Transcriptome Analysis Reveals the Response of Cryptomeria japonica to Feeding Stress of Dendrolimus houi Lajonquière Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Treatments
2.2. RNA Isolation and cDNA Library Construction
2.3. Sequence Assembly and Gene Annotation
2.4. Differential Expression Analysis and Gene Cluster Analysis
2.5. qRT-PCR Validation
3. Results
3.1. Illumina Sequencing and De Novo Assembly
3.2. Functional Annotation of Unigenes
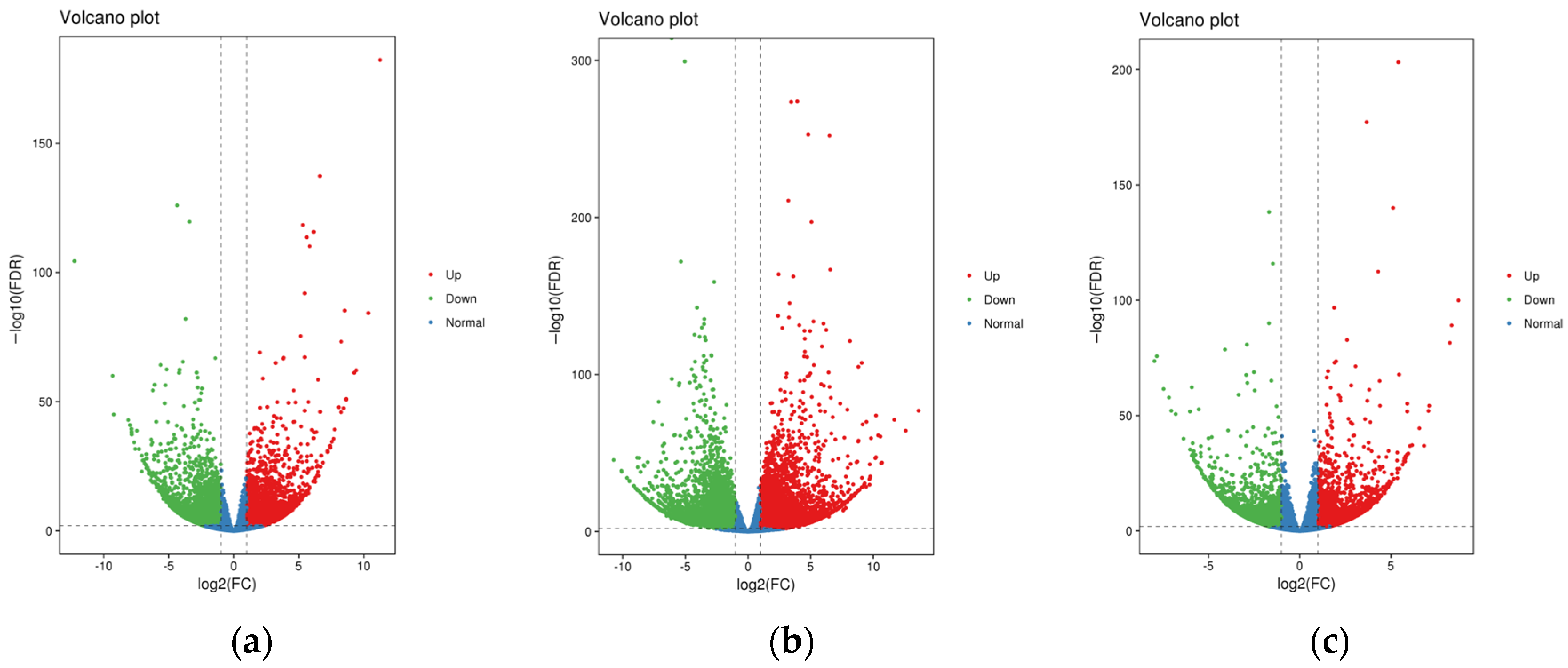
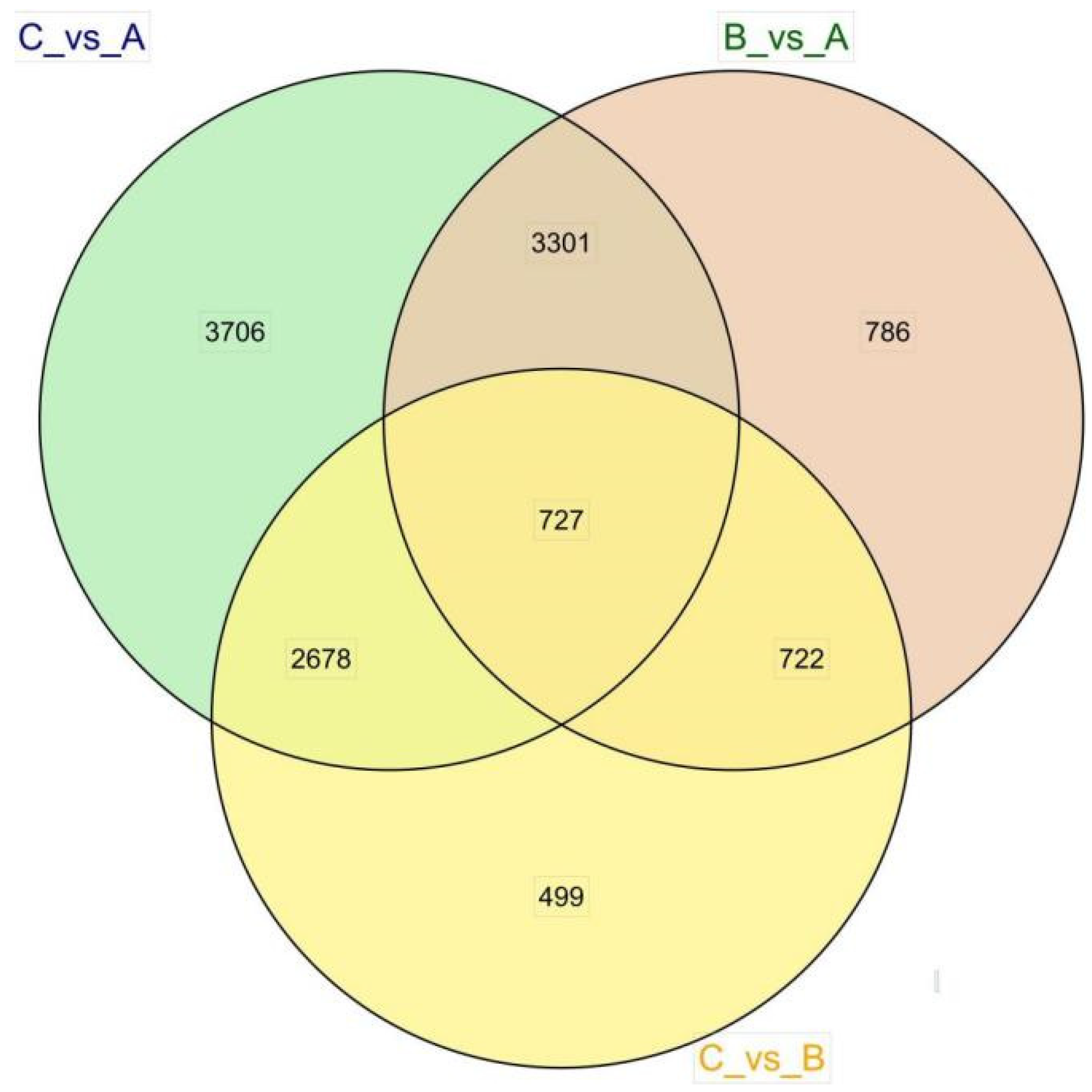
3.3. Differential Expression Analysis
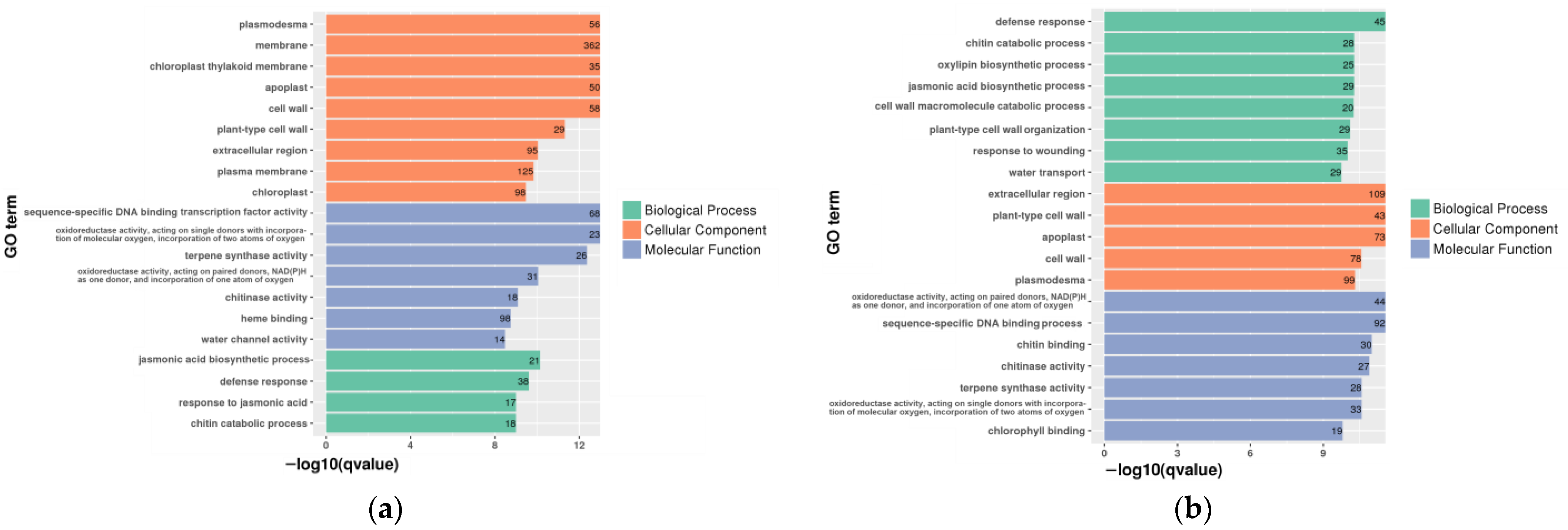
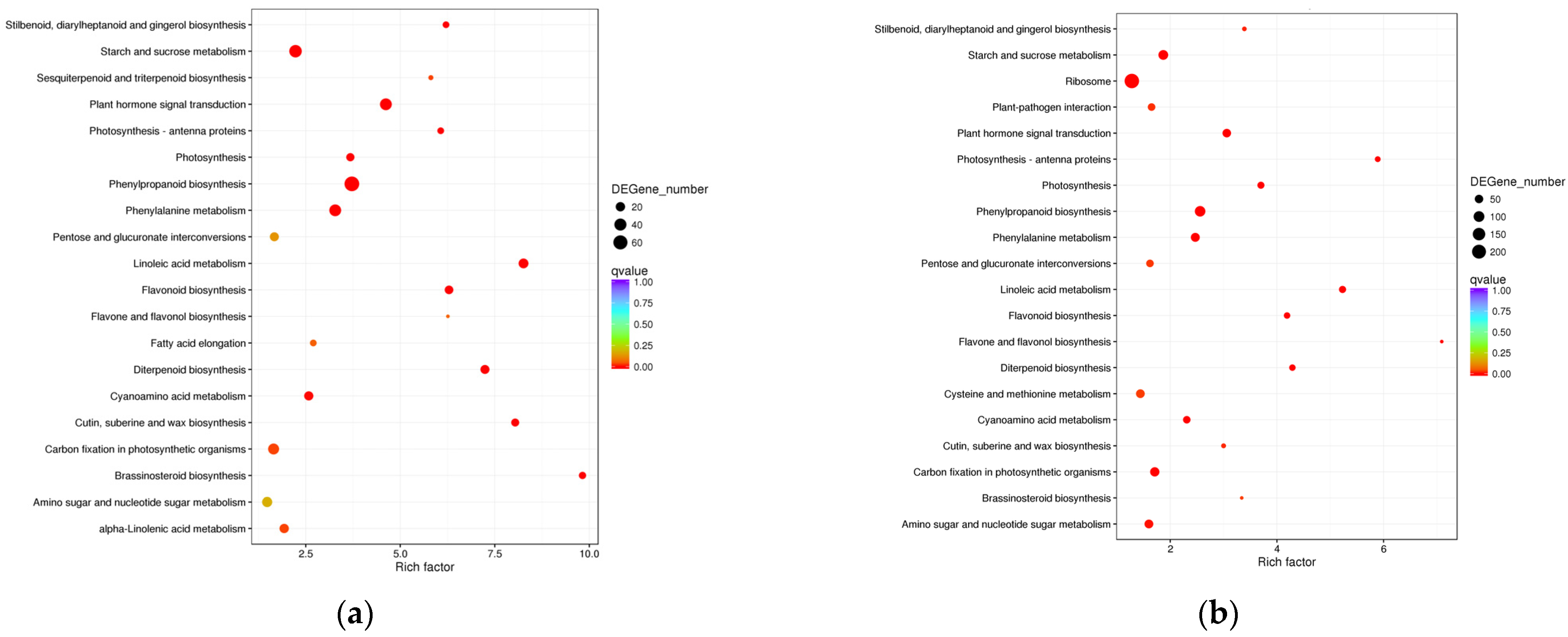
3.4. Classification of Unigenes Function and Metabolic Pathway
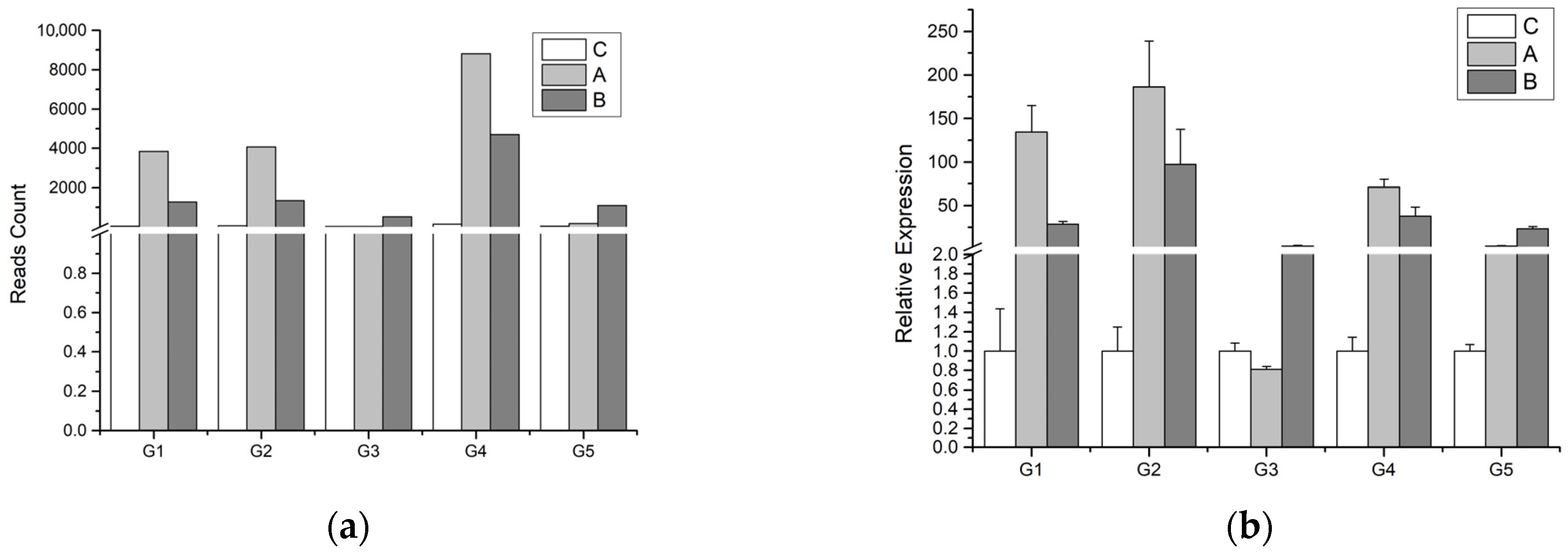
3.5. qRT-PCR Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Yang, L.; Zhang, M.; Yang, J.; Cui, J.; Hu, H.; Xu, J. CfAPX, a cytosolic ascorbate peroxidase gene from Cryptomeria fortunei, confers tolerance to abiotic stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2022, 172, 167–179. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, J.; Ouyang, L.; Shi, J. Study on regeneration system of Cryptomeria clones in vitro culture. J. Nanjing For. Univ. 2015, 58, 55. [Google Scholar]
- Lin, H.Y.; Lu, C.D.; Chen, Z.H.; Zhou, Y.J.; Liang, Y.; Chen, H.; Liang, G.H. A survey on pupae parasitoid species of Dendrolimus houi (Lajonquiere)(Lepidoptera, Lasiocampidae) in China. Biodivers. Data J. 2023, 11, e97878. [Google Scholar] [CrossRef]
- Ouyang, X.; Lin, H.; Bai, S.; Chen, J.; Chen, A. Simulation the potential distribution of Dendrolimus houi and its hosts, Pinus yunnanensis and Cryptomeria fortunei, under climate change in China. Front. Plant Sci. 2022, 13, 1054710. [Google Scholar] [CrossRef]
- Yin, A.; Chuan, R.; Xu, G. A study on the biological characteristics of Dendrolimus houi. J. Southwest For. Coll. 2002, 22, 53–55. [Google Scholar]
- Liu, X. Study on biological characteristics and its integrated control of Dendrolimus latipenis. J. Southwest For. Coll. 2006, 26, 52–54. [Google Scholar]
- Peng, Q.; Yang, Z.; Ke, M.; Yin, G.; Zhou, P. Occurrence and Controlling of Caterpillar moth in Pinus kesiya Forest in Mangshi County, Yunnan Province. For. Inventory Plan. 2014, 39, 32–35. [Google Scholar]
