Abstract
Pine pitch canker, caused by the ascomycete Fusarium circinatum, poses a substantial threat to pine trees and Douglas firs (Pseudotsuga menziesii), and has been identified as a pervasive issue in forests and nurseries worldwide, particularly in regions where susceptible conifers are cultivated. Given its prevalence in the Iberian Peninsula, assessments of the susceptibility of diverse European provenances of Scots pine (Pinus sylvestris)—specifically those from Poland, Lithuania, and Ukraine—have been conducted. Preliminary evaluations of Polish provenances have raised concerns about the potential threat to Scots pine stands in Poland posed by pitch canker. Under controlled conditions, we examined the impact of F. circinatum inoculation on the survival of seeds and seedlings from ten provenances of Scots pine. In response, the initial assessment of F. circinatum pathogenicity was undertaken in a controlled greenhouse environment. This evaluation uncovered a heightened susceptibility of pine seedlings to pitch canker among the tested provenances. Notably, one Lithuanian provenance demonstrated superior resistance to pitch canker, while two Polish provenances exhibited a higher prevalence of symptomless seedlings. These findings underscore the need for further exploration and identification of resilient individuals within these provenances, offering valuable insights for developing strategies to mitigate the impact of pitch canker on Scots pine in Europe.
1. Introduction
A plethora of Fusarium species are acknowledged as causative agents of severe forest diseases, posing significant challenges to forest nurseries and plantations worldwide [1,2]. Among these, Fusarium circinatum Nirenberg & O’Donnell stands out as a prominent instigator of damping-off and root rot in pine seedling nurseries, leading to substantial damage to conifers [3] and posing a significant threat. This fungal pathogen is particularly notorious for its capacity to inflict widespread and destructive losses on numerous conifer species [4], notably through the induction of pitch canker (PPC) of susceptible tree species [5].
Originally believed to have originated in North America, F. circinatum has spread to Europe, with reported occurrences in Portugal, Spain, France, and Italy across various susceptible Pinus species [5]. The fungus has only been reported to infect one known host outside the genus Pinus, which is Pseudotsuga menziesii (Douglas fir), observed to be infected under field conditions in California [6]. Currently, the disease in Europe has only been reported from the Iberian Peninsula, with a high likelihood of further entry and spread within the EU [4]. Anticipated alterations in pine pitch canker distribution, influenced by rapid global climate change and diverse pathways for pathogen entry, underscore the need for proactive measures. The extent and severity of PPC are also expected to depend on host factors and other forest and climatic conditions. Furthermore, the latent phase of infection complicates early identification, posing a significant challenge for timely intervention and containment measures [1]. This latent phase increases the risk of PPC spreading from nurseries to forests, emphasizing the importance of identifying vulnerable and tolerant species or provenances to mitigate its rapid spread into disease-free European countries. While natural infections primarily occur through wounds, the pathogen also has the ability to colonize the roots of mature trees without causing damage to surrounding tissues, thereby enabling plants to avoid exhibiting symptoms [7].
F. circinatum exhibits a considerable capacity to establish itself in various parts of Europe. According to the European Food Safety Authority (EFSA), the impact of PPC in regions conducive to its proliferation could be substantial [8]. PPC affects over 60 species of Pinus, including P. sylvestris L., across all stages of tree development [7]. In adult trees, the disease manifests through symptoms such as pitch flow, the formation of resinous cankers, and branch and crown dieback. In nurseries, symptoms include tip dieback and damping-off of seedlings [7].
The fungus causes resin-soaked cankers on roots and root collars of mature trees, while also being present internally in pine seeds or as a surface contaminant [7]. Although ascospores are presumed to have a minimal role in infection, the pathogen primarily spreads through macroconidia and microconidia carried by rain, wind, or insects [4,7]. While bark-feeding insects may contribute to infection through wounds, recent studies suggest F. circinatum can colonize seedlings and tree branches without wounds [7]. Predicting the environmental suitability for PPC infection is complex due to the influence of various factors on both F. circinatum and its hosts and vectors. High sporulation occurs under conditions of high relative humidity, either through precipitation or fog, coupled with cooler temperatures (15–25 °C), while sporulation diminishes as temperatures rise, and ceases when minimum temperatures approach zero [8]. These patterns suggest that the disease may proliferate more readily in coastal, mild, wet climates rather than in mountainous or continental climates with extreme temperature fluctuations.
Numerous studies have delved into the infection mechanisms and virulence of F. circinatum. Following conidial germination, the pathogen radially colonizes the host stem, progressing towards the pith, potentially facilitated by the release of cell wall-degrading enzymes to extract nutrients from the host tissues [9]. Previous studies have also explored variation in susceptibility within certain Pinus families, clones, and hybrids [5], revealing significant differences in tolerance to F. circinatum among various pine species and provenances. Various phenotypic and molecular characteristics have been linked to F. circinatum pathogenicity or virulence [7]. Our previous study also presented the first investigation into the susceptibility of Polish provenances of Scots pine (Pinus sylvestris) to F. circinatum infection, revealing high susceptibility among all tested pines and highlighting the potential risk of establishment posed by this invasive pathogen in Poland [10]. These findings are of particular interest for forestry in Europe, North America, and South Africa [1,7].
