Possible Factors of Poplar Susceptibility to Large Poplar Borer Infestation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region and Tested Poplar Clones
2.2. Weather Data Analysis
2.3. Field Data
2.4. Data Processing
3. Results
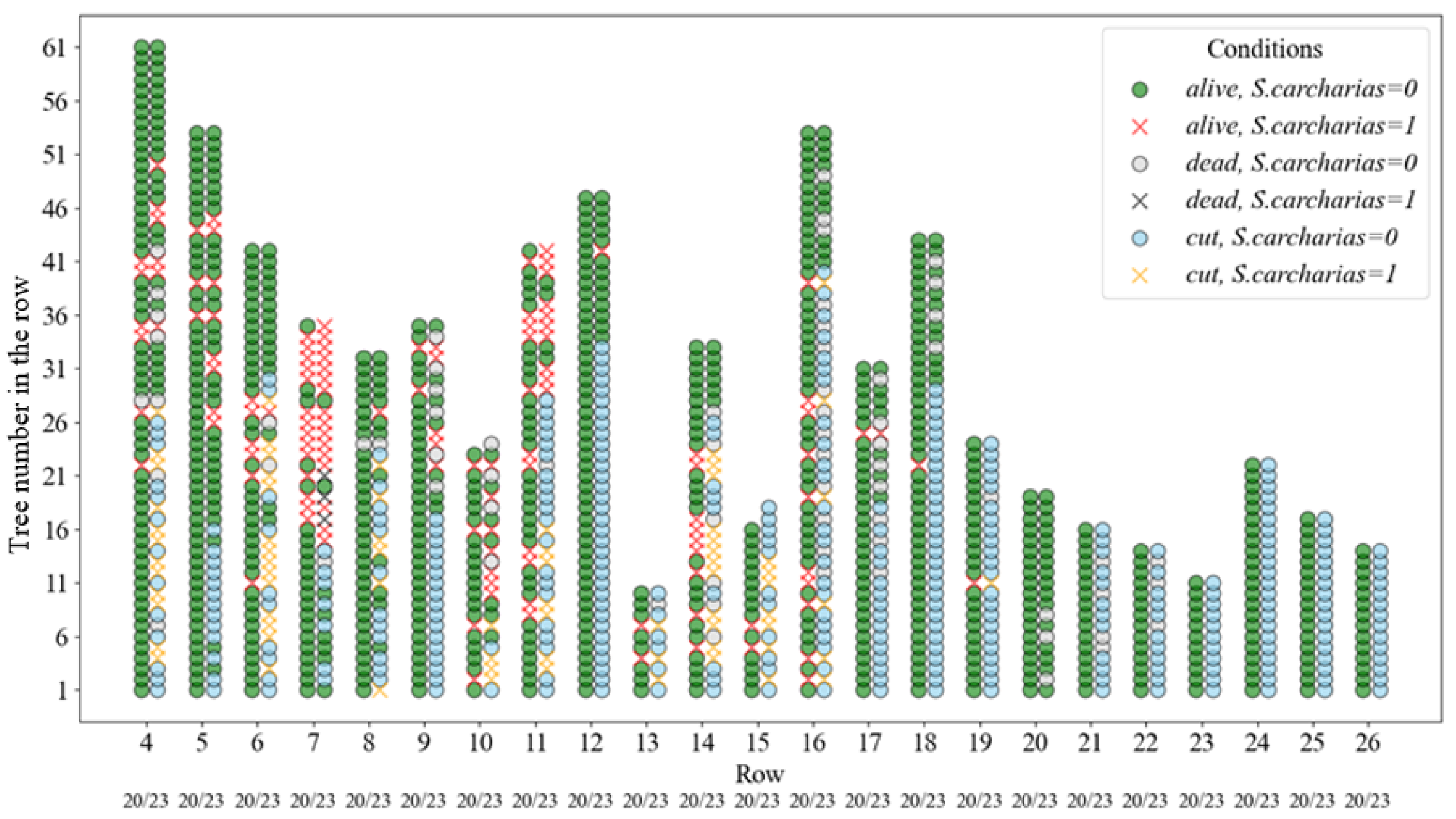
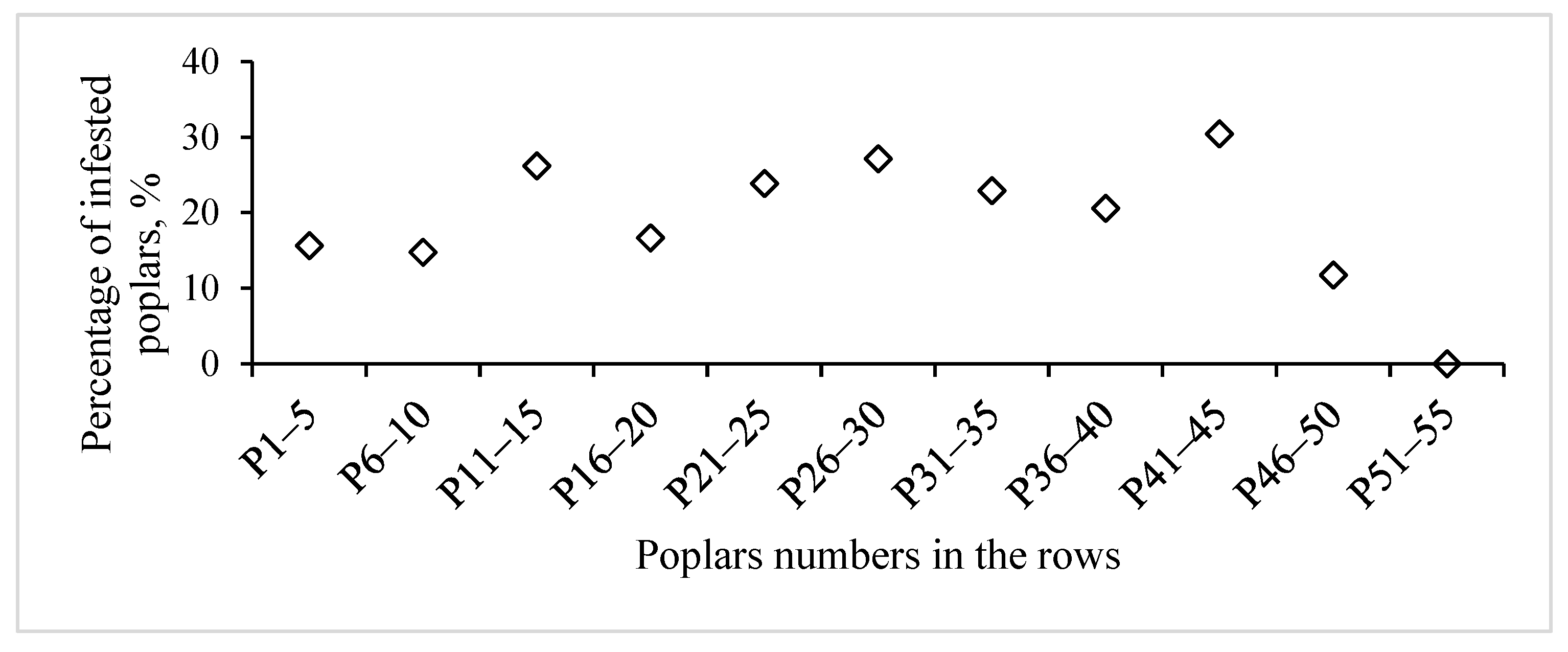
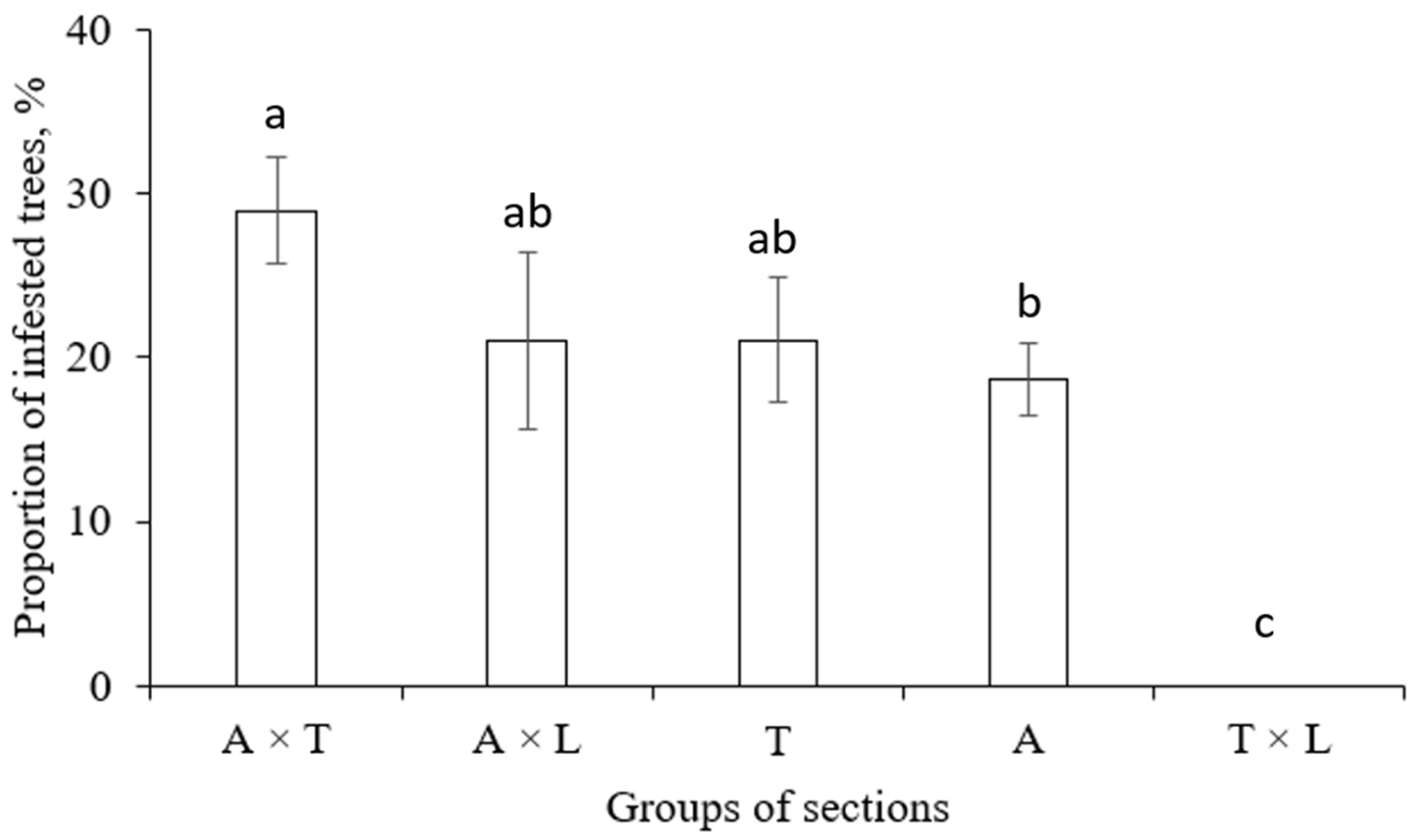
3.1. Clones Infestation by S. carcharias Depending on Placement in Plantation
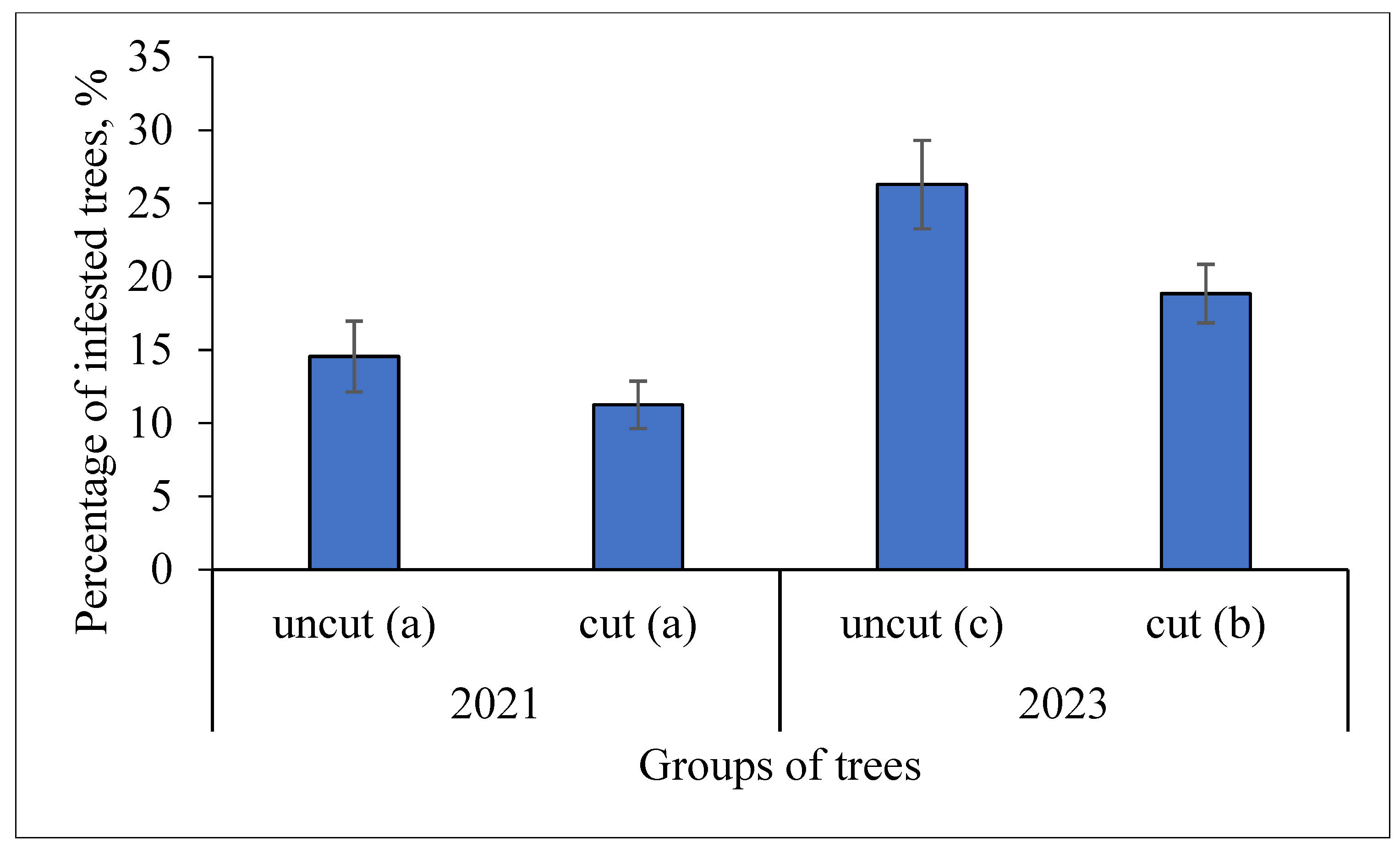
3.2. Effect of Cutting on S. carcharias Infestation
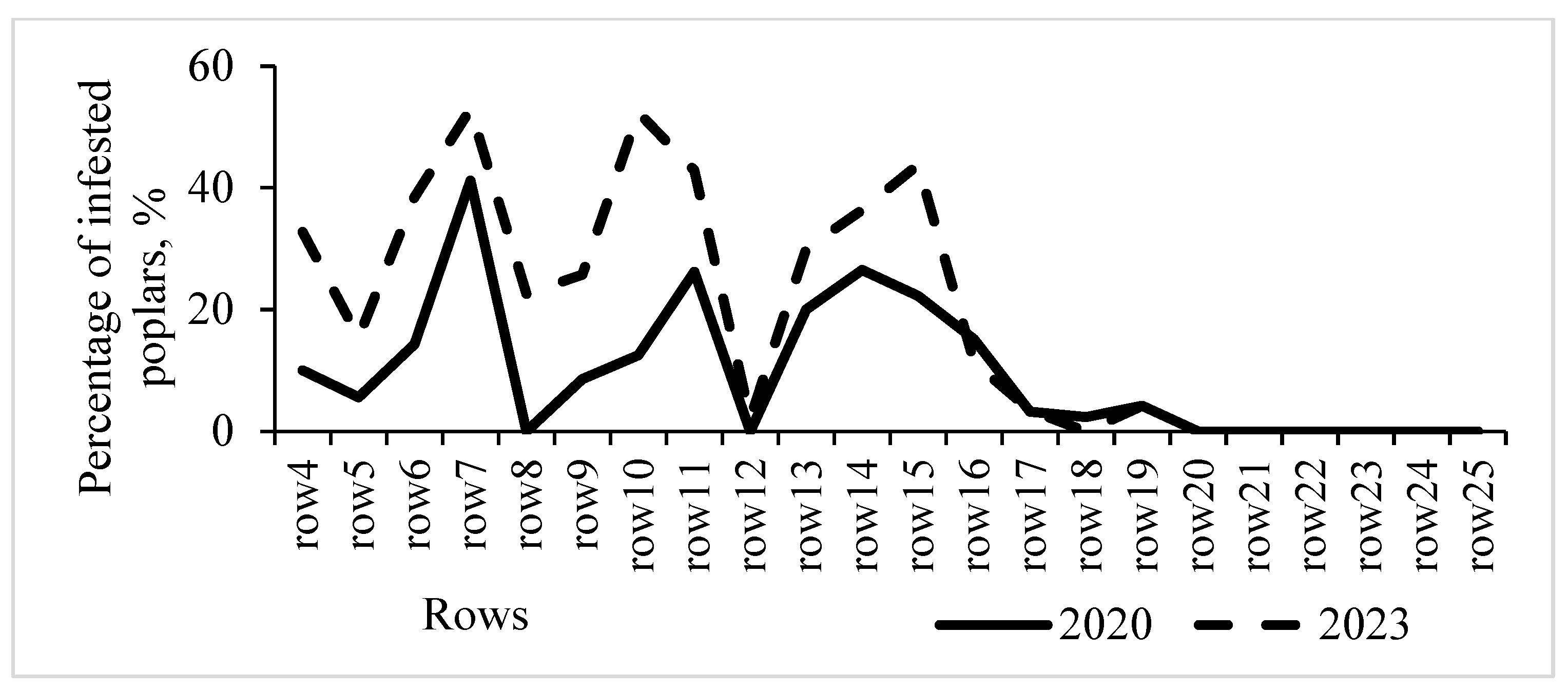
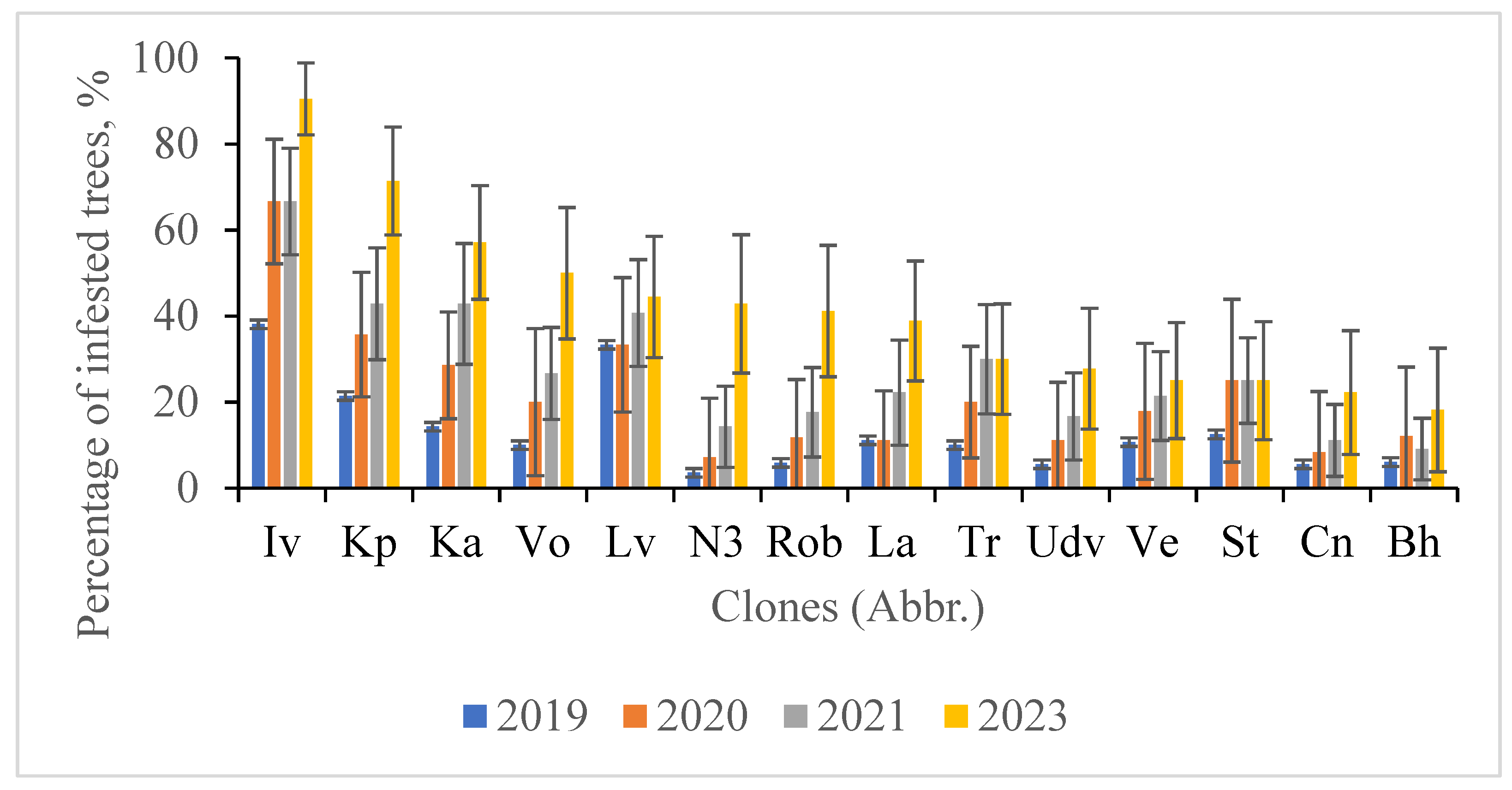
3.3. Dynamics of Poplar Clones Infestation by S. carcharias
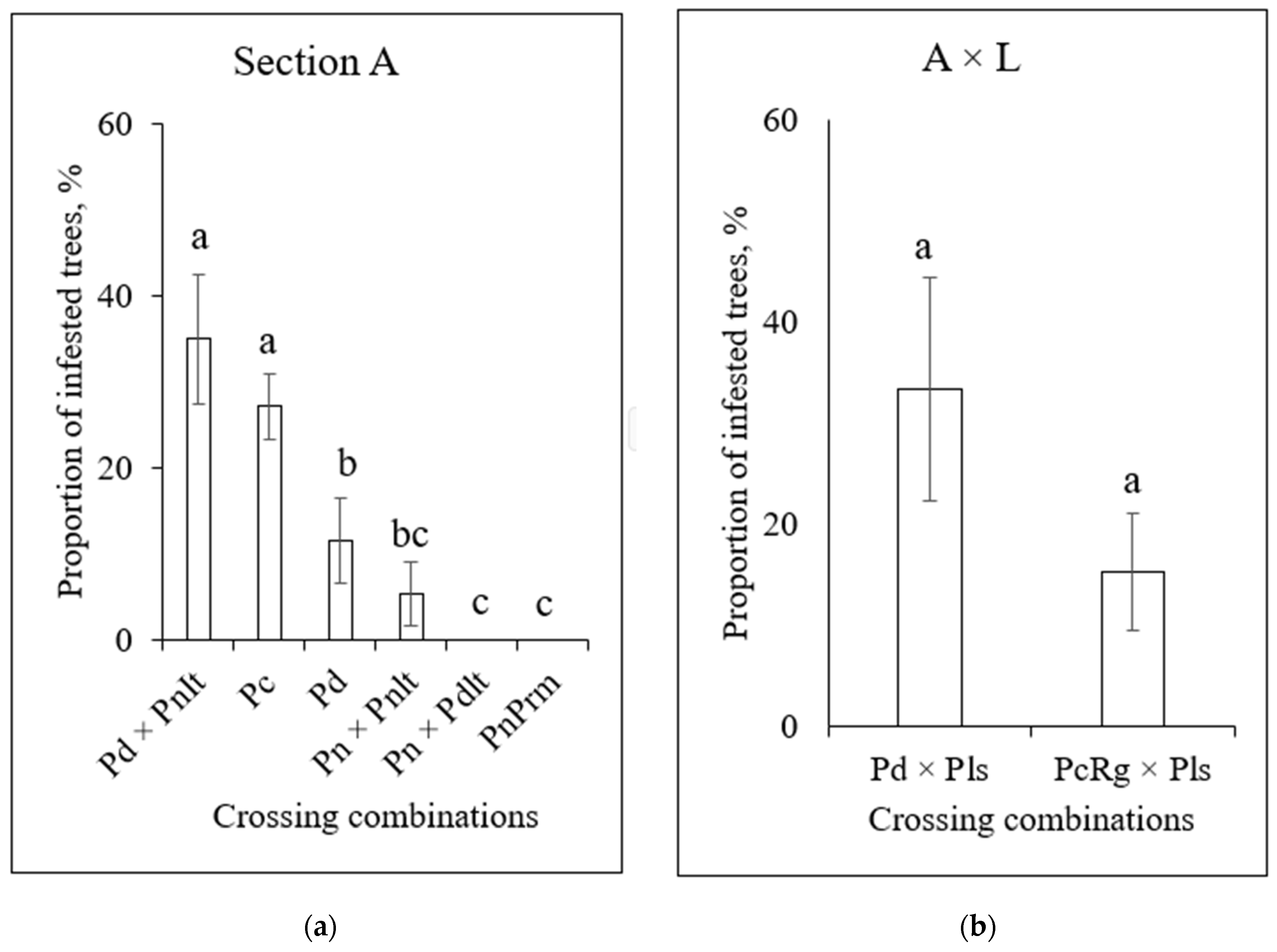
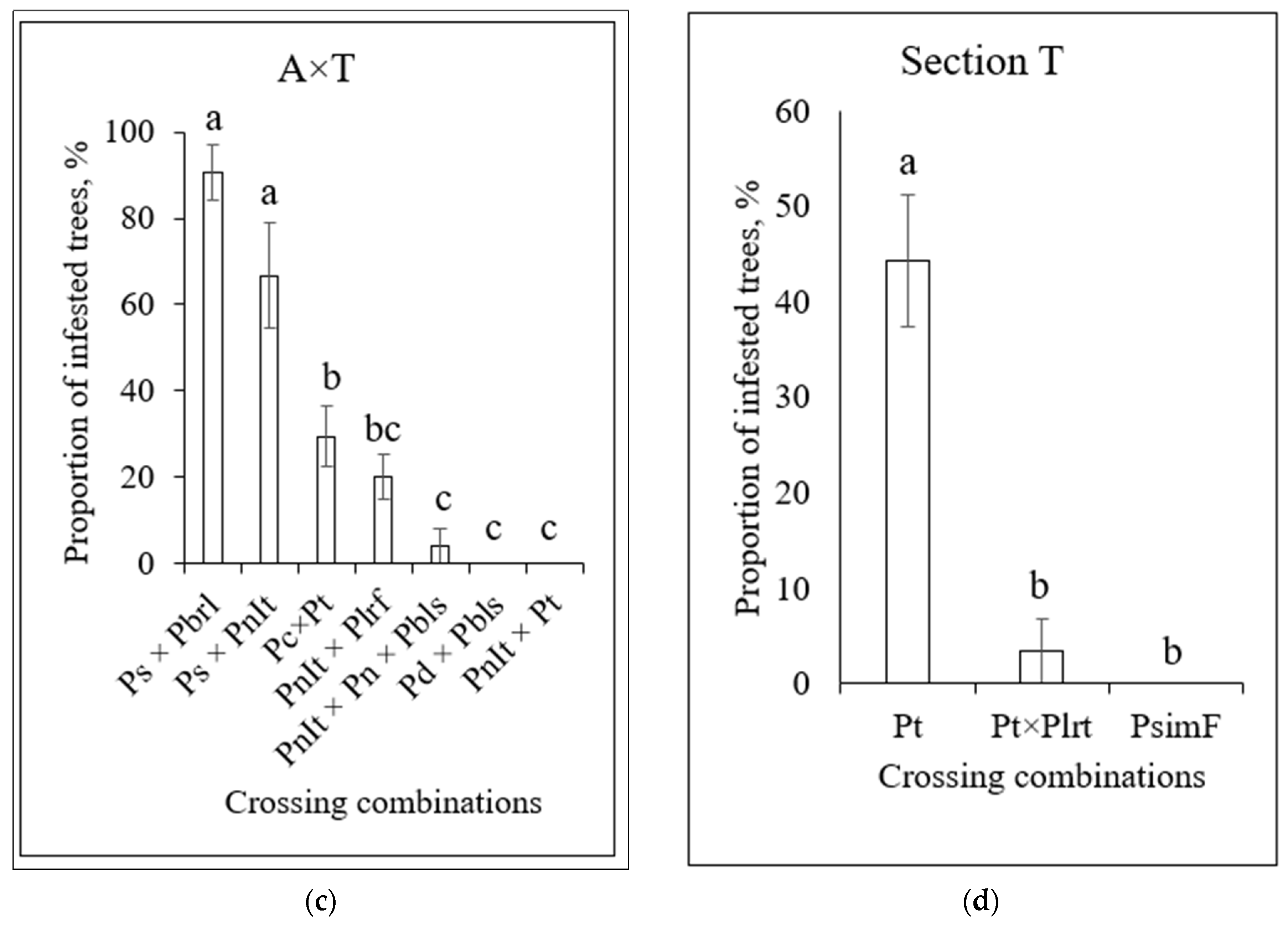
3.4. Infestation of Poplars by S. carcharias Depending on Clone Origin and Crossing Combination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kutsokon, N.K.; Khudolieieva, L.V.; Los, S.A.; Vysotska, N.Y.; Torosova, L.O.; Tkach, V.P.; Nesterenko, O.G.; Rashydov, N.M. Evaluation of growth characteristics of one-year poplar and willow clones in short-rotation plantation in Kharkiv region. Stud. Biol. 2018, 12, 55–64. [Google Scholar] [CrossRef]
- González-González, B.D.; Oliveira, N.; González, I.; Cañellas, I.; Sixto, H. Poplar biomass production in short rotation under irrigation: A case study in the Mediterranean. Biomass Bioenergy 2017, 107, 198–206. [Google Scholar] [CrossRef]
- Fuertes, A.; Oliveira, N.; Cañellas, I.; Sixto, H.; Rodríguez-Soalleiro, R.; Hanewinkel, M.; Sperlich, D. Assessing the potential of poplar short rotation plantations to contribute to a low-carbon bioeconomy under water-limited conditions. J. Environ. Manag. 2023, 347, 119062. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, E.E.A.; Schwarzbauer, P.; Fürtner, D.; Hesser, F. Life Cycle Assessment of Agricultural Wood Production—Methodological Options: A Literature Review. BioEnergy Res. 2021, 14, 492–509. [Google Scholar] [CrossRef]