- Liu, J.; Lin, Q.; Zheng, S.; Li, S. The drug effect experiment of avemectin in controlling Dendrolimus Latipenis walker in forest. J. Biol. 2004, 21, 39–40. [Google Scholar]
- Hua, Y.; Lin, H.; Lu, C.; Shi, Y.; He, H.; Han, X.; Zhang, F.; Liang, G. Research advances in Dendrolimus houi Lajonquiere of China. World For. Res. 2019, 32, 62–68. [Google Scholar]
- Xu, G.; Chai, S.; Xie, K.; Luo, F.; Liu, W.; Feng, Z. A study on the bio ecological characteristics of Dendrolimus houi in Jingdong County. J. Northwest Sci-Tech Univ. Agric. For. 2002, 6, 151–155. [Google Scholar]
- Zhou, Y.; Lin, H.; Hua, Y.; Han, X.; Wei, Z.; Zhang, F.; Liang, G. Occurrence regularity and biological characteristics of Dendrolimus houi in Fujian Province. Subtrop. Agric. Res. 2019, 15, 20–26. [Google Scholar]
- Han, X.; Lu, C.; Geib, S.M.; Zheng, J.; Wu, S.; Zhang, F.; Liang, G. Characterization of Dendrolimus houi Lajonquiere (Lepidoptera: Lasiocampidae) transcriptome across all life stages. Insects 2019, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; He, H.; Lu, C.; Liang, Y.; Wu, H.; Zheng, L.; Wang, X.; Liang, G. Comparative transcriptome analysis of two populations of Dastarcus helophoroides (Fairmaire) under high temperature stress. Forests 2021, 13, 13. [Google Scholar] [CrossRef]
- Fang, X.; Chen, Z.; Chen, Z.; Chen, J.; Zhao, Z.; Wu, P.; Wu, H.; Zhang, F.; Liang, G. Metabolome and Transcriptome Analysis Reveals the Effects of Host Shift on Dendrolimus houi Lajonquière Larvae. Forests 2023, 14, 1307. [Google Scholar] [CrossRef]
- Fu, L. Taxodiaceae. Flora China 1999, 4, 54–61. [Google Scholar]
- Cai, M.; Wen, Y.; Uchiyama, K.; Onuma, Y.; Tsumura, Y. Population genetic diversity and structure of ancient tree populations of Cryptomeria japonica var. sinensis based on RAD-seq data. Forests 2020, 11, 1192. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. In Pesticides: Toxic Aspects; BoD—Books on Demand: Norderstedt, Germany, 2014; Volume 8. [Google Scholar]
- Birch, A.N.E.; Begg, G.S.; Squire, G.R. How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. J. Exp. Bot. 2011, 62, 3251–3261. [Google Scholar] [CrossRef]