Against this backdrop, our study aims to investigate differences in the pathogenicity of F. circinatum in seeds and seedlings of Pinus sylvestris provenances from Poland, Lithuania, and Ukraine. This research seeks to facilitate the rapid selection of PPC-tolerant or less susceptible individuals, offering valuable insights for subsequent tree breeding trials.
2. Materials and Methods
2.1. Fungal Isolate and Plant Material for Testing
The F. circinatum isolate (FcCa6) utilized in this study belongs to mating type 2 (MAT-2) and was isolated from an infected tree of Pinus radiata D. Don. located in Comillas (Cantabria, Northern Spain; GPS: 4°17′17.706″ W; 43°20′05.033″ N) [11]. The isolate was obtained through the quarantine service, and all testing procedures were conducted at the Research Centre of Quarantine, Invasive, and Genetically Modified Organisms, Institute of Plant Protection (Poland). The F. circinatum inoculum was produced on potato dextrose agar (PDA) at 25 °C.
To evaluate the susceptibility of P. sylvestris within its native range, trees were germinated from seeds representing populations in various provenances in Poland, Ukraine, and Lithuania. These seeds were sourced from national gene bank collections (Table 1).

Table 1.
Origin of seeds of P. sylvestris used in this study.
The selected forest stands in Poland originated from six commercial forests spanning four major regions: the central region of the country (Łochów), the eastern border near Ukraine (Józefów), the northern region (Woziwoda) within Bory Tucholskie, and the northwest (Smolarz). These provenances were chosen for their representation of typical pine forests that thrive on post-glacial, poor sandy soils. Despite these conditions, they exhibit robust growth parameters and are emblematic of Polish forests. The choice of Ukrainian (Makariv and Manevychy) and Lithuanian (Šlienava) provenances was primarily justified by their geographic proximity to Poland, facilitating a relevant comparison in terms of environmental conditions and potential pathogen spread. Additionally, these forests in Lithuania and Ukraine represent typical forested areas in their respective countries, ensuring the inclusion of diverse and representative ecosystems in the study.
Seeds from selected mother trees with confirmed optimal phenotypic characteristics were used for the study, sourced from the Forest Gene Bank in Kostrzyca, Nature Research Centre in Lithuania, and Ukrainian Research Institute of Forestry and Forest Melioration. These seeds, of consistent age and quality, were obtained from a separate batch than those used in a previous study.
This assessment aimed to establish a direct correlation with existing data for the trees, including high viability, tolerance to stress, and resistance to pine diseases. Each seed lot included three hundred seeds that were divided, with one portion (two hundred seeds) used in assays conducted at the seedling emergence stage (Experiment 1), as described below, and another portion (one hundred seeds) grown for use in an inoculation assay conducted at four months of age (Experiment 2).
Cultures of FcCa6 were grown on potato dextrose agar (PDA) at 24 °C for 5 days. Spores were then harvested by flooding plates with 25 mL of sterile water and scraping the colonies with sterile glass beads. The resulting suspension was filtered through double layers of sterile cheesecloth. The spore density in the suspension was estimated using a hemacytometer, and subsequently diluted with sterile water to obtain spore densities of 5000 spores per mL.
2.2. In Planta Pathogenicity Test
Each trial encompassed distinct sets of treatments. Stratified seeds of P. sylvestris from diverse provenances in Poland, Ukraine, and Lithuania were enclosed in new mesh bags and immersed in tap water to reduce seed contaminants. The absence of F. circinatum in Poland, Lithuania, and Ukraine meant that this pathogen should be absent. In preparation for planting, all seeds underwent surface sterilization in 30% H2O2 for 30 min, followed by ten rinses with sterile distilled water.
Experiment 1: Two hundred seeds from each trial were germinated at approximately 22 °C on moistened, sterile Whatman No. 3 filter paper within sterile Petri dishes (150 × 20 mm) without any artificial lighting. All tested seeds were inoculated by pipetting 200 µL of a spore solution into Petri dishes and subsequently incubating them at 23–24 °C. Conidial suspensions were applied to each Petri dish without direct contact with the seeds. Control groups for each provenance were inoculated with 200 µL of sterile water. Seed germination progress was monitored daily, with the threshold that seeds were germinated when their primary root reached a length of at least 5 mm. The evaluation period for the F. circinatum impact on germination lasted 30 days for each trial, during which seedling mortality was recorded. Diseased seedlings were excised, and roots were meticulously washed and placed directly on PDA for re-isolation of the inoculated isolates [12]. After 30 days, roots of surviving seedlings were scrutinized for length and condition. Root samples from surviving seedlings were also analyzed for evidence of infection by the inoculated isolates, as previously described. Following the examination of seedlings for disease symptoms, re-isolations onto MEA were performed for all inoculated and control seedlings (Figure 1).