- Saulino, L.; Allevato, E.; Todaro, L.; Rossi, S.; Bonanomi, G.; Saracino, A. Comparative study of hybrid and wild black poplar genotypes in the first three-year cycle of multi-stem short-rotation coppice. Biomass Bioenergy 2019, 122, 17–27. [Google Scholar] [CrossRef]
- Čakšs, R.; Zeltinš, P.; Cakša, L.; Zeps, M.; Jansons, A. The effects of frost cracks and large poplar borer damage on stem rot in hybrid aspen (Populus tremula L. × Populus tremuloides Michx.) clones. Forests 2022, 13, 593. [Google Scholar] [CrossRef]
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Carra, A. Responses to drought stress in poplar: What do we know and what can we learn? Life 2023, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dong, Y.; Zhang, Q.; Xiao, D.; Yang, M.; Wang, J. Expression of multiple exogenous insect resistance and salt tolerance genes in Populus nigra L. Front. Plant Sci. 2020, 11, 546447. [Google Scholar] [CrossRef]
- Biselli, C.; Vietto, L.; Rosso, L.; Cattivelli, L.; Nervo, G.; Fricano, A. Advanced breeding for biotic stress resistance in poplar. Plants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Nordman, E.E.; Robison, D.J.; Abrahamson, L.P.; Volk, T.A. Relative resistance of willow and poplar biomass production clones across a continuum of herbivorous insect specialization: Univariate and multivariate approaches. For. Ecol. Manag. 2005, 217, 307–318. [Google Scholar] [CrossRef]
- Charles, J.G.; Nef, L.; Allegro, G.; Collins, C.M.; Delplanque, A.; Gimenez, R.; Höglund, S.; Jiafu, H.; Larsson, S.; Luo, Y.; et al. Insect and Other Pests of Poplars and Willows. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; FAO: Rome, Italy, 2014; pp. 459–526. [Google Scholar]
- De Tillesse, V.; Nef, L.; Charles, J.; Hopkin, A.; Augustin, S. Damaging Poplar Insects; FAO: Rome, Italy, 2007. [Google Scholar]
- Mattson, W.; Lawrence, R.; Haack, R.; Herms, D.; Charles, P. Defensive strategies of woody plants against different insect feeding guilds in relation to plant ecological strategies and intimacy of association with insects. In Mechanism of Woody Plant Defenses Against Insects, Search for Pattern; Mattson, W., Levieux, J., Bernard-Dagan, J., Eds.; Springer: Berlin, Germany, 1988; p. 416. [Google Scholar]