- Kogan, M.; Ortman, E.F. Antixenosis–a new term proposed to define Painter’s “nonpreference” modality of resistance. Bull. ESA 1978, 24, 175–176. [Google Scholar] [CrossRef]
- Koch, K.G.; Chapman, K.; Louis, J.; Heng-Moss, T.; Sarath, G. Plant tolerance: A unique approach to control hemipteran pests. Front. Plant Sci. 2016, 7, 1363. [Google Scholar] [CrossRef]
- Lu, K.; Cheng, Y.; Li, W.; Ni, H.; Chen, X.; Li, Y.; Tang, B.; Li, Y.; Chen, D.; Zeng, R. Copper-induced H2O2 accumulation confers larval tolerance to xanthotoxin by modulating CYP6B50 expression in Spodoptera litura. Pestic. Biochem. Physiol. 2019, 159, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Manushree, V.; Devaraj, V.; Prasad, D. Expression of Cocculus hirsutus trypsin inhibitor promotes endogenous defensive response against Helicoverpa armigera and enhanced levels of antioxidants. Afr. J. Plant Sci. 2020, 14, 65–82. [Google Scholar] [CrossRef]
- Gandhi, A.; Kariyat, R.R.; Chappa, C.; Tayal, M.; Sahoo, N. Tobacco hornworm (Manduca sexta) oral secretion elicits reactive oxygen species in isolated tomato protoplasts. Int. J. Mol. Sci. 2020, 21, 8297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Nautiyal, C.S. Plant abiotic and biotic stress alleviation: From an endophytic microbial perspective. Curr. Microbiol. 2022, 79, 311. [Google Scholar] [CrossRef]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant defense and insect adaptation with reference to secondary metabolites. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 795–822. [Google Scholar]
- Divekar, P.A.; Rani, V.; Majumder, S.; Karkute, S.G.; Molla, K.A.; Pandey, K.K.; Behera, T.K.; Govindharaj, G.-P.-P. Protease inhibitors: An induced plant defense mechanism against herbivores. J. Plant Growth Regul. 2022, 42, 6057–6073. [Google Scholar] [CrossRef]
- Harish, G.N.; Singh, R.; Sharma, S.; Taggar, G.K. Changes in defense-related antioxidative enzymes amongst the resistant and susceptible soybean genotypes under whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) stress. Phytoparasitica 2023, 51, 63–75. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, J.; Li, Z.; Yang, B.; Wang, J.; Su, Z. Defensive responses of Populus deltoides cl. Beikang to mechanical injury and Anoplophora glabripennis infection. Chin. J. Appl. Ecol. 2021, 11, 4139–4146. [Google Scholar]