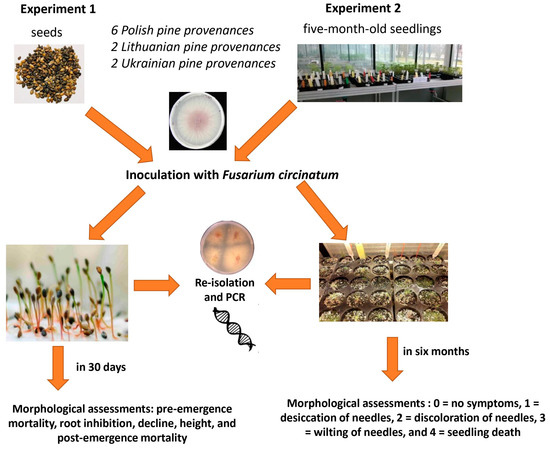
Figure 1.
The experimental designs of Experiments 1 and 2.
Experiment 2: One hundred seeds per treatment were individually sown in 200 mL cylindrical pots containing a sterilized mixture of peat and vermiculite (1:1, v/v). Two seeds per pot were sown at a depth of approximately 3 cm. Emerging seedlings were cultivated in the greenhouse at 22–23 °C with a 14/10 h light/dark photoperiod and watering twice a week. If both seeds germinated in the same pot, the second seedlings were removed within 1–2 days of emergence [13].
Subsequently, five-month-old seedlings for each treatment were inoculated by pipetting 1 mL of a spore solution of F. circinatum (5000 spores mL−1) onto the soil surface or irrigated with 1 mL of sterile water (control). Morphological assessments of all pine seedlings were conducted every three days to assess the severity of typical symptoms of pitch canker. Seedlings were rated for symptom development on a 0–4-point scale: 0 = no symptoms, 1 = desiccation of needles, 2 = discoloration of needles, 3 = wilting of needles, and 4 = seedling death [14,15]. The final disease evaluation was at 180 days post-inoculation. Seedling heights were measured, and half of the seedlings were individually collected for re-isolation [12]. Re-isolation from both symptomatic and asymptomatic seedlings involved surface sterilization in a solution of 1.5% sodium hypochlorite for up to 1 min and rinsing with sterile distilled water. These samples were then placed onpotato dextrose agar (PDA) supplemented with 100 mg/mL streptomycin sulphate. For symptomatic and asymptomatic seedlings, isolation was performed from washed sections below the collar and root. Plates were then incubated at room temperature with day/night light alternation, and typical F. circinatum colonies were transferred to PDA for morphological confirmation and further molecular identification, as described in Section 2.3 [11,16].
The remaining root samples were placed in 2 mL Eppendorf tubes for DNA extraction. The collected samples were homogenized by cutting with surface-sterilized scissors to 10 mm lengths and shaking in 600 mL of extraction buffer, followed by the addition of two glass beads to every Eppendorf tube. Finally, DNA extraction and conventional PCR tests were conducted on all samples to detect F. circinatum using prescribed tests and species-specific primers (CIRC1A and CIRC4A, as described in Section 2.3) (Figure 1).
In this study, the methodology incorporated insights gained from a previous investigation conducted in 2018 [10]. As a result, adjustments were made regarding the age of the seedlings tested. While two-month-old seedlings proved to be overly susceptible and twelve-month-old seedlings too tolerant, we opted to test five-month-old seedlings in Experiment 2 with ratings at six months. This was a compromise compared to the previous study from 2018, where susceptibility was assessed at 60 and 120 days post-inoculation [10].
2.3. Confirmation of Fusarium circinatum by PCR
Fresh or frozen samples (roots and fungal mycelium) were freeze-dried at −85 °C for 72 h using a ScanVac freeze drier (Labogene, Lillerød, Denmark). Lyophilized samples were crushed, and samples up to 700 mg dry weight were homogenized in a high-speed homogenizer machine (Bertin instruments, Montigny-leBretonneux, France). The extraction and purification procedure followed the protocols of the NucleoSpin® Plant II Midi kit (Macherey-Nagel, Düren, Germany). DNA quantification and quality control of the DNA samples were analyzed spectrophotometrically with a NanoDrop™ One spectrophotometer (Thermo Scientific, Rochester, NY, USA) and adjusted to 10 ng/μL.
DNA extracts were subjected to PCR using species-specific primers CIRC1A and CIRC4A to detect F. circinatum in samples. PCR was performed in a 20 μL PCR reaction using 1× PCR buffer supplied with the DNA polymerase, 0.25 mM of each dNTP, 2 mM MgCl2, 0.5 μM of each primer, 0.05 U/μL DNA polymerase and 6.0 μL of template DNA (approximately 1 ng/μL) [10,17].
The intergenic spacer region (IGS) of the rDNA region was amplified using an Applied Biosystems 2720 thermal cycler (Foster City, CA, USA). The PCR cycling program was set as follows: first step at 95 °C for 3 min; followed by 40 cycles of 30 s at 95 °C, annealing at 64 °C for 55 s and 50 s at 72 °C; and final extension at 72 °C for 12 min [10].