- Skrylnyk, Y.Y.; Zhupinska, K.Y.; Koshelyaeva, Y.V.; Meshkova, V.L. Physiological harmfulness of xylophagous insects in poplar and aspen stands in the Left-Bank Forest-Steppe. For. For. Melior. 2023, 142, 147–157. [Google Scholar] [CrossRef]
- Skrylnyk, Y.Y.; Zhupinska, K.Y.; Koshelyaeva, Y.V.; Meshkova, V.L. Xylophagous insects (Insecta: Coleoptera, Hymenoptera, Lepidoptera) of Populus sp. (Malpighiales: Salicaceae) in the eastern regions of Ukraine. Kharkov Entomol. Soc. Gaz. 2023, 31, 24–30. [Google Scholar] [CrossRef]
- Timchenko, G.A.; Treml’, A.G. Pests of poplars in the eastern part of Ukraine and Crimea. Entomol. Rev. 1963, 42, 793–810. [Google Scholar]
- Tomescu, R.; Nef, L. Leaf-eating insect damage on different poplar clones and sites. Ann. For. Sci. 2007, 64, 99–108. [Google Scholar] [CrossRef][Green Version]
- Christersson, L. Biomass production of intensively grown poplars in the southernmost part of Sweden: Observations of characters, traits and growth potential. Biomass Bioenergy 2006, 30, 497–508. [Google Scholar] [CrossRef]
- Chiarabaglio, P.M.; Deidda, A.; Bergante, S.; Castro, G.; Facciotto, G.; Giorcelli, A.; Carbonaro, C. Life Cycle Assessment (LCA): New poplar clones allow an environmentally sustainable cultivation. Ann. Silvic. Res. 2020, 45, 72–82. [Google Scholar]
- Fuchylo, Y.D.; Sbytna, M.V.; Hayda, Y.I.; Kozatska, N.Y. Growth and productivity of hybrid poplar plantations in conditions of western forest-steppe of Ukraine. Sci. Bull. UNFU 2017, 27, 43–47. [Google Scholar] [CrossRef][Green Version]
- Fuchylo, Y.; Maurer, V.; Sbytna, M.; Odarchenko, I.; Fuchylo, D. Features of woody biomass and planting-stock of poplar in “stump” type of plantation management. Sci. Work. For. Acad. Sci. Ukr. 2016, 14, 134–140. [Google Scholar]
- Kang, M.S.; Pham, H.N. Simultaneous selection for high-yielding and stable crop genotypes. Agron. J. 1991, 83, 161–165. [Google Scholar] [CrossRef]