- Movahedi, A.; Almasi Zadeh Yaghuti, A.; Wei, H.; Rutland, P.; Sun, W.; Mousavi, M.; Li, D.; Zhuge, Q. Plant secondary metabolites with an overview of Populus. Int. J. Mol. Sci. 2021, 22, 6890. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Liang, X.; Xie, J.; Xu, H.; Luo, Q.; Yang, Z. Roles of hormones, calcium and PmWRKY31 in the defense of Pinus massoniana Lamb. against Dendrolimus punctatus Walker. For. Res. 2021, 1, 21. [Google Scholar] [CrossRef]
- Kang, J.-H.; Wang, L.; Giri, A.; Baldwin, I.T. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid–isoleucine–mediated defenses against Manduca sexta. Plant Cell 2006, 18, 3303–3320. [Google Scholar] [CrossRef]
- Chen, H.; Wilkerson, C.G.; Kuchar, J.A.; Phinney, B.S.; Howe, G.A. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 2005, 102, 19237–19242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Jasmonic acid-mediated-induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner)(Lepidoptera: Noctuidae). J. Plant Growth Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- Ramaroson, M.-L.; Koutouan, C.; Helesbeux, J.-J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of phenylpropanoids and flavonoids in plant resistance to pests and diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Bergvinson, D.J.; Burt, A.J.; Ramputh, A.I.; Díaz-Pontones, D.M.; Arnason, J.T. The role of pericarp cell wall components in maize weevil resistance. Crop Sci. 2004, 44, 1546–1552. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Kranthi, K.; Jadhav, D.; Kranthi, S.; Chandra, S. Influence of foliar chemical compounds on the development of Spodoptera litura (Fab.) in interspecific derivatives of groundnut. J. Appl. Entomol. 2004, 128, 321–328. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Smith, P.; Peiffer, M.; Helms, A.; Tooker, J.; Felton, G.W. Phytohormones in Fall Armyworm Saliva Modulate Defense Responses in Plants. J. Chem. Ecol. 2019, 45, 598–609. [Google Scholar] [CrossRef]
- Kang, K.; Yue, L.; Xia, X.; Liu, K.; Zhang, W. Comparative metabolomics analysis of different resistant rice varieties in response to the brown planthopper Nilaparvata lugens Hemiptera: Delphacidae. Metabolomics 2019, 15, 62. [Google Scholar] [CrossRef]
