholar] [CrossRef]
- Wilson, D.C. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Variation of endophytic cork oak associated fungal communities in relation to plant health and water stress. For. Pathol. 2011, 41, 193–201. [Google Scholar] [CrossRef][Green Version]
- Marie-Laure, D.L.; Benoit, M.; Louis-Michel, N.; Dominique, P.; Andrea, V. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar]
- Vannini, A.; Lucero, G.; Anselmi, N.; Vettraino, A.M. Response of endophytic Biscogniauxia mediterranea to variation in leaf water potential of Quercus cerris. For. Pathol. 2008, 39, 8–14. [Google Scholar] [CrossRef]
- Sanz-Ros, A.V.; Müller, M.M.; San Martín, R.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol. 2015, 119, 870–883. [Google Scholar] [CrossRef]
- Patejuk, K.; Kaczmarek-Pieńczewska, A.; Baturo-Ciesniewska, A.; Pusz, W. Mycobiota of peat-bog pine (Pinus × rhaetica) needles in the Stołowe Mountains National Park, Poland. Nova Hedwig. 2021, 112, 253–265. [Google Scholar] [CrossRef]
- Pusz, W.; Baturo-Ciesniewska, A.; Kaczmarek-Pieńczewska, A.; Zwijacz-Kozica, T. The health status of sliver fir (Abies alba Mill.) in selected locations of the Tatra national park and the Karkonosze national park. For. Wood Technol. 2020, 19, 145–152. [Google Scholar] [CrossRef]
- Petrini, L.E.; Petrini, O. Xylariaceous fungi as endophytes. Sydowia 1985, 38, 201–265. [Google Scholar] [CrossRef]
- Patejuk, K.; Baturo-Ciesniewska, A.; Pusz, W.; Kaczmarek-Pienczewska, A. Biscogniauxia charcoal canker-A new potential threat for Mid-European forests as an effect of climate change. Forests 2022, 13, 89. [Google Scholar] [CrossRef]
- Petrović, E.; Godena, S.; Cosic, J.; Vrandečić, K. Identification and pathogenicity of Biscogniauxia and Sordaria species isolated from Olive trees. Horticulturae 2024, 10, 243. [Google Scholar] [CrossRef]
- Amand, S.; Langenfeld, A.; Blond, A.; Dupont, J.; Nay, B.; Prado, S. Guaiane sesquiterpenes from Biscogniauxia nummularia featuring potent antigerminative activity. J. Nat. Prod. 2012, 75, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.M.; Nie, F.X.; Zhao, Y.Q.; Zhang, D.W.; Zhou, D.G.; Wu, J.F.; Qu, L.; Xiao, L.; Liu, L.L. A review on plant endophytes in response to abiotic stress. Environ. Pollut. Bioavailab. 2024, 36. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2024, 125, 73–98. [Google Scholar] [CrossRef]
- Nugent, L.K.; Sihanonth, P.; Thienhirun, S.; Whalley, A.J.S. Biscogniauxia: A genus of latent invaders. Mycologist 2005, 19, 40–43. [Google Scholar]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109 Pt 6, 661–686. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Granata, G.; Sidoti, A. Biscogniauxia nummularia: Pathogenic agent of a beech decline. For. Pathol. 2004, 34, 363–367. [Google Scholar] [CrossRef]
- Karami, J.; Kavosi, M.R.; Babanezhad, M.; Kiapasha, K. Integrated management of the charcoal disease by silviculture, chemical and biological methods in forest parks. J. Sustain. For. 2017, 37, 429–444. [Google Scholar] [CrossRef]
- Vasilyeva, L.; Li, Y.; Stephenson, S. Some pyrenomycetous fungi new to China. Mycotaxon 2009, 109, 415–428. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, F.; Zhou, X.; Liu, X.Z.; Liu, S.J.; Cai, L. Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 2017, 39, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Zhang, J.Y.; Xiao, Y.P.; Lu, Y.Z. Biscogniauxia dicranopteridis sp. nov. and B. petrensis isolated from Dicranopteris dichotoma in China. Phytotaxa 2024, 641, 255–266. [Google Scholar] [CrossRef]