Final PCR products were analyzed using gel electrophoresis on a 1% agarose gel that included a lane of Nancy-520 (Sigma–Aldrich, Merck Group, Burlington, MA, USA) DNA Ladders and viewed with an UV illuminator. After PCR testing with the CIRC1A/4A primers, samples that generated a 146 bp fragment were classified as positive and with the presence of F. circinatum confirmed. A DNA template containing DNA from an F. circinatum isolate that yielded a 146 bp fragment after amplification with CIRC1A and CIRC4A primers served as a positive control. A negative control (purified water) and a false control (a DNA template containing DNA from isolates of F. poae and F. oxysporum from a previous study) were also used to confirm the specificity of the F. circinatum primers and to avoid bias from other Fusarium species.
2.4. Statistical Analyses
Average decline and mortality for seedlings were compared using one-way analysis of variance (ANOVA); comparisons were made between provenances; and significant means (p-value = 0.05) were separated using post hoc test Fisher’s Least Significant Difference (LSD) test in Statistica software (STATISTICA® 7.0 (StatSoft, Inc., Tulsa, OK, USA)). Welch’s F-test or the unequal variances test was used to test the hypothesis that all three provenances have equal means when the samples have unequal variances and/or unequal sample sizes. The survival analysis was performed based on the nonparametric estimator Kaplan–Meier with the “Survival” package to test final mortality. These analyses were performed using Survival 3.5-8 in R 4.1.1 (https://www.r-project.org, accessed on 20 December 2023).
3. Results
3.1. Pathogenicity of Fusarium circinatum in Scots Pine Seedlings (Experiment 1)
Based on the contingency table (Table 2), we compared the following: (i) a null model, which assumes that all seedlings react the same in response to F. circinatum infection, regardless of their origin; (ii) a provenance-effects model, which assumes that a difference exists between the provenances (ten provenance groups and control), but no difference occurs among the seedlings within each group in their response; and (iii) a full model, which states that a difference exists between the groups, and it acknowledges differences among the seedlings within each of the groups in their response. This model considered both the overall effect and the variability within each group of seedlings.

Table 2.
Effects of tested Fusarium circinatum on the germination of Pinus sylvestris, root development, and seedling height at 30 days after inoculation, where Lith = Lithuania and Ukr = Ukraine.
Comparing the null model (no difference between the provenances) against the provenance-effects model resulted in the rejection of the null model (p-value 0.00109). To determine whether significant variation in other symptoms of root inhibition, decline, height and post-emergence mortality exists, we compared the full model (which takes into consideration variation within the groups) against the provenance-effects model (accounting for only the variation between provenance groups). Based on this comparison, the provenance-effects model was rejected (p-value = 0), which indicates that there is significant variation between provenances (p-value 0.0153) (Table 2).
In this assay, F. circinatum exhibited a deleterious impact, causing mortality in nearly half of all pine seedlings, and significantly influencing plant development. The root growth of pine seedlings showed a mortality rate ranging from 39.1% to 51.6% by the 30th day (Table 2) compared to the control group.
Statistical analyses with ANOVA followed by Fisher’s Least Significant Difference (LSD) test indicated that pre-emergence mortality levels and seedling height were nearly identical, with no significant differences observed within and between all provenances (p-value = 0.9628 and p-value = 0.95127, respectively). However, strong and significant differences were observed in root inhibition, decline, and post-emergence mortality compared to the control group for each provenance (all p-values < 0.001). Subsequently, means for all groups—Polish, Lithuanian, and Ukrainian—were used for further. Notably, only Lithuanian provenances exhibited significantly lower post-emergence mortality compared to Polish and Ukrainian ones, although seedling decline was significantly 2.0–2.5 times higher for Lithuanian and Ukrainian provenances.
3.2. Pathogenicity of Fusarium circinatum in Scots Pine Seedlings (Experiment 2)
Initial symptoms of decline, such as needle desiccation, became apparent approximately 70–80 days after inoculation, irrespective of the provenance and group. Over time, desiccated needles transitioned to a yellow hue and eventually wilted. The percentage of symptomatic and dead seedlings, as well as symptomless seedlings, did not show significant differences across pine provenances (F = 2.27, 1.95, 3.11 for Polish, Lithuanian, and Ukrainian provenances, respectively; df = 10; p > 0.10. This difference in mortality appears to be solely attributable to a higher number of seedlings displaying symptoms but remaining alive. No dead or weakened seedlings were observed in the control group (Figure 2).