- Uawisetwathana, U.; Graham, S.F.; Kamolsukyunyong, W.; Sukhaket, W.; Klanchui, A.; Toojinda, T.; Vanavichit, A.; Karoonuthaisiri, N.; Elliott, C.T. Quantitative 1 H NMR metabolome profiling of Thai Jasmine rice (Oryza sativa) reveals primary metabolic response during brown planthopper infestation. Metabolomics 2015, 11, 1640–1655. [Google Scholar] [CrossRef]
- De Bruyn, L.; Scheirs, J.; Verhagen, R. Nutrient stress, host plant quality and herbivore performance of a leaf-mining fly on grass. Oecologia 2002, 130, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Castrillón-Arbeláez, P.A.; Martínez-Gallardo, N.; Arnaut, H.A.; Tiessen, A.; Délano-Frier, J.P. Metabolic and enzymatic changes associated with carbon mobilization, utilization and replenishment triggered in grain amaranth (Amaranthus cruentus) in response to partial defoliation by mechanical injury or insect herbivory. BMC Plant Biol. 2012, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Xiong, T.; Zeng, X. Aloe vera L. extracts repel oviposition by the oriental fruit fly (Bactrocera dorsalis). Chin. J. Appl. Entomol. 2017, 54, 468–474. [Google Scholar]
- Wu, L.; Li, L.; Wang, L.; Yuan, Y.; Chen, M. Effects of gallic acid on the nutritional efficiency and detoxificationenzymes in Hyphantria cunea larvae. J. Environ. Entomol. 2020, 42, 471–479. [Google Scholar]
- Ling, R.; Yang, R.; Li, P.; Zhang, X.; Shen, T.; Li, X.; Yang, Q.; Sun, L.; Yan, J. Asatone and isoasatone A against Spodoptera litura fab. by acting on cytochrome P450 monoxygenases and glutathione transferases. Molecules 2019, 24, 3940. [Google Scholar] [CrossRef] [PubMed]
- Razmjou, J.; Davari, M.; Ebadollahi, A. Effect of two plant essential oils and the entomopathogenic fungus, Lecanicillium muscarium (zare & gams) on the cotton aphid, Aphis gossypii glover. Egypt. J. Biol. Pest Control 2016, 26, 775–779. [Google Scholar]
- Wang, T.; Shi, D.; Zhang, S.; Du, K. Effects of four plant secondary metabolites on the growth and development of Tenebrio molitor and the synergistic effect of Vitamin B1. For. Ecol. Sci. 2021, 1, 49–55. [Google Scholar]