- Neyko, I.S.; Kolchanova, O.V. Adaptability and features of poplar varieties growth in conditions of the Podillya region. Sci. Bull. UNFU 2018, 28, 53–56. [Google Scholar] [CrossRef]
- Niemczyk, M.; Kaliszewski, A.; Jewiarz, M.; Wróbel, M.; Mudryk, K. Productivity and biomass characteristics of selected poplar (Populus spp.) cultivars under the climatic conditions of northern Poland. Biomass Bioenergy 2018, 111, 46–51. [Google Scholar] [CrossRef]
- Caldbeck, E.S.; McNabb, H.S., Jr.; Hart, E.R. Poplar clonal preferences of the cottonwood leaf beetle. J. Econ. Entomol. 1978, 71, 518–520. [Google Scholar] [CrossRef]
- Castellani, E.; Freccero, V.; Lapietra, G. Proposal of a scale of differentiation of poplar leaf buds useful for antiparasitic interventions. Plant Biosyst. 1967, 101, 355–360. [Google Scholar]
- Yousuf, P.; Razzak, S.; Parvaiz, S.; Rather, Y.A.; Lone, R. Role of plant phenolics in the resistance mechanism of plants against insects. In Plant Phenolics in Biotic Stress Management; Springer Nature: Singapore, 2024; pp. 191–215. [Google Scholar]
- Jia, S.; Yang, X.; Castagneyrol, B.; Yang, L.; Yin, Q.; He, C.; Hao, Z. Neighbouring tree effects on leaf herbivory: Insect specialisation matters more than host plant leaf traits. J. Ecol. 2024, 112, 189–199. [Google Scholar] [CrossRef]
- Hannon, E.R.; Kittelson, N.T.; Eaton, J.A.; Brown, J.J. Screening hybrid poplar clones for susceptibility to Cryptorhynchus lapathi (Coleoptera: Curculionidae). J. Econ. Entomol. 2008, 101, 199–205. [Google Scholar] [CrossRef]
- Marquina, J.L.; Marlats, R.; Núñez Cresto, M. Cloning susceptibility of poplar (Populus sp.) when attacked by Platypus mutatus in Buenos Aires, Argentina. Revista Bosque 2006, 27, 92–97. [Google Scholar] [CrossRef][Green Version]
- Sepúlveda, S.L.; Neale, D.B.; Holliday, J.A.; Famula, R.; Fiehn, O.; Stanton, B.J.; Guerra, F.P. GWAS on the attack by aspen borer Saperda calcarata on black cottonwood trees reveals a response mechanism involving secondary metabolism and independence of tree architecture. Forests 2023, 14, 1129. [Google Scholar] [CrossRef]