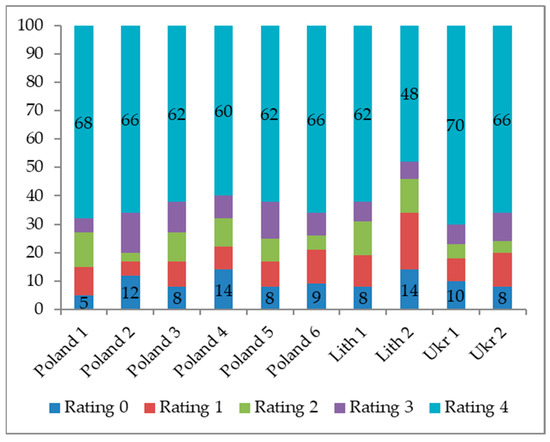
Figure 2.
Distribution of symptoms shown by inoculated Pinus sylvestris seedlings at the end of the experiment following the Vivas et al. [14] scale, where 0 = no symptoms, 1 = desiccation of needles, 2 = discoloration of needles, 3 =wilting of needles, and 4 = seedling dead.
The 11-month-old pine seedling mortality across all groups at 180 days post-inoculation ranged from 60% to 70%, with the exception of one Lithuanian provenance where seedling death reached 48% (F = 9.34, p-value = 0.0014) (Figure 2). Infected pine seedlings exhibited typical decline symptoms, resulting in up to 70% mortality within two months after inoculation. Survival analyses over a six-month period underscored the susceptibility of P. sylvestris to F. circinatum at inoculum levels of 5000 spores mL−1 (Figure 3).
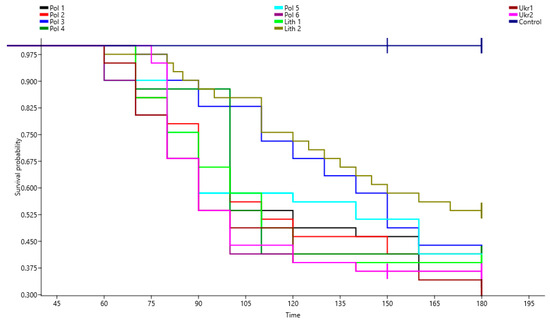
Figure 3.
Plot of survival probability determined using the Kaplan–Meier estimate of the survival function for 10 provenances of Pinus sylvestris seedlings infected with Fusarium circinatum.
Furthermore, the frequency of symptomless seedlings (symptom 0) was notably higher for Lithuanian provenance 2 (14%), as well as for two Polish provenances (Poland 2 and Poland 4) (F = 9.5421; p-value =0.0163).
The final assessment conducted among all provenances at 180 days after inoculation revealed substantial plant mortality, ranging from 60% to 70% for almost all provenances. Notably, pine seedlings originating from Lithuanian provenance 2 demonstrated a significantly lower level of mortality (48%) at the 180 day mark after inoculation (Figure 2).
The inoculated F. circinatum strain was successfully re-isolated from 86%–100% of the seedlings. The nucleotide identity of the samples was 99.8%–100% with F. circinatum strain FCC4880 28S-18S, GenBank: KC147556.1. All re-isolated fungal species and plant samples from the inoculated and control seedlings used for direct amplification of the fungal IGS rDNA region resulted in successful amplification from 100% of the isolates and 87% of the plants. The presence of F. circinatum was confirmed only for seedlings that were inoculated with this pathogen. No F. circinatum was found in the control group.
4. Discussion
Numerous studies have reported significant variability in the susceptibility of conifers to pitch canker, including Pinus sylvestris, emphasizing the high or moderate virulence of the pathogen for both seedlings and mature plants [5,18,19]. Given the absence of cost-effective and efficient treatments for mitigating pitch canker, genetic resistance emerges as the primary strategy to address potential PPC damage. The advancements in genetic resistance integrated into breeding programs can mitigate the risk of infection by different fungal pathogens, based on field observations of tested resistant phenotypes [20,21,22]. While efforts to screen for tolerance to F. circinatum have been undertaken in many locations, such as the USA, Africa, and Europe, there are currently no Scots pine breeding initiatives producing seeds from populations with confirmed pitch canker tolerance [15,23,24]. This is mainly because field data have been found consistent for screening for pitch canker resistance.
Several studies have also highlighted variations in tolerance among Pinus species in different taxonomic sections, as well as differences among species and families within these sections [9,15,20,25]. These studies, along with others involving inoculations, have demonstrated that varieties of P. sylvestris are susceptible to damage but there exists a broad spectrum of the resistance level. Variations in susceptibility to pitch canker have also been observed among pine provenances in research conducted across various countries [14,26]. Preliminary screens of Polish provenances of Scots pine [10] revealed the absence of PPC symptoms in some seedlings from two provenances after inoculation with F. circinatum, similar to findings observed in certain Romanian and Austrian provenances [5,26]. The initial investigation of Polish pine provenances spanned the four-month post-inoculation period [10]; therefore, in present study, the incubation period was extended to six months to explore potential resistance against F. circinatum, considering the variability in susceptibility observed among the Polish provenances [10] and new data from neighboring Ukrainian and Lithuanian provenances. While only two provenances were utilized for Lithuania and Ukraine, six sites were selected for Poland to account for risks of potential pathogen spread. The methodology incorporated lessons from a previous study [10], with adjustments made regarding seedling age and the timing of susceptibility data collection. Despite promising viability post-inoculation, challenges in the survival analysis and the risk of latent infections emphasize the need for continued observation to accurately assess susceptibility. Certain provenances from Poland and Lithuania have not been deemed completely resistant to pitch canker caused by F. circinatum. The inability of F. circinatum to induce disease in certain seedlings may be attributable to various factors, such as individual antifungal activity or the upregulation of proteins associated with carbohydrate and amino acid metabolism, as well as secondary metabolism. These molecular responses could be interpreted as a strategy to compensate for the energy deficit incurred during infection and to withstand the pathogen’s impact. This study highlights the effectiveness of such an approach in preparing foresters for emerging diseases. Despite promising viability post-inoculation, challenges in the survival analysis and the risk of latent infection underscore the importance of ongoing observation to accurately assess susceptibility. The high susceptibility of P. sylvestris underscores the significant threat posed by the introduction of F. circinatum, especially in nursery facilities conducive to its global spread.