- Puri, S.; Singh, S.; Sohal, S.K. Inhibitory effect of chrysin on growth, development and oviposition behaviour of melon fruit fly, Zeugodacus cucurbitae (Coquillett)(Diptera: Tephritidae). Phytoparasitica 2022, 50, 151–162. [Google Scholar] [CrossRef]
- Bruinsma, M.; Posthumus, M.A.; Mumm, R.; Mueller, M.J.; van Loon, J.J.; Dicke, M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: Effects of time and dose, and comparison with induction by herbivores. J. Exp. Bot. 2009, 60, 2575–2587. [Google Scholar] [CrossRef]
- Heil, M.; Greiner, S.; Meimberg, H.; Krüger, R.; Noyer, J.-L.; Heubl, G.; Eduard Linsenmair, K.; Boland, W. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 2004, 430, 205–208. [Google Scholar] [CrossRef]
- Kessler, A. The information landscape of plant constitutive and induced secondary metabolite production. Curr. Opin. Insect Sci. 2015, 8, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, C.; Wu, Z.; Zhang, X.; Luo, C.; Cheng, X. Molecular Regulation of Plant Flavonoid Biosynthesis Pathway. North Hortic. 2011, 4, 204–207. [Google Scholar]
- Huang, H.J.; Ye, Z.X.; Lu, G.; Zhang, C.X.; Chen, J.P.; Li, J.M. Identification of salivary proteins in the whitefly Bemisia tabaci by transcriptomic and LC–MS/MS analyses. Insect Sci. 2021, 28, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zou, J.; Lin, X.; Zhang, H.; Yu, N.; Liu, Z. Nilaparvata lugens salivary protein NlG14 triggers defense response in plants. J. Exp. Bot. 2022, 73, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Wang, Y.-Z.; Li, L.-L.; Lu, H.-B.; Lu, J.-B.; Wang, X.; Ye, Z.-X.; Zhang, Z.-L.; He, Y.-J.; Lu, G. Planthopper salivary sheath protein LsSP1 contributes to manipulation of rice plant defenses. Nat. Commun. 2023, 14, 737. [Google Scholar] [CrossRef] [PubMed]
- Zogli, P.; Pingault, L.; Grover, S.; Louis, J. Ento(o)mics: The intersection of ‘omic’approaches to decipher plant defense against sap-sucking insect pests. Curr. Opin. Plant Biol. 2020, 56, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Brenya, E.; Chen, Z.-H.; Tissue, D.; Papanicolaou, A.; Cazzonelli, C.I. Prior exposure of Arabidopsis seedlings to mechanical stress heightens jasmonic acid-mediated defense against necrotrophic pathogens. BMC Plant Biol. 2020, 20, 548. [Google Scholar] [CrossRef]