- Zhou, Y.; Ge, X.; Zou, Y.; Guo, S.; Wang, T.; Zong, S. Prediction of the potential global distribution of the Asian longhorned beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) under climate change. Agric. For. Entomol. 2021, 23, 557–568. [Google Scholar] [CrossRef]
- Palm, T. The wood borers and bark beetles of southern and central Swedish deciduous trees. Opusc. Entomol. 1959, 15–16, 334–335. [Google Scholar]
- Latzel, M.J.; Gruppe, A.; Lemme, H. Analysis of larval galleries of the large poplar longhorn beetle Saperda carcharias L. 1758 (Coleoptera, Cerambycidae). Mitteilungen Der Dtsch. Ges. Für Allg. Und Angew. Entomol. 2018, 21, 83–87. [Google Scholar]
- Ritchie, W. The structure, bionomics, and economic importance of Saperda carcharias L., the large poplar longhorn. Ann. Appl. Biol. 1920, 7, 299–343. [Google Scholar] [CrossRef]
- Šrot, M. Bionomics of poplar borer (Saperda carcharias L.). Rep. For. Res. Inst. Czechoslov. 1962, 25, 85–114. [Google Scholar]
- Zeps, M.; Senhofa, S.; Zadina, M.; Neimane, U.; Jansons, A. Stem damages caused by heart rot and large poplar borer on hybrid and European aspen. For. Stud. 2017, 66, 21–26. [Google Scholar] [CrossRef][Green Version]
- Välimäki, S.; Heliövaara, K. Hybrid aspen is not preferred by the large poplar borer (Saperda carcharias). Arthropod-Plant Interact. 2007, 1, 205–211. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Richardson, J. (Eds.) Poplars and Willows: Trees for Society and the Environment; CABI: Surrey, UK, 2014. [Google Scholar]
- Schneiderowa, J. Economical meaning of the large poplar borer (Saperda carcharias L.—Cerambycidae, Coleoptera). Pr. IBL 1961, 234, 3–98. [Google Scholar]
- Petitpierre, E. Saperda carcharias (L., 1758) nueva especie para la fauna balear (Coleoptera: Cerambycidae). Rev. Gaditana Entomol. 2018, 9, 191–192. [Google Scholar]
- Nuorteva, M.; Patomäki, J.; Saari, L. Large poplar longhorn, Saperda carcharias (L.), as food for white-backed woodpecker, Dendrocopos leucotos (Bechst.). Silva Fenn. 1981, 15, 208–221. [Google Scholar] [CrossRef][Green Version]