In the present study, F. circinatum was re-isolated from all inoculated pine seedlings, despite the apparent resistance of seedlings to F. circinatum infection. The frequency of re-isolation varied among and within provenances, with no variation observed in re-isolation between certain sites. Although certain seedlings appeared resistant to F. circinatum in this study, the fungus was still re-isolated from them, suggesting that some degree of growth into host tissues may occur without necessarily resulting in decline and mortality. Therefore, while certain provenances may exhibit resistance to F. circinatum infection, the presence of the fungus within host tissues indicates the potential for colonization without visible disease symptoms. Further research is needed to elucidate the mechanisms underlying the resistance of some provenances to pitch canker and the dynamics of F. circinatum colonization in host species, especially genotypes that are infected but apparently asymptomatic.
Contrary to the traditional view of pitch canker inducing damping-off symptoms, our study underscores the severe negative impact of F. circinatum on the decline, root inhibition, and post-emergent mortality of P. sylvestris seedlings. Surviving seedlings, however, did not exhibit a subsequent increase in root and shoot biomass or growth even six months post-inoculation compared to the control group. Weakened seedlings showed reduced root development without an enhancement of resistance to stem infections. In contrast, asymptomatic seedlings (score 0, showing no symptoms of wilt or damping-off) displayed comparable root and height parameters to the control group. This suggests that while F. circinatum may not cause the death of certain seedlings, it might even induce resistance, potentially influencing their field survival and subsequent forest dynamics.
Climate is also considered a limiting factor for the spread of F. circinatum in Europe [7,26]. Colder climates are considered restrictive, although the climatic change scenarios of the potential distribution of PPC in Europe includes the Netherlands and Denmark [26,27,28]. Climatic constraints are less restrictive for F. circinatum inducing massive damping-off in forest nurseries, particularly under protected cultivation. Moreover, any international trade in live plant material further increases the risk of pathogen dissemination to disease-free regions of Europe where Scots pine plantations and native forests are present. Our results align with the emerging concept that high-pathogenicity agents such as F. circinatum may serve varied ecological functions extending beyond the immediate impacts of the disease [29]. Nevertheless, our findings unequivocally indicate the susceptibility of the majority of Pinus sylvestris provenances from Poland, Lithuania, and Ukraine to F. circinatum, with the pathogen capable of adversely affecting and killing pine seedlings. However, some provenances and seedlings may exhibit potential resistance.
The dearth of data regarding resistance to F. circinatum constrains our capacity to strategically deploy resources and enhance tolerance, which has not been a primary focus for breeding populations of P. sylvestris. As certain groups have explored the potential benefits of employing genomic tools to characterize pitch canker tolerance in other pine species, these analyses could be effectively integrated into P. sylvestris breeding programs to enhance tolerance [25,30,31].
Despite these observations, it is crucial to recognize that most tests and experiments involving invasive quarantine pathogens like F. circinatum, which rely on greenhouse inoculation data, require further validation. In both nursery and stand environments, multiple pathogens affecting roots, collars, foliage, and vasculature may coexist with F. circinatum [8]. While our data are preliminary, it is conceivable that the results could be influenced by volatiles or other compounds in plants or soil that enhance seedling or tree growth [29,30]. Therefore, considering the unique characteristics of Lithuanian provenance, along with the inherent resistance observed in certain seedlings from two Polish provenances and the variation in susceptibility observed in previous studies, our findings imply potential considerations for future genetic breeding programs of P. sylvestris. In future investigations, expanding the scope to include more varieties from other Eastern European countries would be beneficial. The high susceptibility of P. sylvestris underscores the potential threat of F. circinatum establishment in Eastern and Northern Europe, underscoring the serious risks associated with its introduction to Poland, Ukraine, and Lithuania.