- Huang, T.; Jander, G.; de Vos, M. Non-protein amino acids in plant defense against insect herbivores: Representative cases and opportunities for further functional analysis. Phytochemistry 2011, 72, 1531–1537. [Google Scholar] [CrossRef]
- Lim, G.-H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid–and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Adão, P.; Reboleira, J.; Teles, M.; Santos, B.; Ribeiro, N.; Teixeira, C.M.; Guedes, M.; Pessoa, J.C.; Bernardino, S. Enhancement of the antioxidant and antimicrobial activities of porphyran through chemical modification with tyrosine derivatives. Molecules 2021, 26, 2916. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.; Chen, W.; Zhang, G.; Wang, Q. Characterization of highly active tyrosine ammonia lyase and its application in biosynthesis of p-coumaric acid. Chin. J. Biotechnol. 2022, 38, 4553–4566. [Google Scholar]
- Vallverdú-Queralt, A.; Verbaere, A.; Meudec, E.; Cheynier, V.; Sommerer, N. Straightforward method to quantify GSH, GSSG, GRP, and hydroxycinnamic acids in wines by UPLC-MRM-MS. J. Agric. Food Chem. 2015, 63, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Saadat, S.; Abbey, L. Effect of exogenous amino acids application on growth and nutritional value of cabbage under drought stress. Sci. Hortic. 2020, 272, 109561. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Z.; Tian, H.; Li, X.; Zhang, Y. Arabidopsis CALMODULIN-BINDING PROTEIN 60b plays dual roles in plant immunity. Plant Commun. 2021, 2, 100213. [Google Scholar] [CrossRef]
- Lv, Z.-Y.; Sun, W.-J.; Jiang, R.; Chen, J.-F.; Ying, X.; Zhang, L.; Chen, W.-S. Phytohormones jasmonic acid, salicylic acid, gibberellins, and abscisic acid are key mediators of plant secondary metabolites. World J. Tradit. Chin. Med. 2021, 7, 307–325. [Google Scholar]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Oliveira, D.; Isaias, R.; Fernandes, G.; Ferreira, B.; Carneiro, R.; Fuzaro, L. Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J. Insect Physiol. 2016, 84, 103–113. [Google Scholar] [CrossRef]
- Takeda, S.; Hirano, T.; Ohshima, I.; Sato, M.H. Recent progress regarding the molecular aspects of insect gall formation. Int. J. Mol. Sci. 2021, 22, 9424. [Google Scholar] [CrossRef]