| Locus | PCR Primers (Forward/Reverse) | PCR: Thermal Cycles: (Annealing Temperature in Bold) |
|---|---|---|
| ITS | ITS5/ITS4 | 95 °C: 5 min, (95 °C: 30 s, 55 °C: 30 s, 72 °C: 90 s) ×33 cycles, 72 °C: 10 min |
| ACT | ACT-512F/ACT-783R | 95 °C: 2 min, (95 °C: 30 s, 61 °C: 30 s, 72 °C: 60 s) ×35 cycles, 72 °C: 5 min |
| TUB2 | Bt-2a/Bt-2b | 95 °C: 2 min, (95 °C: 30 s, 55 °C: 30 s, 72 °C: 60 s) ×35 cycles, 72 °C: 5 min |
| RPB2 | fRPB2-5F/fRPB2-7cR | 95 °C: 2 min, (95 °C: 30 s, 54 °C: 30 s, 72 °C: 60 s) ×35 cycles, 72 °C: 5 min |
| Species | Locality | Host | Strain Number 1 | GenBank Accessions | Reference | |||
|---|---|---|---|---|---|---|---|---|
| ITS | ACT | TUB2 | RPB2 | |||||
| Annulohypoxylon cohaerens | France | Fagus | YMJ 310 = BCRC34013 ab | EF026140 | AY951766 | AY951655 | GQ844766 | [29] |
| Biscogniauxia anceps | France | Fagus | YMJ 123 = BCRC34029 ab | EF026132 | AY95178 | AY951671 | JX507777 | [29] |
| B. anceps | Spain | Eucalyptus sp. | CBS 149687 a | OQ990096 | NA | NA | NA | [30] |
| B. anceps | England | unknown | KM 201862 a | MZ159582 | NA | NA | NA | submitted directly |
| B. arima | Mexico | wood | YMJ 122 * = BCRC34030 ab | EF026150 | AY951784 | AY951672 | GQ304736 | [29] |
| B. atropunctata | Ecuador | Pinus | NY8660c a | HQ108028 | NA | NA | NA | submitted directly |
| B. atropunctata | unknown | Switchgrass | IUB:B.Whitaker:OTU41 a | MH178710 | NA | NA | NA | submitted directly |
| B. atropunctata | Canada | Acer | KC2031E5 a | KX589181 | NA | NA | NA | submitted directly |
| B. atropunctata | unknown | unknown | KoLRI_053373 a | MZ855376 | NA | NA | NA | submitted directly |
| B. atropunctata | unknown | milk thistle | G411 a | KM215648 | NA | NA | NA | submitted directly |
| B. atropunctata | unknown | unknown | K135 a | MK304352 | NA | NA | NA | submitted directly |
| B. atropunctata | unknown | unknown | C226 a | MK304016 | NA | NA | NA | submitted directly |
| B. atropunctata | unknown | unknown | W498 a | MK247673 | NA | NA | NA | submitted directly |
| B.atropunctata | Iran | Celtis australis | BIAT72 a | MF497753 | NA | NA | NA | submitted directly |
| B. atropunctata | Mexico | Taxus globosa | SGSGf17 a | EU715651 | NA | NA | NA | [31] |
| B. atropunctata | USA | wood | YMJ 128 ab | JX507799 | AY951785 | AY951673 | JX507778 | [13] |
| B. atropunctata var. intermedia | Costa Rica | Quercus sp. | B70M = GB 4796 a | AJ390412 | NA | NA | NA | [31] |
| B. bartholomaei | USA | branches | ATCC 38992 a | AF201719 | NA | NA | NA | [32] |
| B. bartholomaei | USA | branches | 108898191 a | ON692782 | NA | NA | NA | [32] |
| B. capnodes | China | wood | YMJ 138 = BCRC34032 b | EF026131 | AY951787 | AY951675 | JX507779 | [29] |
| B. capnodes | unknown | unknown | S74 a | OR237607 | NA | NA | NA | submitted directly |
| B. capnodes | unknown | unknown | FS39 a | MF770835 | NA | NA | NA | submitted directly |
| B. capnodes | Gabon | unknown | GAB038 a | KY250379 | NA | NA | NA | submitted directly |
| B. capnodes var. capnodes | New Zealand | Lophozonia menziesii | 11682 a | MH410019 | NA | NA | NA | submitted directly |
| B. cinereolilacina | Norway | unknown | CBS 10496 a | MH862567 | NA | NA | NA | [33] |
| B. citriformis | USA | Casuarina equisetifolia | YMJ 129 = BCRC34034 b | JX507801 | AY951789 | AY951678 | JX507781 | [13] |