5. Conclusions
In conclusion, our study provides valuable insights into the susceptibility of Polish, Lithuanian, and Ukrainian provenances of Scots pine (Pinus sylvestris) to F. circinatum. The findings suggest a high vulnerability of the tested pine provenances, as evidenced by the significant mortality rates observed among all provenances at 180 days after inoculation, ranging from 60% to 70%. However, pine seedlings from a Lithuanian provenance exhibited a notably lower mortality rate (48%), highlighting the importance of continued investigations to understand and mitigate the impact of this pathogen on forest ecosystems. Our results underscore the urgent need for proactive measures to manage the risk of F. circinatum introduction and spread, particularly in nursery settings where its global dissemination is plausible.
Author Contributions
Conceptualization, J.J.D.C. and T.O.; data curation, K.D. and J.A.N.; formal analysis, K.D.; investigation, K.S., J.A.N. and K.R.; methodology, N.Ł.-S., S.M., D.B., O.S. and J.M.-G.; project administration, J.J.D.C.; resources, J.A.N., S.M., D.B. and O.S.; software, K.D.; supervision, T.H. and T.O.; validation, N.Ł.-S., K.S. and K.R.; visualization, J.M.-G.; writing—original draft, K.D.; writing—review & editing, T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This article was developed under the Scholarship Fund of the Forest Research Institute in Poland (Agreement on Realization of Scholarship for Kateryna Davydenko from the Scholarship Fund of the Forest Research Institute concluded on 24 September 2018 in Sękocin Stary, Appendix No. 3). The research of K.D. was funded in part by the Carl Tryggers Stiftelse (CTS), project number CTS 23:2906. K.D. was supported by the Ministry of Education and Science of Ukraine within joint Ukrainian–Latvian project No. M/92-2023.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Gordon, T.; Swett, C.; Wingfield, M. Management of Fusarium Diseases Affecting Conifers. Crop Prot. 2015, 73, 28–39. [Google Scholar] [CrossRef]
- van Diepeningen, A.D.; de Hoog, G.S. Challenges in Fusarium, a Trans-Kingdom Pathogen. Mycopathologia 2016, 181, 161–163. [Google Scholar] [CrossRef]
- Gordon, T. Pitch Canker Disease of Pines. Phytopathology 2006, 96, 657–659. [Google Scholar] [CrossRef]
- Bezos, D.; Martinez-Alvarez, P.; Fernandez, M.; Diez, J. Epidemiology and Management of Pine Pitch Canker Disease in Europe—A Review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Gordon, T.; Kirkpatrick, S.; Aegerter, B.; Wood, D.; Storer, A. Susceptibility of Douglas Fir (Pseudotsuga Menziesii) to Pitch Canker, Caused by Gibberella circinata (anamorph = Fusarium circinatum). Plant Pathol. 2006, 55, 231–237. [Google Scholar] [CrossRef]
- Drenkhan, R.; Ganley, B.; Martín-García, J.; Vahalík, P.; Adamson, K.; Adamcíková, K.; Ahumada, R.; Blank, L.; Bragança, H.; Capretti, P.; et al. Global Geographic Distribution and Host Range of Fusarium circinatum, the Causal Agent of Pine Pitch Canker. Forests 2020, 11, 724. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH). Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. Efsa J. 2010, 8, 1620. [Google Scholar] [CrossRef]
- Amaral, J.; Valledor, L.; Alves, A.; Martin-Garcia, J.; Pinto, G. Studying Tree Response to Biotic Stress Using a Multi-Disciplinary Approach: The Pine Pitch Canker Case Study. Front. Plant Sci. 2022, 13, 916138. [Google Scholar] [CrossRef]
- Davydenko, K.; Nowakowska, J.A.; Kaluski, T.; Gawlak, M.; Sadowska, K.; Garcia, J.M.; Diez, J.J.; Okorski, A.; Oszako, T. A Comparative Study of the Pathogenicity of Fusarium circinatum and Other Fusarium Species in Polish Provenances of P. sylvestris L. Forests 2018, 9, 560. [Google Scholar] [CrossRef]
- Vainio, E.; Martinez-Alvarez, P.; Bezos, D.; Hantula, J.; Diez, J. Fusarium circinatum Isolates from Northern Spain Are Commonly Infected by Three Distinct Mitoviruses. Arch. Virol. 2015, 160, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Abdo, Z.; Dumroese, R.K.; Klopfenstein, N.B.; Kim, M.S. Virulence of Fusarium oxysporum and F. commune to Douglas-Fir (Pseudotsuga menziesii) Seedlings. For. Pathol. 2012, 42, 220–228. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Raposo, R.; García-Serna, I.; Mesanza, N.; Kirkpatrick, S.C.; Gordon, T.R. Resistance levels of Spanish conifers against Fusarium circinatum and Diplodia pinea. For. Pathol. 2013, 43, 488–495. [Google Scholar] [CrossRef]