- Wu, H.X. Benefits and risks of using clones in forestry—A review. Scand. J. For. Res. 2019, 34, 352–359. [Google Scholar] [CrossRef]
- Fritz, R.S.; Moulia, C.; Newcombe, G. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu. Rev. Ecol. Syst. 1999, 30, 565–591. [Google Scholar] [CrossRef]
- Villari, C.; Herms, D.A.; Whitehill, J.G.; Cipollini, D.; Bonello, P. Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer, a model for wood-boring insects that kill angiosperms. New Phytol. 2016, 209, 63–79. [Google Scholar] [CrossRef]
- Patlai, I.M.; Rudenko, V.N. Selection of fast-growing forest trees in Ukraine. For. For. Melior. 1990, 81, 3–7. [Google Scholar]
- Starova, N.V. Selection of Salicaceae; Forest Industry: Moscow, Russia, 1980; pp. 1–208. [Google Scholar]
- Los, S.A.; Tereshchenko, L.I.; Gayda, Y.I.; Ustimenko, P.M. State of Forest Genetic Resources in Ukraine; PLANETA-PRINT: Kharkiv, Ukraine, 2014; 138p, ISBN 978-617-7229-06-2. [Google Scholar]
- Torosova, L.A.; Vysotska, N.Y.; Los, S.A.; Orlovska, T.V.; Zolotyh, I.V. Studies of morphological characters for representatives of Populus genus. For. For. Melior. 2015, 126, 148–157. [Google Scholar]
- Kutsokon, N.; Rakhmetov, D.; Rakhmetova, S.; Khudolieieva, L.; Rashydov, N. Nursery screening of poplar and willow clones for biofuel application in Ukraine. Iforest-Biogeosci. For. 2022, 15, 401. [Google Scholar] [CrossRef]
- Krupej, N.S. Inheritance of crown pyramidality in pyramidal poplar hybrids. For. For. Melior. 1970, 23, 71–79. [Google Scholar]
- Nazarenko, V.V.; Pasternak, V.P. Patterns of Formation of Forest Types of Forest-Steppe of the Kharkiv Region; KhNAU: Kharkiv, Ukraine, 2016; 190p. [Google Scholar]
- Zepner, L.; Karrasch, P.; Wiemann, F.; Bernard, L. ClimateCharts. Net—An interactive climate analysis web platform. Int. J. Digit. Earth 2021, 14, 338–356. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Meteofor: Weather in Kharkiv. Available online: https://meteofor.com.ua/ru/weather-kharkiv-14410 (accessed on 2 March 2024).