| B. citriformis | China | wood | YMJ 88113012 ab | JX507800 | AY951790 | AY951677 | JX507780 | [13] |
| B. cylindrispora | China | Cinnamomum | YMJ 89092701 = BCRC33717 ab | EF026133 | AY951791 | AY951679 | JX507782 | [34] |
| B. dendrobii | China | Dendrobium aphyllum | MFLUCC 17 2607 * a | NR172733 | NA | NA | NA | [35] |
| B. formosana | China | Bark | YMJ 89032201 * = BCRC33718 ab | JX507802 | AY951792 | AY951680 | JX507783 | [34] |
| B. formosana | unknown | unknown | Gjav1ITS2OTU03662 a | KY588559 | NA | NA | NA | submitted directly |
| B. granmo | Austria | Prunus padus | YMJ 135 = BCRC34035 ab | JX507803 | AY951793 | AY951681 | JX507784 | [13] |
| B. glaucae | China | Quercus glauca | GMBC 0007 ab | MT624046 | MT622656 | MT622654 | MT622652 | [36] |
| B. latirima | China | Bark | YMJ 89101101 = BCRC33729 ab | JX507804 | AY951794 | AY951682 | JX507785 | [13] |
| B. latirima | China | Bark | YMJ 90080703 = BCRC34036 ab | EF026135 | AY951795 | AY951683 | JX507786 | [29] |
| B. magna | Thailand | Unidentified | MFLU 18-0850 a | MW240616 | NA | NA | NA | [37] |
| B. mangiferae | Thailand | Mangifera indica | MFLU 18-0827 *a | MN337232 | NA | NA | NA | [38] |
| B. marginata | Switzerland | Sorbus aucuparia | ZT-Myc-64233 a | MW489534 | NA | NA | NA | submitted directly |
| B. maritima | Russia | unknown | LE 262845 a | JQ247198 | NA | NA | NA | submitted directly |
| B. maritima | Republic of Korea | unknown | KoLRI_EL004708 a | MN844502 | NA | NA | NA | submitted directly |
| B. maritima | Republic of Korea | Parmotrema | KoRLI046050 a | MN341558 | NA | NA | NA | submitted directly |
| B. maritima | Republic of Korea | unknown | KACC 410582 a | OR886237 | NA | NA | NA | submitted directly |
| B. marginata | Germany | Malus sp. | CBS 124505 ab | KU684016 | KU684036 | KU684124 | KU684310 | [36] |
| B. mediterranea | Italy | Q. pubescens | B × 70 a | KT253502 | NA | NA | NA | [39] |
| B. mediterranea | Italy | Q. pubescens | B × 85 a | KT253503 | NA | NA | NA | [39] |
| B. mediterranea | France | Fagus sp. | YMJ 147 = BCRC34037 b | EF026134 | AY951796 | AY951684 | GQ844765 | [39] |
| B. mediterranea | Japan | Malus sp. | F8012 a | AB693903 | NA | NA | NA | submitted directly |
| B. mediterranea | USA | Acer | VH04 a | OR778793 | NA | NA | NA | submitted directly |
| B. mediterranea | USA | wood | Kern805 a | OP038087 | NA | NA | NA | [40] |
| B. nothofagi | New Zealand | Lophozonia menziesii | NZFS 3015 a | MN007010 | NA | NA | NA | Hood, I.A submitted directly. |
| B. mediterranea var. microspora | Thailand | unknown | BCC 1198 a | AB376712 | NA | NA | NA | [41] |
| B. nummularia | England | Salix alba | H86 = CCF 3919 b | GQ428318 | GQ428312 | GQ428324 | FR715504 | [39] |
| B. nummularia | France | Fagus sp. | MUCL 51395 * a | JX658444 | NA | NA | NA | [39] |
| B. nummularia | China | unknown | F8422 a | ON332160 | NA | NA | NA | submitted directly |
| B. nummularia | Poland | Viscum album | 122H a | OP699770 | NA | NA | NA | [42] |
| B. nummularia | Poland | Pinus | Bn5L-19Pu a | MN588203 | NA | NA | NA | submitted directly |
| B. nummularia | Iran | Zelkova | BINU72 a | MF358880 | NA | NA | NA | submitted directly |
| B. cf. nummularia | unknown | Lewinskya | 2010_26_NT2 a | MW907969 | NA | NA | NA | submitted directly |
| B. nummularia var. merrillii | Thailand | bark | BCC 1213 a | AB376714 | NA | NA | NA | [41] |