- Murakami, R.; Ushima, R.; Sugimoto, R.; Tamaoki, D.; Karahara, I.; Hanba, Y.; Wakasugi, T.; Tsuchida, T. A new galling insect model enhances photosynthetic activity in an obligate holoparasitic plant. Sci. Rep. 2021, 11, 13013. [Google Scholar] [CrossRef]



| Gene | Forward Primer | Reverses Primer |
|---|---|---|
| G1:DN215200_c3_g9 | ATTGCGTGTGAATTGCGGTT | TTGCCCCTCTGGTACAGACT |
| G2:DN164112_c1_g1 | TATGAGCAGCCTGCATACGG | GGCAGAACTTTCTCCGACCA |
| G3:DN218129_c5_g3 | AGCTACTCGTTCGCCATTGT | ATCCGGAAATAGTGACCCGC |
| G4:DN222603_c3_g2 | ACGATCCGGACATTGTGGAC | ACGATGCGATCTCTCCCAAC |
| G5:DN226353_c0_g1 | GTGGGGACCGTCAGAACAAA | TTAGAGCTCTTGCACACGGG |
| G6:DN228798_c1_g1 | CGGCATTTAAGGGAGCGGTA | ACTCTCCTCCAGTAGCCTCG |
| Anno_Database | Annotated_Number | 300 ≤ Length < 1000 | Length ≥ 1000 |
|---|---|---|---|
| COG | 87,299 | 19,007 | 6891 |
| GO | 99,644 | 25,238 | 11,486 |
| KEGG | 35,133 | 9827 | 5240 |
| KOG | 69,706 | 19,321 | 10,061 |
| Pfam | 105,308 | 26,872 | 14,318 |
| Swissprot | 110,935 | 29,302 | 13,806 |
| Nr | 144,601 | 38,060 | 16,934 |
| All | 177,418 | 43,641 | 17,085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Que, Y.; Xie, W.; Fang, X.; Xu, H.; Ye, S.; Wu, S.; Zheng, Y.; Lin, X.; Zhang, F.; Liang, G. Transcriptome Analysis Reveals the Response of Cryptomeria japonica to Feeding Stress of Dendrolimus houi Lajonquière Larvae. Forests 2024, 15, 85. https://doi.org/10.3390/f15010085
Que Y, Xie W, Fang X, Xu H, Ye S, Wu S, Zheng Y, Lin X, Zhang F, Liang G. Transcriptome Analysis Reveals the Response of Cryptomeria japonica to Feeding Stress of Dendrolimus houi Lajonquière Larvae. Forests. 2024; 15(1):85. https://doi.org/10.3390/f15010085
Chicago/Turabian StyleQue, Yuwen, Weiwei Xie, Xinyuan Fang, Han Xu, Shuting Ye, Shanqun Wu, Yican Zheng, Xiaochun Lin, Feiping Zhang, and Guanghong Liang. 2024. "Transcriptome Analysis Reveals the Response of Cryptomeria japonica to Feeding Stress of Dendrolimus houi Lajonquière Larvae" Forests 15, no. 1: 85. https://doi.org/10.3390/f15010085
APA StyleQue, Y., Xie, W., Fang, X., Xu, H., Ye, S., Wu, S., Zheng, Y., Lin, X., Zhang, F., & Liang, G. (2024). Transcriptome Analysis Reveals the Response of Cryptomeria japonica to Feeding Stress of Dendrolimus houi Lajonquière Larvae. Forests, 15(1), 85. https://doi.org/10.3390/f15010085