- Meshkova, V.L. Seasonal Development of Foliage Browsing Insects; Novoe Slovo: Kharkov, Ukraine, 2009; pp. 1–396. ISBN 978-966-2046-69-4. [Google Scholar]
- Selyaninov, G.T. Methodology of Agricultural Climate Characteristics. In World Agro-Climatic Reference Book; Hydrometeoizdat: Leningrad-Moscow, Russia, 1937; pp. 5–29. [Google Scholar]
- Atramentova, L.A.; Utevskaya, O.M. Statistical Methods in Biology; Likhtar: Gorlovka, Ukraine, 2008; 248p, ISBN 978-966-2129-26-7. [Google Scholar]
- Glen, S. Z Test: Definition & Two Proportion Z-Test. Statistics HowTo.com: Elementary Statistics for the Rest of Us! 2020. Available online: https://www.statistics-howto.com/z-test/. (accessed on 2 March 2024).
- Turok, J.; Lefèvre, F.; Cagelli, L.; De Vries, S.M.G. Populus Nigra Network. Report of the Second Meeting, 10–12 September 1995, Casale Monferrato, Italy; International PlantGenetic Resources Institute: Rome, Italy, 1996; 26p. [Google Scholar]
- Python Software Foundation, Python Language Reference. Available online: http://www.python.org (accessed on 2 March 2024).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Peck, R.; Short, T.; Olsen, C. Introduction to Statistics and Data Analysis; Cengage Learning: Boston, MA, USA, 2020. [Google Scholar]
- StatisticsLectures.com. Z-Test for Proportions, Two Samples. 2017. Available online: http://www.statisticslectures.com/topics/ztestproportions/ (accessed on 2 March 2024).
- Cochran, W.G. Some methods on strengthening the common Chi-square tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Jaworski, T.; Hilszczański, J. The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the context of expected climate change. For. Res. Pap. 2013, 74, 345–355. [Google Scholar] [CrossRef]
- Tichy, V. The influence of birds on the reduction of large poplar longhorn beetle (Saperda carcharias L.) population. Rep. For. Res. Inst. Czechoslov. 1963, 26, 49–84. [Google Scholar]
- Ayoub, L.; Yaqoob, M.; Zahoor, S.; Wani, F.F.; Irshad, S.S.; Gull, A.; Sheikh, M.A. Role of Induced Resistance in Insect-Pest Management. In Plant Resistance to Insects in Major Field Crops; Springer Nature: Singapore, 2024; pp. 249–277. [Google Scholar]
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of Cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Jactel, H.; Moreira, X.; Castagneyrol, B. Tree diversity and forest resistance to insect pests: Patterns, mechanisms, and prospects. Annu. Rev. Entomol. 2021, 66, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Broberg, C.L.; Borden, J.H. Hybrid poplar clones with Populus maximowiczii parentage demonstrate postoviposition antibiosis to Cryptorhynchus lapathi (Coleoptera: Curculionidae). J. Econ. Entomol. 2005, 98, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Kalischuk, A.R.; Gom, L.A.; Floate, K.D.; Rood, S.B. Intersectional cottonwood hybrids are particularly susceptible to the poplar bud gall mite. Can. J. Bot. 1997, 75, 1349–1355. [Google Scholar] [CrossRef]
- Moore, L.M.; Wilson, L.F. Impact of the poplar-gall saperda, Saperda inornata (Coleoptera: Cerambycidae) on a hybrid Populus plantation in Michigan. Great Lakes Entomol. 1986, 19, 163–167. [Google Scholar] [CrossRef]


| Section Abbr. * | Hybrids/Crossing Combination (Abbr. in Parentheses) | Clone Name (Abbr. and Number of Trees in Parentheses) | Ref. ** |
|---|---|---|---|
| A | P. × canadensis (Pc) | ‘Bachelieri’ (Bh; 33), ‘Constanta’ (Cn; 36), ‘Robusta’ (Rob; 17), ‘Robusta 16’ (Rob16; 8), ‘Sakrau45-51’ (Sk45; 26), ‘Sakrau79’ (Sk79; 8), ‘Tronco’ (Tr; 10), ‘Veryla’ (Ve; 28) | [39,46,49] |
| A | P. deltoides (Pd) | ‘Gulliver’ (Gu; 22), ‘Deltopodibna’ (De; 14), ‘Karolinska 162’ (Ka; 7) | [46,49] |
| A | P. deltoides × P. nigra cv. ‘Italica’ (Pd × PnIt) | ‘Strilopodibna’ (St; 40) | [47] |
| A | P. nigra × P. deltoides (Pn × Pd) | ‘Gradizka’ (Gr), ‘Keliberdynska’ (Ke) | [39,46] |
| A | P. nigra × P. nigra cv. ‘Italica’ (Pn × PnIt) | ‘Addita’ (Ad; 14), ‘Pioner’ (Pi; 13), ‘Rosijska’ (Ro; 12) | [39,49] |
| A | P. nigra cv. ‘Pyramidalis’ (PnPrm) | ‘Slava Ukrayiny’ (Sl; 17) | [46] |
| T | P. trichocarpa (Pt) | ‘Lada’ (La; 18), ‘Volosystoplidna’ (Vo; 30) | [49] |
| T | P. trichocarpa × P. laurifolia (Pt × Plrf) | ‘Druzhba’ (Dr; 29) | [49] |
| T | P. simonii f. fastigiata (PsimF) | ‘Rohanska’ (Rh; 32) | [49] |
| A × L | P. × canadensis cv. ‘Regenerata’ × P. lasiocarpa (PcRg × Pls) | ‘Perspektyvna’ (Pe; 41) | [46] |
| A × L | P. deltoides × P. lasiocarpa (Pd × Pls) | ‘Udyvytelnaya’ (Udv; 18) | [46] |
| A × T | P. × canadensis × P. trichocarpa (Pc ×Pt) | ‘Lvivska’ (Lv; 27), ‘Mobilna’ (Mo; 17) | [46] |