| B. petrensis | Thailand | Dendrobium harveyanum | MFLUCC 14-0151 * a | MK951680 | NA | NA | NA | [38] |
| B. cf. petrensis | Ecuador | wood | RLC_1445.1_iNat_7002890 a | OQ878456 | NA | NA | NA | [43] |
| B. petrensis | Japan | Tea | ST253 a | LC685807 | NA | NA | NA | submitted directly |
| B. petrensis | China | Osmanthus sp. | HKAS 102388 a | MW240615 | NA | NA | NA | [37] |
| B. petrensis | unknown | unknown | X2P3HRa a | ON921656 | NA | NA | NA | submitted directly |
| B. philippinensis var. microspora | China | Bark | YMJ 89041101 * = BCRC33720 ab | EF026136 | AY951797 | AY951685 | JX507787 | [39] |
| B. repanda | USA | Fagus sp. | ATCC 62606 a | KY610383 | NA | NA | NA | [44] |
| B. repanda | unknown | unknown | B75A a | AJ390418 | NA | NA | NA | [45] |
| B. rosacearum | Italy | P. domestica | Bx26 = CBS141046 * a | KT253493 | NA | NA | NA | [39] |
| B. rosacearum | Italy | P. domestica | Bx4 | KT253491 | NA | NA | NA | [39] |
| B. rosacearum | Khorramabad | Quercus. brantii | IRAN 4288C a | MZ359664 | NA | NA | NA | [45] |
| B. rosacearum | Iran | almond tree | IRNBS68 a | MW452324 | NA | NA | NA | [45] |
| B. rosacearum | unknown | unknown | CSN1052 a | MT813910 | NA | NA | NA | [46] |
| Biscogniauxia sinensis | China | Pinus thunbergii | SC1 = CFCC59648 ab | OR803753 | OR832182 | OR832177 | OR832187 | This study |
| Biscogniauxia sinensis | China | P. thunbergii | SC2 = CFCC59649 ab | OR803754 | OR832183 | OR832178 | OR832188 | This study |
| Biscogniauxia sinensis | China | P. thunbergii | SC3 = CFCC59650 * ab | OR803755 | OR832184 | OR832179 | OR832189 | This study |
| Biscogniauxia sinensis | China | P. thunbergii | SC4 = CFCC59651 ab | OR803756 | OR832185 | OR832180 | OR832190 | This study |
| Biscogniauxia sinensis | China | P. thunbergii | SC5 = CFCC59652 ab | OR803757 | OR832186 | OR832181 | OR832191 | This study |
| B. simplicior | France | Rhamnus cathartica | YMJ 136 = BCRC34038 ab | EF026130 | AY951798 | AY951686 | JX507788 | [39] |
| B. simplicior | unknown | unknown | B73C a | AJ3904916 | NA | NA | NA | [31] |
| B. uniapiculata | China | Bark | YMJ 90080608 = BCRC34039 ab | JX507805 | AY951799 | AY951687 | JX507789 | [39] |
| B. aff. uniapiculata | Gabon | unknown | GAB220 a | KY250378 | NA | NA | NA | submitted directly |
| B. whalleyi | Thailand | wood | SWUF 13-85 ab | MW403821 | MZ466385 | MZ466386 | MZ466387 | Jantaharn, P. submitted directly. |
| Hypoxylon rubiginosum | UK | Fraxinus | YMJ 24 = BCRC34116 b | EF026143 | AY951862 | AY951751 | JX507791 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, C.; Zhao, R.; Li, D.; Ding, X. A New Species of Biscogniauxia Associated with Pine Needle Blight on Pinus thunbergii in China. Forests 2024, 15, 956. https://doi.org/10.3390/f15060956
Qiao C, Zhao R, Li D, Ding X. A New Species of Biscogniauxia Associated with Pine Needle Blight on Pinus thunbergii in China. Forests. 2024; 15(6):956. https://doi.org/10.3390/f15060956
Chicago/Turabian StyleQiao, Changxia, Ruiwen Zhao, Dewei Li, and Xiaolei Ding. 2024. "A New Species of Biscogniauxia Associated with Pine Needle Blight on Pinus thunbergii in China" Forests 15, no. 6: 956. https://doi.org/10.3390/f15060956
APA StyleQiao, C., Zhao, R., Li, D., & Ding, X. (2024). A New Species of Biscogniauxia Associated with Pine Needle Blight on Pinus thunbergii in China. Forests, 15(6), 956. https://doi.org/10.3390/f15060956