- Vivas, M.; Zas, R.; Solla, A. Screening of Maritime pine (Pinus pinaster) for Resistance to Fusarium circinatum, the Causal Agent of Pitch Canker Disease. Forestry 2012, 85, 185–192. [Google Scholar] [CrossRef]
- Amaral, J.; Correia, B.; Antonio, C.; Rodrigues, A.; Gomez-Cadenas, A.; Valledor, L.; Hancock, R.; Alves, A.; Pinto, G. Pinus Susceptibility to Pitch Canker Triggers Specific Physiological Responses in Symptomatic Plants: An Integrated Approach. Front. Plant Sci. 2019, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Ioos, R.; Aloi, F.; Piskur, B.; Guinet, C.; Mullett, M.; Berbegal, M.; Braganca, H.; Cacciola, S.; Oskay, F.; Cornejo, C.; et al. Transferability of PCR-Based Diagnostic Protocols: An International Collaborative Case Study Assessing Protocols Targeting the Quarantine Pine Pathogen Fusarium circinatum. Sci. Rep. 2019, 9, 8195. [Google Scholar] [CrossRef]
- Schweigkofler, W.; O’Donnell, K.; Garbelotto, M. Detection and Quantification of Airborne Conidia of Fusarium circinatum, the Causal Agent of Pine Pitch Canker, from Two California Sites by Using a Real-Time PCR Approach Combined with a Simple Spore Trapping Method. Appl. Environ. Microbiol. 2004, 70, 3512–3520. [Google Scholar] [CrossRef]
- Bonello, P.; Gordon, T.; Storer, A. Systemic Induced Resistance in Monterey pine. For. Pathol. 2001, 31, 99–106. [Google Scholar] [CrossRef]
- Slinski, S.L.; Kirkpatrick, S.C.; Gordon, T.R. Inheritance of virulence in Fusarium circinatum, the cause of pitch canker in pines. Plant Pathol. 2016, 65, 1292–1296. [Google Scholar] [CrossRef]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2010, 41, 601–607. [Google Scholar] [CrossRef]
- Vivas, M.; Martin, J.; Gil, L.; Solla, A. Evaluating Methyl Jasmonate for Induction of Resistance to Fusarium oxysporum, F. circinatum and Ophiostoma novo-ulmi. For. Syst. 2012, 21, 289–299. [Google Scholar] [CrossRef]
- Liziniewicz, M.; Tolio, B.; Cleary, M. Monitoring of Long-Term Tolerance of European ash to Hymenoscyphus fraxineus in Clonal Seed Orchards in Sweden. For. Pathol. 2022, 52, e12773. [Google Scholar] [CrossRef]
- Fulton, J.C.; Yu, P.L.; Smith, K.E.; Huguet-Tapia, J.C.; Hudson, O.; Meeks, A.; Quesada, T.; McKeever, K.; Brawner, J.T. Comparative Genomics of Fusarium circinatum Isolates Used to Screen Southern Pines for Pitch Canker Resistance. Mol. Plant-Microbe Interact. 2022, 35, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Schmale, D.; Gordon, T. Variation in Susceptibility to Pitch Canker Disease, Caused by Fusarium circinatum, in Native Stands of Pinus muricata. Plant Pathol. 2003, 52, 720–725. [Google Scholar] [CrossRef]
- De Vos, L.; van der Nest, M.A.; Santana, Q.C.; van Wyk, S.; Leeuwendaal, K.S.; Wingfield, B.D.; Steenkamp, E.T. Chromosome-Level Assemblies for the Pine Pitch Canker Pathogen Fusarium circinatum. Pathogens 2024, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.; Flores-Pacheco, J.A.; Munoz-Adalia, E.J.; Martínez-Alvarez, P.; Martín-García, J.; Diez, J.J. Susceptibility of Germinating Seedlings of European and Eurasian Populations of Pinus sylvestris to Damping-Off Caused by Fusarium circinatum. For. Pathol. 2022, 52, e12749. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; Ganley, R.; Kriticos, D.; Manning, L. Dothistroma Needle Blight and Pitch Canker: The Current and Future Potential Distribution of Two Important Diseases of Pinus species. Can. J. For. Res. 2011, 41, 412–424. [Google Scholar] [CrossRef]
- Moykkynen, T.; Capretti, P.; Pukkala, T. Modelling the Potential Spread of Fusarium circinatum, the Causal Agent of Pitch Canker in Europe. Ann. For. Sci. 2015, 72, 169–181. [Google Scholar] [CrossRef]
- Swett, C.; Gordon, T. Exposure to a Pine Pathogen Enhances Growth and Disease Resistance in Pinus radiata Seedlings. For. Pathol. 2017, 47, e12298. [Google Scholar] [CrossRef]
- Chen, Z.P.; Yang, X.; Xia, H.M.; Wu, C.P.; Yang, J.; Dai, T.T. A Frontline, Rapid, Nucleic Acid-Based Fusarium circinatum Detection System Using CRISPR/Cas12a Combined with Recombinase Polymerase Amplification. Plant Dis. 2023, 107, 1902–1910. [Google Scholar] [CrossRef]
- Swett, C.; Kirkpatrick, S.; Gordon, T. Evidence for a Hemibiotrophic Association of the Pitch Canker Pathogen Fusarium circinatum with Pinus radiata. Plant Dis. 2016, 100, 79–84. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).