| A × T | P. deltoides × P. balsamifera (Pd × Pbls) | ‘Kanadska×balsamichna’ (KB; 14) | [49] |
| A × T | P. nigra cv. ‘Italica’ × P. laurifolia (PnIt × Plrf) | ‘Novoberlinska-3’ (N3; 28); ‘Novoberlinska-7’ (N7; 18) | [46,49] |
| A × T | P. nigra cv. ‘Italica’ × P. trichocarpa (PnIt × Pt) | ‘Lubenska’ (Lu; 13) | [46] |
| A × T | (P. nigra cv. ‘Italica’ × P. nigra) × P. balsamifera (PnIt × Pn × Pbls) | ‘Versia’ (Vr; 24) | [46] |
| A × T | P. suaveolens ×P. × berolinensis (Psv × Pbrl) | ‘Ivantiivska’ (Iv; 20) | [47,49] |
| A × T | P. simonii × P. nigra cv. ‘Italica’ (Psim × PnIt) | ‘Kytaiska × pyramidalna’ (Kp; 14) | [47] |
| T × L | P. trichocarpa × P. lasiocarpa (Pt × Pls) | ‘Nocturne’ (No; 17) | [47,49] |
| Clone Name | Abbr. | Row | Place in the Row * | Number of Plants | Infestation ** |
|---|---|---|---|---|---|
| ‘Bachelieri’ | Bh | 4 | end | 33 | 1 |
| ‘Constanta’ | Cn | 5 | end | 36 | 1 |
| ‘Sakrau79’ | Sk79 | 8 | end | 8 | single |
| ‘Robusta’ | Rob | 10 | end | 17 | 1 |
| ‘Veryla’ | Ve | 11 | start | 28 | 1 |
| ‘Tronco’ | Tr | 13 | start | 10 | 1 |
| ‘Sakrau45-51’ | Sk45 | 14 | end | 7 | 0 |
| ‘Sakrau45-51’ | Sk45 | 20 | start | 19 | 0 |
| Robusta 16’ | Rob16 | 25 | start | 8 | 0 |
| ‘Deltopodibna’ | De | 7 | start | 14 | 0 |
| ‘Karolinska 162’ | Ka | 10 | start | 7 | 1 |
| ‘Gulliver’ | Gu | 23 | start | 22 | 0 |
| ‘Strilopodibna’ | St | 16 | start | 40 | 1 |
| ‘Rosijska’ | Ro | 6 | end | 12 | 0 |
| ‘Addita’ | Ad | 12 | end | 14 | single |
| ‘Pioner’ | Pi | 17 | end | 13 | single |
| ‘Keliberdynska’ | Ke | 21 | start | 22 | 0 |
| ‘Gradizka’ | Gr | 22 | start | 11 | 0 |
| ‘Slava Ukrayiny’ | Sl | 9 | start | 17 | 0 |
| ‘Perspektyvna’ | Pe | 8 | start | 25 | single |
| ‘Perspektyvna’ | Pe | 20 | end | 16 | 0 |
| ‘Udyvytelnaya’ | Udv | 9 | end | 18 | 1 |
| ‘Versia’ | Vr | 19 | start | 24 | single |
| ‘Lvivska’ | Lv | 14 | start | 27 | 1 |
| ‘Mobilna’ | Mo | 24 | start | 17 | 0 |
| ‘Kanadska×balsamichna’ | KB | 18 | end | 14 | 0 |
| ‘Novoberlinska-3’ | N3 | 4 | start | 28 | 1 |
| ‘Novoberlinska-7’ | N7 | 17 | start | 18 | 0 |
| ‘Lubenska’ | Lu | 16 | end | 13 | 0 |
| ‘Kytaiska × pyramidalna’ | Kp | 11 | end | 14 | 1 |
| ‘Ivantiivska’ | Iv | 7 | end | 21 | 1 |
| ‘Rohanska’ | Rg | 12 | start | 33 | 0 |
| ‘Volosystoplidna’ | Vo | 6 | start | 30 | 1 |
| ‘Lada’ | La | 15 | start | 18 | 1 |
| ‘Druzhba’ | Dr | 18 | start | 29 | 0 |
| ‘Nocturne’ | No | 5 | start | 17 | 0 |
| Climatic Indicators * | 1990–2020 | 2019 | 2020 | 2021 | 2023 |
|---|---|---|---|---|---|
| Air temperature, T °C | |||||
| —for year | 8.8 | 10.8 | 10.4 | 9.2 | 10.3 |
| —for the growing season | 17.4 | 19.2 | 17.9 | 17.8 | 18.2 |
| Date of stable transition of temperature | |||||
| —over 5 °C | 29/03 | 19/03 | 9/03 | 2/04 | 16/03 |
| —over 10 °C | 16/04 | 9/04 | 24/04 | 23/04 | 10/04 |
| Precipitation, mm | |||||
| —for year | 535.2 | 342.3 | 494.6 | 399.0 | 694.1 |
| —for the growing season | 287.2 | 159.4 | 285.1 | 217.8 | 354.2 |
| Hydrothermal index, HTI | 0.90 | 0.45 | 0.87 | 0.67 | 1.06 |
| Clone Name | Abbr. | Zobserved | p-Value (Two-Tailed) |
|---|---|---|---|
| ‘Ivantiivska’ | Iv | 3.39 | 0.001 |
| Kytaiska × pyramidalna’ | Kp | 2.66 | 0.01 |
| ‘Karolinska 162’ | Ka | 1.76 | 0.08 |
| ‘Volosystoplidna’ | Vo | 3.51 | <0.0001 |
| ‘Lvivska’ | Lv | 0.98 | 0.33 |
| ‘Novoberlinska-3’ | N3 | 3.94 | <0.0001 |
| ‘Robusta’ | Rob | 2.62 | 0.01 |
| ‘Lada’ | La | 2.21 | 0.03 |
| ‘Tronco’ | Tr | 1.19 | 0.24 |
| ‘Udyvytelnaya’ | Udv | 1.90 | 0.6 |
| ‘Veryla’ | Ve | 1.41 | 0.16 |
| ‘Strilopodibna’ | St | 1.28 | 0.20 |
| ‘Constanta’ | Cn | 2.19 | 0.03 |
| ‘Bachelieri’ | Bh | 1.35 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshkova, V.; Zhupinska, K.; Borysenko, O.; Zinchenko, O.; Skrylnyk, Y.; Vysotska, N. Possible Factors of Poplar Susceptibility to Large Poplar Borer Infestation. Forests 2024, 15, 882. https://doi.org/10.3390/f15050882
Meshkova V, Zhupinska K, Borysenko O, Zinchenko O, Skrylnyk Y, Vysotska N. Possible Factors of Poplar Susceptibility to Large Poplar Borer Infestation. Forests. 2024; 15(5):882. https://doi.org/10.3390/f15050882
Chicago/Turabian StyleMeshkova, Valentyna, Kateryna Zhupinska, Oleksandr Borysenko, Olga Zinchenko, Yuriy Skrylnyk, and Natalia Vysotska. 2024. "Possible Factors of Poplar Susceptibility to Large Poplar Borer Infestation" Forests 15, no. 5: 882. https://doi.org/10.3390/f15050882
APA StyleMeshkova, V., Zhupinska, K., Borysenko, O., Zinchenko, O., Skrylnyk, Y., & Vysotska, N. (2024). Possible Factors of Poplar Susceptibility to Large Poplar Borer Infestation. Forests, 15(5), 882. https://doi.org/10.3390/f15050882






